-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Dermatology and Venereology
2012; 1(2): 6-23
doi: 10.5923/j.ajdv.20120102.01
Adiponectin, IL-10, IL-23 and Trace Elements Serum Levels in Patients with Psoriasis
Amina Hamed Alobaidi 1, Zaid Mothana 1, Wesam Suhail Najem 2, Abdulghani Mohamed Alsamarai 2, 3
1Department of Biochemistry, Tikrit University College of Medicine, Tikrit, Iraq
2Department of Medicine, Tikrit University College of Medicine, Tikrit, Iraq
3Asthma & Allergy Centre, Tikrit Teaching Hospital, Tikrit, Iraq
Correspondence to: Abdulghani Mohamed Alsamarai , Department of Medicine, Tikrit University College of Medicine, Tikrit, Iraq.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Psoriasis is a multifactorial, chronic, inflammatory skin disease of unknown aetiology, associated with cytokines and adipokines serum levels changes that may influence disease pathogenesis. A case control study was performed to determine the serum levels of IL-10, IL-23, adiponectin, Zn, Mg and Cu in psoriasis patients. IL-10 and IL-23 mean serum level were higher in psoriatic patients than in control. Moderate and severe cases of psoriasis were with lower serum IL-10 mean level as that in mild form of disease and controls. Mean IL-23 serum level was significantly higher in obese psoriatic patients than in psoriatic patients with BMI <25 and in control individuals with BMI of >25. Serum levels of IL-23 highly significantly negatively correlated with disease duration. An interesting finding of this study was that serum zinc levels were significantly negatively correlated with BMI and the correlation coefficient was more in psoriatic obese or overweight patients (with BMI >25). A highly significant positive correlation between zinc serum levels in psoriatic patients and serum adiponectin levels. A significant correlation demonstrated between IL-10 & IL-23; Mg & IL-23; Adiponectin & BMI; Zn & Cu. Our findings indicated the influence of obesity and disease severity on psoriasis markers.
Keywords: Psoriasis, IL-10, IL-23, Adiponectin, Zn, Mg, Cu, PASI, BMI
Cite this paper: Amina Hamed Alobaidi , Zaid Mothana , Wesam Suhail Najem , Abdulghani Mohamed Alsamarai , "Adiponectin, IL-10, IL-23 and Trace Elements Serum Levels in Patients with Psoriasis", American Journal of Dermatology and Venereology, Vol. 1 No. 2, 2012, pp. 6-23. doi: 10.5923/j.ajdv.20120102.01.
Article Outline
1. Introduction
- Psoriasis is a multifactorial, chronic, inflammatory skin disease of unknown aetiology[1]. T cells secrete or stimulate the production of powerful immune factors called cytokines[2]. Which are peptides, proteins or glycoprotein that has a fundamental role in communication within the immune system and in allowing the immune system and host tissues cells to exchange information[3]? At the present time, one of the main areas of research in the psoriasis field concerns the role of cytokines in the pathogenesis of this disease[1]. Psoriatic lesions have a type 1 cytokine profile (i.e., interleukin (IL)-2, interferon (IFN)-γ, and tumour necrosis factor (TNF)-α), without a significant component of type 2 cytokines (i.e., IL-4, IL-5, and IL-10)[4]. IL-10 is an important immunoregulatory cytokine. One of its mainbiological functions seems to be the limitation and termination of inflammatory responses. Induction of IL-10 expression was found by conventional antipsoriatic therapies, suggesting that IL-10 may be a key cytokine in psoriasis and that application of this cytokine may have therapeutic effects[5].There are growing evidences that a recently recognizedsubset of T cells, Th17 cells, may play an important role in the pathogenesis of psoriasis. The Th17 lineage has emerged from discovery of a new family of cytokines, the IL-17 family that comprises IL-17 and IL-22. Th17 cells differentiate from naïve CD4+ T cells under the stimulation of IL-1, IL-6, and transforming growth factor-β, and their proliferation is driven by IL-23[6]. IL-23 is produced by activated dendritic cells, macrophages and keratinocytes[7].Adipokines are peptide hormones or cytokines secreted from adipose tissues and involved in the pathogenesis of metabolic syndrome[8]. More recently, psoriasis has also been reported to be associated with metabolic disorders including obesity, dyslipidemia and diabetes[9]. Adiponectin as a one of the adipokines is an adipocyte specific secretory protein abundantly present in the circulation. Plasma levels of adiponectin are decreased in obesity, insulin resistance and type 2 diabetes, and hypoadiponectinaemia is assumed to be closely associated with the metabolic syndrome[10].Zinc (Zn) is essential for the survival and function of all cells. The effects of zinc deficiency are particularly obvious in the skin, seen as an erythematous rash, scaly plaques, and ulcers[11]. Zinc also plays important roles in immune functions which regulate several functions of lymphocytes, such as mitogenesis, antibody synthesis, the activation of T cells and natural killer cells, and more specifically cellular immunity[12]. The decreased production of Th1 cytokines by leukocytes in the healthy elderly person is correlated with low zinc serum level. Interestingly, zinc induces cytokine production by isolated leukocytes. Zinc induces monocytes to produce IL-1, IL-6 and TNF-α in peripheral blood mononuclear cells and separated monocytes[13].Increased levels of proinflammatory cytokines (IL-6, TNF-α) have been reported in animals under Magnesium (Mg) deprivation for 3 weeks. In fact, the secretion of certain cytokines such as IL-2, IL-4, IL-5, IL-10, IL-12, IL-13 and IFN-γ has been shown to be induced by plasma substance P (SP) treatment (a well-known cytokine production stimulator)[14].Copper (Cu) is known to play an important role in the development and maintenance of the immune system. The IL-2 is mainly secreted by activated blood T-lymphocytes, where play a central role in regulating the host response to pathogenic challenges. In humans, impaired secretion of IL-2 receptor (a marker of early T cell activation) by peripheral blood mononuclear cells from healthy subjects who received a low-Cu diet has been described[15]. This study was conducted to evaluate the serum levels of IL-10, IL-23 and adiponectin in psoriatic patients and their modulation by therapy in order to explore the immunopathogenesis in psoriasis. The study protocol was approved by Ethical Committee of Tikrit University College of Medicine.
2. Patients and Methods
2.1. Study Design
- This is a hospital-based, case control study conducted in Tikrit Teaching Hospital from January 1, 2011 to July 31, 2011. Gender and age matched controls were recruited from individuals attending Dermatology Department accompanying their relative patients.
2.2. Study Population
- The patients were recruited from outpatient clinic of Department of Dermatology. The study included 50 patients with psoriasis vulgaris were diagnosed by consultant dermatologists according to standard criteria[16]. The PASI for each patient was determined by the same physicians. Of the total, 29 (58 %) were females, and 21(42%) were males and their ages ranging from 12 to 60 years. The history as well as personal information about patients was obtained by questionnaire. All patients would not take any psoriasis treatments for at least one week before venous blood collection. Fifty apparently healthy individual who's matching the patients group in age, sex and BMI, with no history of allergic and medical diseases were chosen from Tikrit city citizens. Verbal agreement of the chosen patients was obtained before involvement in the study. Blood sample were obtained from the veins of tourniquet forearm of the patients, control and the therapeutic modulation groups, serum collected and stored at -30°C until the time of analysis. Samples showing haemolysis was discarded.
2.3. Measurements
- The IL-10, IL-23, adiponectin, Zn, Cu and Mg in serum for all patients, control and therapeutic modulation groups measurement were performed in Postgraduate Research Laboratory, Tikrit University College of Medicine. Adiponectin, IL-10 and IL-23 serum levels determined by ELISA method. While serum levels of zinc, magnesium and copper determined using colorimetric method.
3. Results
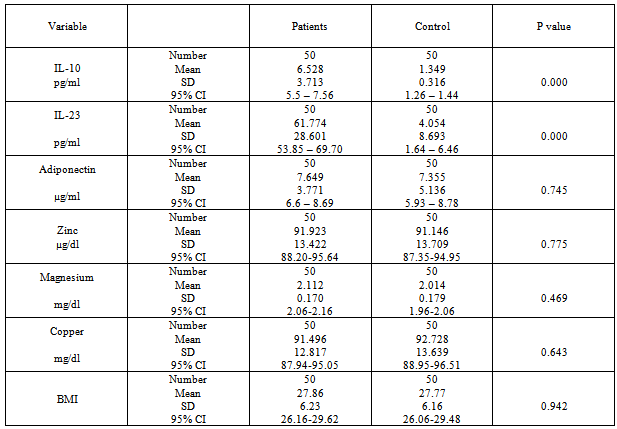
- The highest percentage of disease (26%) was found in age group of (21-30) years, while lowest percentage of disease (14%) was found in age group of (51-60) years. In control also the highest percentage (30%) was found in age group (21-30) years and lowest percentage (8%) was found in age group of (51-60) years. Of the total, 21 (42%) individuals were male and 29 (58%) were female (58%) for both patients and control groups. The highest percentage of woman in psoriatic patients (27.6%) was (11-20) while in man (47.6%) was between (21-30). In control, the highest percentage of woman in (31%) was (31-40) while in man (47.6%) was (21-30). Table 1.
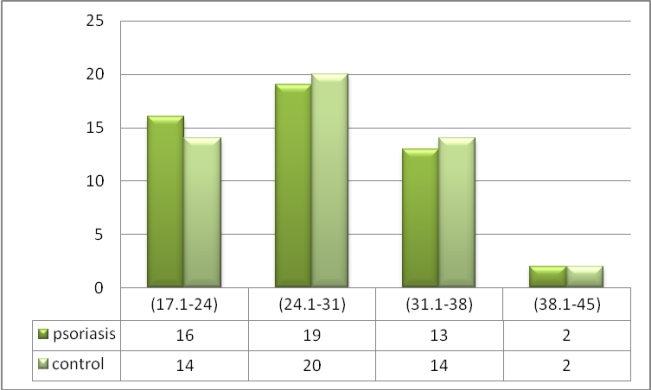 | Figure 1. The Distribution of Psoriatic Patients and control According to BMI intervals |
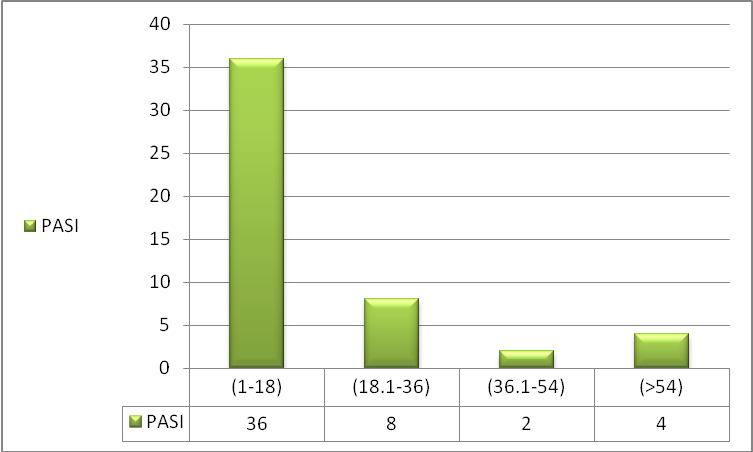 | Figure 2. The Distribution of Psoriatic Patients According to PASI |
|
|
|
|
|
3.1. Interleukin-23 (IL-23)
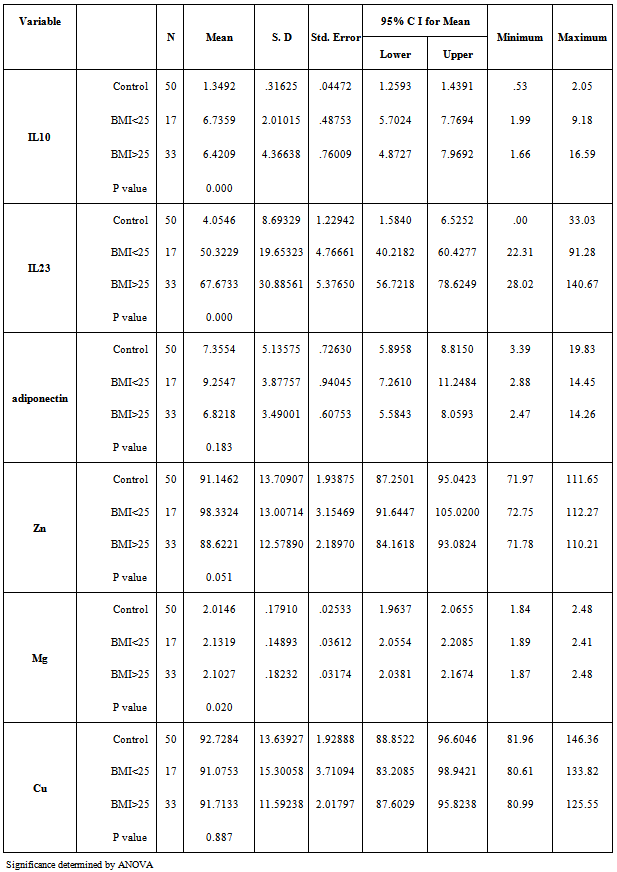
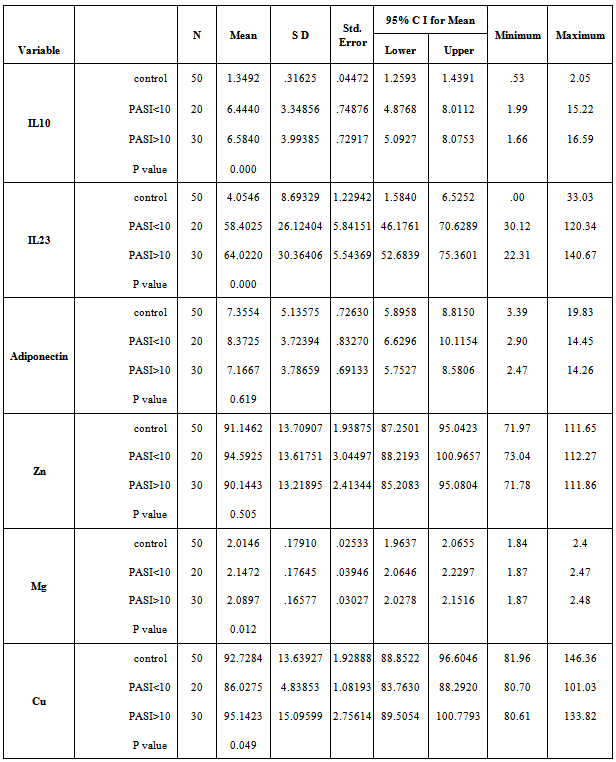
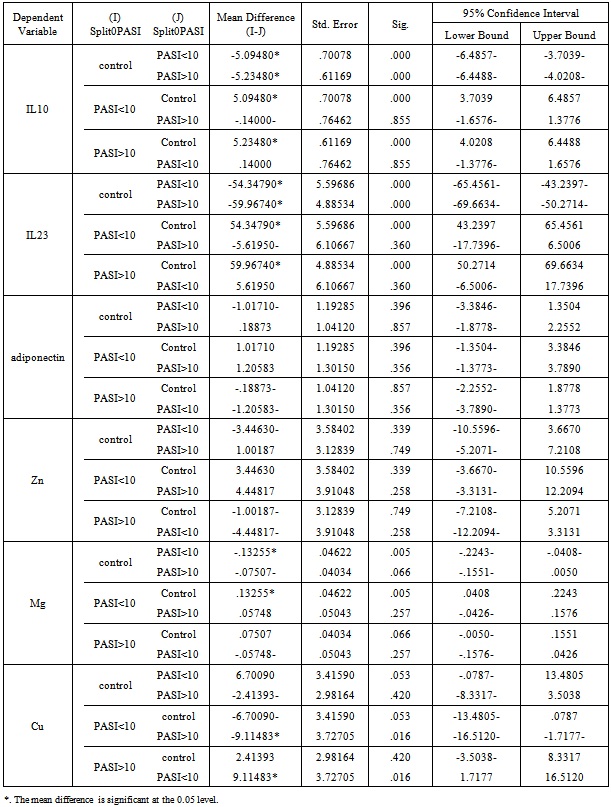
- The mean serum levels of IL-23 for psoriatic patients were (61.77±28.6) pg/ml, while it was (4.05±8.69) pg/ml for control. Table (2), the difference was statistically highly significant (p=0.01). Psoriatic patients with PASI<10 the mean of IL-23 was (58.402±26.12) pg/ml, while the corresponding value in controls was (3.812±9.68) pg/ml with highly significant difference (p=0.00). In psoriatic patients with PASI>10, the mean of IL-23 was (64.022±30.36) pg/ml, while the corresponding value controls was (4.216±8.13) pg/ml, with highly significant difference (p=0.00). Table 5 & 6.A highly significant difference (p=0.00) was found in IL-23 serum levels of the psoriatic patients (50.322±19.65 pg/ml) whom underweight or normal weight (BMI<25) as compared to control individuals (0.463±1.96 pg/ml) with same BMI. In obesity and overweight patients (BMI>25), the IL-23 was (67.673±30.88) pg/ml, while the corresponding value in controls was (6.074±10.27) pg/ml, indicating a highly significant difference (p=0.00). Table (3 & 4).
3.2. Adiponectin
- In table (2) the mean serum levels of adiponectin in psoriatic patients was (7.649±3.77) μg/ml, while in controls group was (7.35±5.13) μg/ml, the difference in serum mean values was statistically not significant (p=0.745).The mean serum levels of adiponectin in psoriatic patients with (PASI<10) was (8.372±3.72) μg/ml, while in controls group was (9.235±6.36) μg/ml, there was no significant difference between the two groups (P=0.605), While in patients with (PASI>10) the adiponectin serum level was (7.166±3.78) μg/ml and in corresponding controls was (6.102±3.73) μg/ml, and there was no significant difference between the two groups (P=0.278).Table 5 & 6.The mean serum levels of adiponectin in psoriatic patients with (BMI<25) was (9.254±3.87) μg/ml, while in controls group was (6.127±4.71) μg/ml, there was significant difference between the two groups (P=0.039). While in patients with (BMI>25) the adiponectin serum level was (6.821±3.49) μg/ml and in corresponding controls was (8.045±5.3) μg/ml, there was no significant difference between the two groups (P=0.278). Table 3 & 4.
3.3. Zinc
- The mean serum levels of zinc in psoriatic patients was (91.92±13.42) μg/dl, while in controls group was (91.14±13.7) μg/dl, there was no significant difference between the two groups (P=0.775).Table (2). The mean serum levels of zinc in psoriatic patients with (PASI<10) was (94.592±13.61) μg/dl, while in controls group was (96.073±12.25) μg/dl, there was no significant difference between the two groups (P=0.720). While in patients with (PASI>10) the zinc serum level was (90.144±13.21) μg/dl and in corresponding controls was (87.861±13.82) μg/dl, there was no significant difference between the two groups (P=0.516). Table 5 & 6. The mean serum levels of zinc in psoriatic patients with (BMI<25) was (98.332±13) μg/dl, while in controls group was (91.2±14.28) μg/dl, there was no significant difference between the two groups (P=0.132). While in patients with (BMI>25) the zinc serum level was (88.622±12.57) μg/dl and in corresponding controls was (91.115±13.6) μg/dl, there was no significant difference between the two groups (P=0.446). Table 3 & 4.
3.4. Magnesium
- Mean serum levels of Mg for psoriatic patients were (2.11±0.17) mg/dl, while the mean serum levels of Mg for control was (2.01±0.17) mg/dl. The difference in mean serum Mg levels was statistically not significant. (P=0.469). Table 2. The mean serum levels of Mg in psoriatic patients with (PASI<10) was (2.147±0.17) mg/dl, while in controls group was (2.113±0.23) mg/dl, there was no significant difference between the two groups (P=0.616). While in patients with (PASI>10) the Mg serum level was (2.089±0.16) mg/dl and in corresponding controls was (1.948±0.07) mg/dl, there was highly significant difference between the two groups (P=0.00). Table 5 & 6. The mean serum levels of Mg in psoriatic patients with (BMI<25) was (2.131±0.14) mg/dl, while in controls group was (2.096±0.22) mg/dl, there was no significant difference between the two groups (P=0.581). While in patients with (BMI>25) the Mg serum level was (2.102±0.18) mg/dl and in corresponding controls was (1.968±0.12) mg/dl, there was highly significant difference between the two groups (P=0.001). Table 3 & 4.
3.5. Copper
- The mean serum levels of copper in psoriatic patients was (91.49±12.81) μg/dl, while in controls group was (92.72±13.63) μg/dl, the difference in mean serum levels of copper between patients and control was statistically not significant (p=0.643), table 2. The mean serum levels of copper in psoriatic patients with (PASI<10) was (86.027±4.83) μg/dl, while in controls group was (95.476±16.84) μg/dl, there was significant difference between the two groups (P=0.025). While in patients with (PASI>10) the copper serum level was (95.142±15.09) μg/dl and in corresponding controls was (90.896±10.94) μg/dl, there was no significant difference between the two groups (P=0.218). Table 5 & 6. The mean serum levels of copper in psoriatic patients with (BMI<25) was (91.075±15.3) μg/dl, while in controls group was (98.453±20.84) μg/dl, there was no significant difference between the two groups (P=0.240). While in patients with (BMI>25) the copper serum level was (91.713±11.59) μg/dl and in corresponding controls was (89.507±5.09) μg/dl, there was no significant difference between the two groups (P=0.324). Table 3 & 4.
3.6. Correlations between Variables
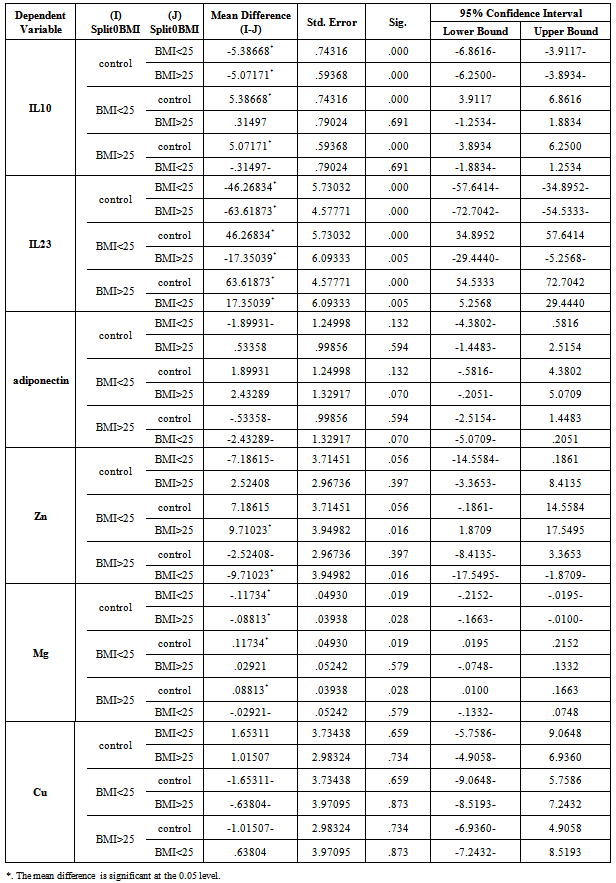
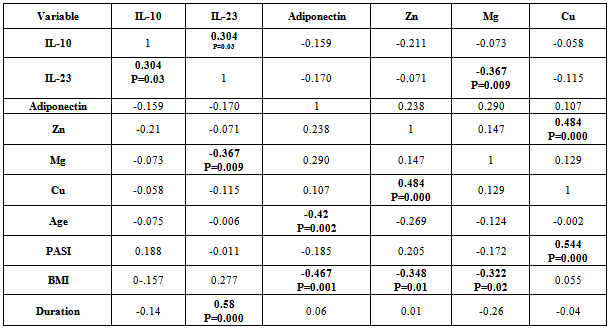
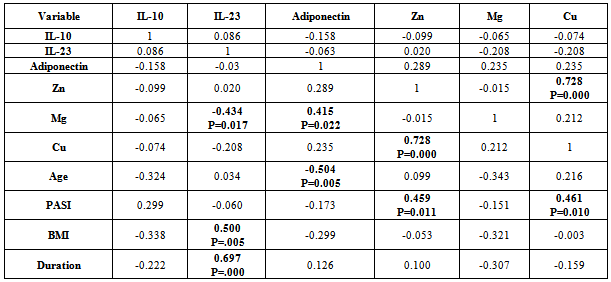
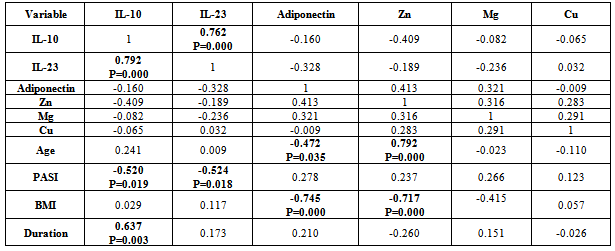
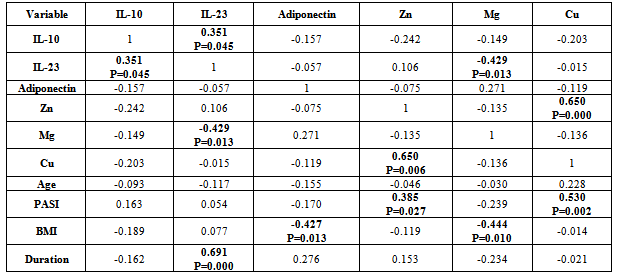
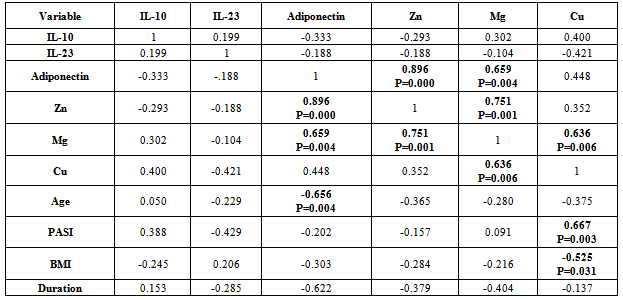
- There was positive significant correlation between IL-10 and IL-23 (r=0.304, p=0.032). Table 7. In patients with PASI<10, there was a positive highly significant correlation between IL-10 and IL-23 serum levels (r=0.762, p=0.00). In addition, IL-10 serum levels were with a positive highly significant (r=0.637, P=0.003) with disease duration. A negative highly significant correlation between IL-10 serum levels and PASI was found (r=-0.52, p=0.019) Table 9. In overweight and obese patients (BMI>25), IL-10 serum levels were with a positive significant correlation with IL-23 serum levels (r=0.351, p=0.045).There was inverse (negative) highly significant correlation between IL-23 and Mg (r=-367, p=0.009). In patients with (PASI>10) a significant negative correlation between IL-23 and Mg (r=-0.434, p=0.017) was found. However, IL-23 serum levels were with highly significant positive correlation with disease duration (r=0.697, P=0.000) and BMI (r=0.500, p=0.005). In addition, there was a significant negative correlation between IL-23 serum levels and PASI (r=-0.524, p=0.018). While in overweight and obesity patients (BMI>25), IL-23 serum levels were significantly negatively correlated to Mg (r= -0.429, P=0.013)However, adiponectin serum levels have a highly significant negative correlations with BMI (r=-0.467, p=0.001) and age (r=-0.410, p=0.003). Table (7). In patients with (PASI>10) adiponectin serum levels were positively significantly correlated with Mg serum levels (r=0.415, p=0.022). In addition, serum levels adiponectin were negatively correlated with age (r=-0.504, p=0.005). Table 8. While in patients with PASI <10, significant negative correlations between adiponectin and BMI was found (r=-0.745, p=0.00) and with age (r=-0.472, p=0.035). Table 9.Adiponectin serum levels have positive significant correlations with Zn (r=0.896, p=0.00), and Mg (r=0.659, p=0.004) in underweight or normal weight patients (BMI<25). In addition, negative significant correlations were demonstrated between adiponectin serum levels with between with age (r= -0.656, p=0.004), and duration (r= -0.622, P=0.008). Table 11. Furthermore, adiponectin serum levels were negatively correlated with BMI (r= -0.427, P=0.013) in patients with BMI >25. Table 10.
|
|
|
|
|
4. Discussion
- IL-10, a major anti-inflammatory cytokine, plays an important role in down-regulating inflammatory and immune responses. IL-10 exerts its anti-inflammatory effects on various cell types (TH1 cells, monocytes /macrophages) and also regulates several PMN functional responses[17]. The present study indicated that serum of psoriatic patients had highly significant increase in IL-10 mean level than in control, this finding agree with Borska L. et al.[18] study. In addition, Elkhayam et al[19] reported a significantly higher plasma level of IL-10 in patients with psoriatic arthritis. Also, Deeva et al.[20] had reported higher level of serum IL-10 in mild to moderate psoriasis patients, when compared to healthy controls. However, the present study finding was not agreed with studies reported by Jacob et al,[21] as in their study, IL-10 was undetectable in serum of psoriatic patients. Furthermore, some studies not determined a statistically significant difference between psoriatic patients and healthy controls in IL-10 serum levels[22, 23] In addition, other studies revealed that IL-10 significantly decreased in psoriasis serum as compared to controls[24,25]. The IL-10 promoter contains several transcription factor-responsive elements. Thus macrophages, the major source of IL-10, are stimulated to produce IL-10 by several endogenous and exogenous factors such as endotoxin, TNF-α, catecholamines, and cAMP-elevating drugs[25]. Reported studies[26, 25] indicated that TNF-α serum levels increased significantly in psoriatic patients in comparison to controls. In addition, TNF-α production showed a positive relation to clinical severity of disease[25, 26]. This may explain the high IL-10 serum levels in this study.A depression of monocytic TNF-a and IL-12 secretion capacity as well as decreasing plasma levels of IL-12 which is critically important for the generation of cell-mediated immunity[27]. In contrast, increased production of monocytes TNF-α and IL-12 as well as increasing plasma levels of them may upregulate overproduction of IL-10. This lead to increase in serum/plasma levels of IL-10 in mild to moderate but not in severe psoriasis cases[20]. The present study findings indicated that moderate and severe cases of psoriasis[PASI >10] were with lower serum IL-10 mean level[5.54 ±2.72] as compared to mean[6.33±3.41] in mild form[PASI <10] of disease and controls. Furthermore, in a recent reported research[28], weight loss lead to increase in IL-10 production.Thus in the present study, IL-10 serum level was increased in our patients series which may be due to inducer cells activation by IL-12. These information collected together suggested that IL-10 production was influenced by its pleiotropic property and polymorphism, which leads to variable production sets, increased production in some cases or reduced in others. This could explain the variability in IL-10 levels in psoriatic patients between the reported studies. Not all substances (biologic and drugs) are associated with increase of internal IL-10 production[29, 30,31] as some suggest[32], thus IL-10 may be increased in some patients and reduced in others, whether the changes due to biologic substances or drugs.The present study indicated that mean serum IL-10 was not significantly different when psoriatic patients divided into those who are with BMI of <25 (6.74 pg/ml) or >25 (6.42pg/ml). However, patients with BMI of less than 20 were with mean serum IL-10 level of 8.83 pg/ml, indicating that obesity may have a reflection on serum IL-10. This needs a future research in a large number of patients to reach accepted conclusion. Furthermore, both groups (<25 & >25) are with mean IL-10 serum levels that were significantly higher than in control.Patients with PASI of <10 with no significant mean serum level of IL-10 from those with PASI >10. However, mean IL-10 serum level in patients with moderate and severe form (PASI >10) were with significantly lower value (5.54±2.72 ) as compared to mild (6.33±3.41) cases of psoriasis. There are possibilities that IL-10 might be involved in the early phase of psoriasis development and reduced subsequently in accordance with disease severity. In addition, both PASI groups were with significantly higher serum IL-10 mean level as compared to control (P=0.000). IL-10 serum levels were positively correlated to IL-23 (r= 0.304, P=0.032), PASI (r= 0.188, P=0.19) and sex (r=0.186, P=0.19). IL-10 serum levels were also negatively correlated with Zinc (r=-0.211, P=0.142), adiponectin (r=-0.159, P=0.26), BMI (r=-0.157, P=0.27) and duration (r=-0.139, P=0.33). However, there was a weak negative correlation with Age, Mg and Cu. In addition, patients group with PASI of > 10, showed a negative correlation between IL-10 and age (r=-0.324, P=0.08), BMI (r=-338, P=0.06), and duration (r=-222, P=0.23). These correlation values were more than when the patients collected in one group. Furthermore, correlation value between IL-10 and PASI increased from 0.188 in total to 0.299 in PASI group of >10. In addition, Pearson correlation reduced between IL-10 and IL-23 from 0.304 in total to 0.08 in PASI of >10 group. In patients group with PASI of <10, Pearson correlation value in contrast to patients group of >10, increased from 0.304 in total to 0.762 between IL-10 and IL-23 (P=0.000). IL-10 was positivity correlated with disease duration (r=0.637, P=0.003) and negatively correlated with PASI (r=-0.52, P=0.019). Takahashi et al[25], reported a negative correlation between IL-10and PASI. Interestingly, a strong correlations were found between IL-10 and IL-23, disease duration and PASI in patients with mild severity (PASI<10).In psoriasis and other autoimmune inflammatory diseases the IL-23 stimulates survival and proliferation of Th17 cells, and thus serves as a key master cytokine regulator for these diseases. IL-23 is overproduced by dendritic cells and keratinocytes, and this cytokine stimulates Th17 cells within dermis to make IL-17A and IL-22[33]. The highly significant increase in IL-23 serum levels in psoriatic patients in this study agree with Coimbra et al[34] and Hadidi et al[7] studies who found there was statistically highly significant increase in serum IL-23 levels of the psoriatic patients in comparison with controls. These two studies refer to the important role of the IL-23 in pathogenesis of psoriasis. However, the present study finding not agreed with that reported by Nakajima et al[8], who not detected IL-23 in serum of their psoriatic patients and controls.An interesting finding of this study is that IL-23 mean serum level was significantly (P=0.000) higher (67.67 pg/ml) in obese psoriatic patients (BMI>25) than in psoriatic patients with BMI <25 (50.32 pg/ml). In addition, IL-23 mean serum value was statistically highly significant (P=0.000) in psoriatic patients with BMI of >25 as compared to control individuals with BMI of >25 (0.463 pg/ml). Furthermore, IL-23 serum mean value was significantly higher (P=0.000) in psoriatic patients with BMI of > 25 as compared to control individuals with BMI of > 25 (6.07 pg/ml). Thus IL-23 mean serum levels were higher in psoriatic patients than in age, sex and BMI matched non –psoriatic control. This finding indicated that obesity could be an underlying cause for psoriasis or it may be a consequence that follows disease initiation. Thus a future research is warranted and to be performed in obese children as a follow up research study design to elucidate this association. The finding of this study was consistent with that reported by Sumarac-Dumanovic et al[35,36], which indicate a higher blood concentration of IL-23 in obese as compared to lean women.The present study clarify a negative correlation between IL-23 serum levels and Mg serum level was found (r=-0.367, p<0.01) in psoriatic patients. In addition, a negative correlation was demonstrated between serum IL-23 levels and adiponectin (r=-0.17, p=0.23) and Cu (r=-115, P=0.42). However, a weak correlation between serum IL-23 levels and Zn (r=-0.071, P=0.62). To our knowledge, this is the first report that studied the correlations between serum IL-23 levels and Mg, Cu, Zn, thus we are unable to compare it with other works.Serum IL-23 levels demonstrated a positive correlation with BMI, which is just with marginal P value (=0.051). This indicated the role of obesity in provoking psoriasis immune-pathogenesis, since IL-23 play an important role in pathogenesis of psoriasis. The present study finding that support this assumption is that correlation between serum IL-23 levels and BMI was higher in psoriatic patients with BMI of >25 as compared to that with BMI <25. Although numerous clinical studies have suggested that obesity has an adverse effect on psoriasis,[37-43] information is lacking about potential pathophysiological pathways that may be responsible for this association. We can now perhaps begin to envision some links between increases in the volume of adipose tissue and severity of psoriasis. Thus, increased adiposity is associated with raised levels of circulating cytokines, including IL-23, which may promote activation of T cells and monocytes, driving both Th1 and Th17 immune responses and at the same time impairing the function of regulatory T cells. High concentrations of IL-23 may furthermore induce local production of cytokines which, together stimulated production of CXCL8, could help to drive the keratinocyte proliferation which is characteristic for psoriasis.Serum levels of IL-23 highly significantly negatively correlated (r= -0.58, P=0.000) with disease duration. IL-23 was higher the earlier the lesion. This may suggest that it could be an important early mediator in induction of psoriatic 1esion.It might also have a role in stimulation of innate immunity. Previous studies have reported that IL-23 stimulates IL-22 production by Th17 cells. IL-22 activation of keratinocytes stimulates a variety of antimicrobial activities, such as psoriasin, calgranulin A, calgranulin B, B-defensin 2, and S-defensin 3[44]. This finding suggests that IL-23 is the master regulator cytokines at the early stage of psoriatic disease induction and was replaced by other cytokines such as IL-12, IL-22, and IL-17. This explanation derived from this study findings that indicated a higher correlation (r=-0.524, P=0.019) in patients with mild (PASI<10) as compared to those with moderate and severe (r=-0.06, P=0.75) disease form. Furthermore, the correlation between IL-23 serum levels with BMI was highly significant in patients with severe disease (PASI>10). IL-23 was significantly higher in psoriatic patients than in controls, a finding consistent to that reported by Hadidi et al[7]. These findings indicate an overreaction of the immune system suggesting that IL-23 might be important in the maintenance of immune responses. Thus IL-23 level is related to disease severity and extent of tissue involvement, however, its production during disease natural history and activity, may be ameliorated by therapy, stress and extent of tissue involvement.Adiponectin levels did not differ significantly between psoriasis patients and controls as this study indicated. That agree with Nakajima et al.[8] and Corbetta[45] studies which demonstrate that serum levels of adiponectin in psoriatic patients had not significantly different from controls. However, other studies[10,46] reported a statistically significant decrease in mean plasma level of adiponectin as compared to control. A studies reported that serum adiponectin levels in normal weight psoriasis patients were rather increased as compared to healthy control[8,47], which was consistent with our result. This study indicated that serum adiponectin mean serum level was higher (9.25 µg/ml) in psoriatic patients with normal weight (BMI <25), as compared to overweight psoriatic patients (6.82 µg/ml) and healthy control (7.35 µg/ml). Adiponectin is an adipocyte specific secretory protein abundantly present in the circulation. Plasma levels of adiponectin are decreased in obesity, insulin resistance and type 2 diabetes[48], and hypoadeponectinaemia is assumed to be closely associated with metabolic syndrome[49]. A recent study indicates that adiponectin inhibits TNF-alpha production and TNF-alpha inhibits adiponectin production, antagonizing each other function[50]. It is known that TNF-α has a critical role in the pathogenesis of psoriasis[51]. In a recent study, Takahashi et al[10] demonstrated a negative correlation between the plasma levels of adiponectin and those of TNF –α in psoriasis. All together, these findings consolidated the hypothesis that TNF-α inhibits the production of adiponectin. Komai et al[52] recently reported that anti-TNF-α therapy increased serum adiponectin level with the improvement of rheumatoid arthritis, confirming the relation between adiponectin and TNF-α. These study findings strongly suggest that adiponectin exert a significant role in the pathogenesis of psoriasis and serum level of adiponectin might be useful for the objective evaluation of the severity of psoriasis.In the present study, adiponectin serum levels were negatively correlated to BMI, and this correlations were highly significant in psoriatic patients with mild (PASI<10) psoriasis (r= - 0.745, P=0.000), while it was non- significant correlation (r= - 0.299, P=0.109) in psoriatic patients with moderate and severe disease form (PASI>10). In addition, adiponectin mean serum level was higher in psoriatic patients with mild severity (PASI <10), as compared to patients with moderate and severe disease form (PASI>10), and control. This could explain that adiponectin production was inhibited by TNF-α, which is significantly increased and correlated to PASI[25].In psoriatic patients adiponectin levels were significantly negatively correlated to the BMI (r=-0.467, p<0.01), agreed with the finding of Coimbra S. et al study where patients presented significantly higher BMI and significantly lower adiponectin values[34]. However, the present study correlation coefficient was higher as to that reported by Nakajima et al[8]. Circulating adiponectin is increased in obesity and diabetes and it is negatively correlates with BMI[53]. In accordance with this, the present study shows that mean serum adiponectin level was higher in underweight or normal weight psoriatic patients as compared to obese and overweight psoriatic patients. In addition, the correlation coefficient value was more in psoriatic obese patients (BMI of >25) as to that who are underweight or normal (BMI<25). Furthermore, the correlation coefficient was higher in psoriatic patients with mild form as compared to that with moderate to severe form. This finding indicated that metabolic changes are increased with time resulting in inflammatory and autoimmune changes in psoriasis. Adiponectin levels were negatively associated with PASI in our study, in agreement to previous report[34, 54] and in contrast to others[8,10]. Interestingly, a strong negative correlation was found between serum adiponectin levels and psoriatic patients age, and the correlation coefficient increased when we limited to moderate to severe psoriasis patients (PASI>10).Adiponectin serum levels showed positive correlation with the other biochemical variables, such as with Zn, Mg, and Cu. The present study indicated that psoriasis disease severity and body obesity do influence the correlations coefficient values between different biochemical markers. Furthermore, the correlation coefficients were higher in patients with underweight or normal weight as compared to obese and overweight patients for all variables with the exception of BMI. This may be due to activation of cells to promote the secretion of proinflammatory cytokines[55] and downregulates the production of anti-inflammatory cytokines[56,57]Zinc plays an important role in cell-mediated immune function. Altered cellular immune response resulting from zinc deficiency leads to altered cytokine production[58]. In this study Zn mean serum level did not differ significantly between psoriasis patients and controls, this finding agreed with that reported by some studies[59,60,61,62]. However, the present study finding was in contrast to that reported significantly lowered plasma zinc concentrations were found in patients with psoriasis as compared to control[63]. In addition, this study finding not agreed to other studies which reported increase in mean serum levels in psoriatic patients.[67, 68].Positive correlation between zinc serum levels and copper serum levels (r=0.484, p<0.01) was found in this study. While with BMI zinc serum levels was showed negative correlation (r=-0.348, p<0.05). No significant correlation between severity of psoriasis with serum levels of zinc in psoriatic patients was found, also zinc levels showed no significant correlation with cytokines (IL-10, IL-23 and adiponectin) in this study.The immune system requires copper to perform several functions, of which little is known about the direct mechanism of action. Some of research showed that IL-2 is reduced in copper deficiency and is likely the mechanism by which T cell proliferation is reduced. In present study the serum levels of Cu in psoriatic patients showed a non significant increase in comparison to Cu serum levels in healthy controls. This disagrees with other studies[69,70,71] where serum Cu was found low in active psoriasis patients than the healthy controls the differences were significant. In contrast, other studies reported a significant increase in serum mean levels in psoriatic patients as compared to controls[ 64,65,67,72].The present study showed positive correlation between Cu serum levels and the severity of psoriasis (r=0.544, p<0.01) in psoriatic patients group. This finding agreed with that reported by Nigam[64]. Meanwhile the Cu serum levels had no significant correlation with age and BMI. The cytokines (IL-10, IL-23 and adiponectin) in this study, showed no significant correlation with Cu serum levels. There was high tendency for serum copper levels to rise with the extent of psoriatic surface area which was highly significant. However, this finding not consistent with that reported by other[72]. It would thus appear that the elevated serum copper levels in psoriasis are simply a reflection of the extent of involvement. This assumption is consolidated with present study finding in which copper mean serum values was significantly higher in psoriatic patients with moderate and severe form as compared to those with mild disease form.However, probably a better evaluation of the relation of the serum copper levels to disease activity would be obtained by a study of serum copper levels in the same patients during different stages of the disease. In the present study, 70% (14/20) of psoriatic treated patients demonstrate a decrease in serum copper levels. This series of patients would permit a conclusion as to the relation of disease activity to serum copper values.Magnesium deficiency has been shown to increase substance P release and induce a pro-inflammatory response that can be attenuated with the administration of a substance P-antagonist[73]. This “neuronal” tachykinin is thought to be released from neural tissues, and it is known to stimulate production of certain cytokines, including IL-1, IL-6, and TNF-α[74]. IL-6 highly expressed by CD31+ endothelial cells and CD11c+ dermal dendritic cells in lesional psoriatic skin. Exposure to high IL-6 in lesional tissue may lead to the dampened Treg function observed in psoriasis patients[75]. While TNF-α increases production of pro-inflammatory molecules (e.g. IL-1, IL-6, IL-8, NF-κB). These inflammatory cytokines can be synthesized by stimulated T-lymphocytes, mononuclear phagocytic cells, and keratinocytes[75]. The magnesium serum levels in psoriatic patients showed small (not significant) increase in comparison magnesium serum levels in healthy controls. Basavaraj et al[67] reported that serum Mg mean value was significantly higher in Psoriasis as than in control. However, Madadi et al[65] reported a reduction in serum magnesium mean value as compared to control.Negative correlation was found between magnesium serum levels and BMI (r=-0.322, p<0.05) in psoriatic patients. While with age and severity of disease no significant correlation was found, also this study showed no significant correlation between magnesium levels with (IL-10 and adiponectin) levels in psoriatic patients.Both zinc and copper are known to be involved in a number of cellular metabolic activities[76]. It has been established by number of analytical and experimental observations that both zinc and copper play an essential role in the normal keratinization process of animal skin[77]. Psoriasis is a multifactorial disease with a genetic predisposition characterized by a defect in keratinization; many workers have observed significantly low serum levels of zinc and copper in psoriatic patients[69-71], while others[78] have found normal levels. A significant lowering of the serum zinc concentration in psoriasis without an improvement in serum zinc levels after oral zinc therapy also observed[70].The present study indicated higher mean serum level of zinc in psoriatic patients with mild form (PASI<10) as compared to patients group with moderate or severe (>PASI), and control. This finding agreed with that reported by McMillan and Rowe[71] and Nigam[64]. The reduction of serum zinc mean levels in moderate and severe forms of the disease may be due to depletion secondary to loss of zinc through exfoliation. An alternative explanation is that serum zinc low levels lead to development of severe skin lesions.An interesting finding of this study was that serum zinc levels were significantly negatively correlated with BMI and the correlation coefficient was more in psoriatic obese or overweight patients (with BMI >25). In addition, zinc serum mean level was significantly higher in normal weight as compared to obese and overweight psoriatic patients. Zinc is required for many biological functions including DNA synthesis, cell division, gene expression and the activity of many enzymes in humans and animals. A deficiency of zinc due to nutritional factors and several disease states has now been recognized. The high phytate content of cereal proteins is known to decrease the availability of zinc, thus the prevalence of zinc deficiency is likely to be high in a population consuming large quantities of cereal proteins[79]. On the other hand, zinc concentrations in the plasma and erythrocytes are lower and urinary zinc excretion and serum insulin levels are higher in obese subjects[80]. Leptin, the product of the obesity gene, is expressed mainly in adipose tissue. Obese individuals, with or without essential hypertension, have hyperleptinemia and hypozincemia, with an inverse correlation between the plasma values of zinc and leptin[81,82].In the present study there was a highly significant positive correlation between zinc serum levels in psoriatic patients and serum adiponection levels. This data with that reported by others indicate a correlation between serum levels on zinc in one side and adiponectin and leptin in other side. This correlation in their serum levels may for circulatory and tissue network that influence the process of psoriatic pathogenesis.Lower serum zinc mean value in obese psoriatic patients as this study indicated was in consistent to that reported in overweight persons in Thailand (BMI>25) and were lower than those of normal controls[83]. In animal models, a reduction in adipose zinc concentrations and a negative correlation between serum leptin and adipose zinc concentrations were found in mice fed a high-fat diet[84]. DiToro et al. also reported that a weight-loss program based on a hypocaloric diet led to a decrease in weight and increase in zinc levels in the erythrocytes and plasma in 55 obese children and adolescents[85].In a reported study, a 6-month weight-loss program, based on a hypocaloric balanced diet, reduced the body weight, BMI and percentage and amount of body fat, with a slight lowering of blood pressure and of plasma levels of triglyceride. Interestingly, the plasma concentrations of zinc were markedly enhanced at the end of the program.[86]. Our findings, concerning zinc and adiponectin, strongly suggest that zinc and adiponectin exert a significant role in the pathogenesis of psoriasis, may be through down and upregulation of leptin and/or TNF-α.
5. Conclusions
- Serum IL-10 and IL-23 levels were significantly elevated in psoriasis patients indicating their role in disease pathogenesis. A significant correlation demonstrated between IL-10 & IL-23; Mg & IL-23; Adiponectin & BMI; Zn & Cu. Findings that indicated the influence of obesity and disease severity on psoriasis markers.
References
| [1] | Abanmi A, Alharthi F, Alagla R, Khan HA, Tariq M. Serum levels of proinflammatory cytokines in psoriasis patients from Saudi Arabia. Int J Dermatol; 44(1):82-83, 2005. |
| [2] | Kunkel SL, Chensue SW, Strieter RM, Lynch JP, Remick DG. Cellular and molecular aspects of granulomatous inflammation. Am J Respir Cell Mol Biol. 1(6):439-47, 1989. |
| [3] | Firestein GS, Budd RC, Harris ED, McInnes LB, Ruddy S, Sergent JS. Kkelley's textbook of rheumatology. 8th ed. Philadelphia: Saunders; 2008. |
| [4] | Uyemura K, Yamamur M, Fivenson DF, Modlin RL, Nickoloff BJ. The cytokine network in lesional and lesion-free psoriatic skin is characterized by a T-helper type 1 cell-mediated response. J Invest Dermatol21;101:701-705,1993. |
| [5] | Asadullah K, Volk HD, Sterry W. Novel immunotherapies for psoriasis. Trends in Immunology.23:47-53, 2002. |
| [6] | Caproni M, Antiga E, Melani L, Volpi W, Bianco ED, Fabbri P. Serum levels of IL-17 and IL-22 are reduced by Etanercept, but not by Acitretin, in patients with psoriasis: a randomized-controlled trial. J Clin Immunol 29:210-214, 2008. |
| [7] | El Hadidi H, Grace BDM ,Gheita T, Shaker O. Involvement of IL-23 in psoriasis and psoriatic arthritis patients; possible role in pathogenesis. J Egypt worn Dermatol Soc; 5(2):70-75, 2008. |
| [8] | Nakajima H, Nakajima K, Tarutani M, Morishige R, Sano S. Kinetics of circulating Th17 cytokines and adipokines in psoriasis patients. Arch Dermatol Res August; 303(6): 451–455, 2011. |
| [9] | Gisondi P, Tessari G, Conti A, Piaserico S, Schianchi S, Peserico A, Gianetti A, Girolomoni G. Prevalence of metabolic syndrome in patients with psoriasis. Br J Dermatol 157:68-73, 2007. |
| [10] | Takahashi H, Tsuji H, Takahashi I, Hashimoto Y, Yamamoto AI, Iizuka H. Plasma adiponectin and leptin levels in Japanese patients with psoriasis. Br J Dermatol; 159(5):1207-1208, 2008. |
| [11] | Wilson D, Varigos G, Ackland ML. Apoptosis may underlie the pathology of zinc-deficient skin. Immunol Cell Biol 84:28-37, 2006. |
| [12] | Bao B, Prasad AS, Beck FWJ, Godmere M. Zinc modulates mRNA levels of cytokines. Am J Physiol Endocrinol Metab 17; 285:1095-1102, 2003. |
| [13] | Rink L, Kirchner H. Zinc-altered immune function and cytokine production. J Nutr 130(5):1407-1411, 2000. |
| [14] | Tam M, Gómez S, González M, Marcos A. Possible roles of magnesium on the immune system. Eur J Clin Nutr; 57:1193-1197, 2003. |
| [15] | Muñoz C, López M, Olivares M, Pizarro F, Arredondo M, Araya M. Differential response of interleukin-2 production to chronic copper supplementation in healthy humans. Eur Cytokine Netw 16(4):261-265, 2005. |
| [16] | Nast A, Boehncke WH, Mrowietz U, Ockenfels HM, Philipp S. Guidelines on the treatment of psoriasis vulgaris, Update JDDG 9(S2):S1-S95,2011. |
| [17] | Dang PM, Elbim C, Marie JC, Chiandotto M, Pocidalo MA, El-Benna J. Anti-inflammatory effect of interleukin -10 on human neutrophil respiratory burst involves inhibition of GM-CSF-induced p47PHOX phosphorylation through a decrease in ERK1/2 activity. FASEB J 20:E698-E709, 2006. |
| [18] | Borska L, Andrys C, Krejsek J, Hamakova K, Kremlacek J, Ettler K, Fiala Z. Serum levels of the proinflammatory cytokine interleukin-12 and the anti-inflammatory cytokine-10 in patients with psoriasis treated by the Goeckerman regimen. Int J Dermatol 47:800-805, 2008. |
| [19] | Elkhayam O, Yaron I, Shirazi I, Yaron M, Caspi D. Serum levels of IL-10, IL-6, IL-1ra, and sIL-2R in patients with psoriatic arthritis. Rheumatol Internat 19:101-105, 2000. |
| [20] | Deeva I., Mariani S., De Luca C., Pacifico V., Leoni L., Raskovic D., Kharaeva Z., Korkina L., Pastore S. Wide-spectrum profile of inflammatory mediators in the plasma and scales of patients with psoriatic disease. Cytokine. 49(2):163-70, 2010. |
| [21] | Jacob SE, Nassiri M, Kerdel FA, Vincek V. Simultaneous measurement of multiple Th1 and Th2 serum cytokines in psoriasis and correlation with disease severity. Mediat Inflamm. 12(5):309–13, 2003. |
| [22] | Antiga E, Del Bianco E, Difonzo E, Fabrri P, Caproni M. Serum levels of the regulatory cytokines transforming growth factor-β and interleukin-10 are reduced in patients with discoid lupus erythematouses. Lupus 20:556-560, 2010. |
| [23] | Jadali Z, Izad M, Eslami MB, Mansouri P, Safari R, Bayatian P, Mirshafiey A and Salehi Nodeh AR. Th1/Th2 cytokines in psoriasis. Iranian J Public Health 36:87-91, 2007. |
| [24] | Verghese B, Bhatnagar S, Tanwar R, Bhattacharjee J. Serum cytokine profile in psoriasis: a case control study in a tertiary care hospital from northern india. Ind J Clin Biochem 26:373-377, 2011. |
| [25] | Takahashi H, Tsuji H, Hashimato Y, Yamamoto AI, Iizuka H. Serum cytokines and growth factor levels in Japanese patients with psoriasis. Clin Exp Dermatol 35:645-649, 2010. |
| [26] | Mitzutani H, Ohmoto Y, Mizutani T, Murata M, Shimizu M. Role of increased production of monocytes TNF-α, IL-1β and IL-6 in psoriasis: relation to focal infection, disease activity and responses to treatment. J Dermatol Sci 14:145-153, 1997. |
| [27] | Germann, T., and E. Rude-E. Interleukin-12. Int. Arch. Allergy Immunol. 108:103–112, 1995. |
| [28] | Jung SH, Park HS, Kim KS, Choi WH, Ahn CW, Kim BT, Kim SM, Lee SY, Ahn SM, Kim YK, Kim HJ, Kim DJ, Lee KW. Effect of weight loss on some serum cytokines in human obesity: increase in IL-10 after weight loss. J Nutr Biochem 19:371–375,2008. |
| [29] | Toichi E, Torres G, McCormick TS, Chang T, Mascelli MA, Kauffman CL, Aria N, Gottlieb AB, Everitt DE, Frederick B, Pendley CE, Cooper KD. An anti-IL-12p40 antibody down-regulates type 1 cytokines, chemokines, and IL-12/IL-23 in psoriasis. J Immunol 177:4917-26, 2006. |
| [30] | Trepicchio, W. L., M. Ozawa, I. B. Walters, T. Kikuchi, P. Gilleaudeau, J. L. Bliss, U. Schwertschlag, A. J. Dorner, and J. G. Krueger. Interleukin-11 therapy selectively downregulates type I cytokine proinflammatory pathways in psoriasis lesions. J. Clin. Invest. 104: 1527–1537, 1999. |
| [31] | Olaniran, A. K., B. S. Baker, D. G. Paige, J. J. Garioch, A. V. Powles, and L. Fry. Cytokine expression in psoriatic skin lesions during PUVA therapy. Arch Dermatol. Res. 288: 421–425, 1996. |
| [32] | Asadullah, K., W. Sterry, K. Stephanek, D. Jasulaitis, M. Leupold, H. Audring, H. D. Volk, and W. D. Docke. IL-10 is a key cytokine in psoriasis: proof of principle by IL-10 therapy: a new therapeutic approach. J. Clin. Invest. 101:783–794, 1998. |
| [33] | Yawalkar N, Karlen S, Hunger R, Brand CU, Braathen LR. Expression of interleukin-12 is increased in psoriatic skin. J Invest Dermatol 111:1053-1057, 1998. |
| [34] | Coimbra S, Oliveira H, Reis F, Belo L, Rocha S, Quaintanilha A, Figueiredo A, Teixeira F, ... Teixeira F, Castro E, Rocha-Pereira P, Santos-Silva A. Interleukin (IL)-22,IL-17, IL-23, IL-8, vascular endothelial growth factor and tumor necrosis factor-α levels in patients with psoriasis before, during and after psoralen ultraviolet A and narrowband ultraviolet B therapy. Br J Dermatol 163:1282-1290, 2010. |
| [35] | Sumarac-Dumanovic M, Stevanovic D, Ljubic A, Jorga J, Simic M, Stamenkovic-Pejkovic D, Starcevic, V.; Trajkovic, V.; Micic, D. Increased activity of interleukin-23/interleukin-17 proinflammatory axis in obese women. Int J Obes 33:151-156, 2009. |
| [36] | Sumarac-Dumanovic M, Micic D, Simic M, Stamenkovic-Pejkovic D, Stevanovic D, Jorga J, Cvijovic G, Zoric S, Trajkovic V. Relationship between obesity, IL-17 and IL-23 and insulin resistance. Endocr Abst 16:568, 2008. |
| [37] | Naldi L, Chatenoud L, Linder D, Belloni Fortina A, Peserico A, Virgili AR, Bruni PL, Ingordo V, Lo Scocco G, Solaroli C, Schena D, Barba A, Di Landro A, Pezzarossa E, Arcangeli F, Gianni C, Betti R, Carli P, Farris A, Barabino GF, La Vecchia C. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case–control study. J Invest Dermatol 125:61–67, 2005. |
| [38] | Henseler T, Christophers E. Disease concomitance in psoriasis. J Am Acad Dermatol 32:982–986, 1995. |
| [39] | Raychaudhuri SP, Gross J. Psoriasis risk factors: role of lifestyle practices. Cutis 66:348–352, 2000. |
| [40] | Marino MG, Carboni I, De Felice C, Maurici M, Maccari F, Franco E. Risk factors for psoriasis: a retrospective study on 501 outpatient's clinical records. Ann Ig 16:753–758, 2004. |
| [41] | Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand GM. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol 55:829–835, 2006. |
| [42] | Sommer DM, Jenisch S, Suchan M, Christophers E, Weichenthal M. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch Dermatol Res 298:321–328, 2006. |
| [43] | Herron MD, Hinckley M, Hoffman MS, Papenfuss J, Hansen CB, Callis KP, Krueger GG. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol 141:1527–1534, 2005. |
| [44] | Wok K. Witte E, Wallace E, Docke WD, Kunz S, Asadullah K, Volk HD, Sterry W, and Sabat R: IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur. J. Immunol. 36:1309-1323,2006. |
| [45] | Corbita S, Angioni R, Cattaneo A, Peccoz PB, Spada A. Effects of retinoid therapy on insulin sensitivity, lipid profile and circulating adipokines. Eur J Endocrinol 154:83-86, 2006. |
| [46] | Shibatta S, Saek H, Tada Y, Karakawa M, Komine M, Tamaki K. Serum high molecular weight adiponectin levels are decreased in psoriasis patients. J Dermatol Sci 55:62-63, 2009. |
| [47] | Kaur S, Zilmer K, Kairane C, Kals M, Zilmer M. Clear differences in adiponectin level and glutathione redox status revealed in obese and normal weight patients with psoriasis. Br J Dermatol 159:1364-1367, 2008. |
| [48] | Matsuzaka Y. The metabolic syndrome and adipokines. FEBS Lett 580:2917-2921, 2006. |
| [49] | Hulthe J, Hulten LM, Fagerberg B. Low adipocyte-derived plasma protein adiponectin concentrations are associated with metabolic syndrome and small dense low density lipoprotein particles: atherosclerosis and insulin resistance study. Metabolism 52:1612-1614, 2003. |
| [50] | Campfield LA, Smith FJ, GuisezY, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science 269:546-549, 1995. |
| [51] | Boehncke S, Thaci D, Beshmann H, Ludwig RJ, Ackermann H, Badenhoop K, Boehncke WH. Psoriasis patients show signs of insulin resistance. Br J Dermatol. 157:1249-1251,2007. |
| [52] | Komai N, Morita Y, Sakuta T, Kuwabara A, Kashihara N. Anti-tumor necrosis factor therapy increase adiponectin levels with the improvement of endothelial dysfunction in patients with rheumatoid arthritis. Mod Rheumatol 17:385-390, 2007. |
| [53] | Ouchi N, Walsh K. Adiponectin as anti-inflammatory factor. Clin Chim Acta 380:24-30, 2007. |
| [54] | Sommer DM, Jenisch S, Suchan M, Christopher E, Weichenthal M. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch Dermatol Res 298:321-328, 2006. |
| [55] | Silswal N, Singh AK, Aruna B, Mukhoppadhayay S, Gosh S, Ehtesham NZ. Human resisten stimulates the pro-inflammatory cytokines TNF-α and IL-12 in macrophages by NF-κ B- dependent pathway. Biochem Biophys Res Commun 334:1092-1101, 2005. |
| [56] | Gerdes S, Rostami-Yanzdi M, Mrowietz U. Adipokines and psoriasis. Exp Dermatol 20:81-87, 2011. |
| [57] | Takahashi H, Tsuji H, Hashimoto Y, Ishida-Yamamoto A, Iizuka H. Serum cytokine and growth factor levels in Japanese patients with psoriasis. Clin Exp Dermatol 35:645-649, 2009. |
| [58] | Bao B, Prasad AS, Beck FWJ, Godmere M. Zinc modulates mRNA levels of cytokines. Am J Physiol Endocrinol Metab 285:1095-1102, 2003. |
| [59] | Molokhia MM, Portnoy B. Neutron activation analysis of trace elements in skin.V. copper and zinc in psoriasis. Br J Dermatol 83:376-381, 1970. |
| [60] | Hinks LJ, Young S, Clayton B. Trace element status in eczema and psoriasis. Clin Exp Dermatol 12:93-97, 1987. |
| [61] | Ozturk G, Erbas D, Gelier E, Gulekon A, Imir T. Natural killer cell activity, serum immunoglobulins, complement proteins, and zinc levels in patients with psoriasis vulgaris. Immunol Invest 30:181-190, 2001. |
| [62] | Kreft B, Wohlrab J, Fischer M, Uhlig H, Skolziger R, Marsch WC. Analysis of serum zinc level in patients with atopic dermatitis, Psoriasis vulgaris and in probands with healthy skin. Hautazt 51:931-934, 2000. |
| [63] | Greaves M, Boyde TRC. Plasma zinc concentrations in patients with psoriasis, other dermatoses, and venous legulceration. Lancet 290:1019-1020, 1967. |
| [64] | Nigam PK. Serum zinc and copper levels and Cu: Zn ratio in psoriasis. Indian J Dermatol Venereol Leprol 71:205-205, 2005. |
| [65] | Madadi A, Sethi N, Bhandari S. A study of serum zinc, copper, magnesium, proteins and enzyme superoxide dismutase in psoriasis. Indian J Dermatol Venereol Leprol 60:319-322, 1994. |
| [66] | Arora PN, Dhillon KS, Rajan SR, Sayal SK, Das AL. Serum zinc levels in cutaneous disorders. MJAFI 58: 304-306, 2002. |
| [67] | Basavaraj KH, Darshan MS, Shanmugavelu P, Rashmi R, Mahatre AY, Dhanabal SP, Rao KS... Study on the levels of trace elements in mild and severe psoriasis. Clinica Chimica Acta 405:66-70, 2009. |
| [68] | Butnaru C, Pascu M, Mircea C, Agoroaei L, Solovastru L, Vata D, Butnaru E, Petrescu Z. Serum zinc and copper levels in some dermatological diseases. Rev Med Chir Soc Med Nat LasiLasi 112:253-257, 2008. |
| [69] | Bhatnagar M, Bapna A, Khare AK. Serum proteins, trace metals and phosphatases in psoriasis. Indian J Dermatol Vevereol Leprol 60:18-21, 1994. |
| [70] | Saxena N, Sharma RP, Singh VS. Study of serum zinc levels in leprosy. Int J Lep 60:556-61, 1988. |
| [71] | McMillan EM, Rowe D. Plasma zinc in psoriasis in relation to surface area involvement. Br J Derm 100; 1301-1305, 1983. |
| [72] | Rashmi R, Yuti AM, Basavaraj KH. Relevance of copper and ceruloplasmin in Zackheim HS, Wolf P. Serum copper in psoriasis and other dermatoses. J Invest Dermatol 58:28-32, 1972. |
| [73] | Vink R, Donkin JJ, Cruz MI, Nimmo AJ, Cernak I. A substance P antagonist increases brain injury in rats. Am Coll Nut 23:538S-540S, 2004. |
| [74] | Goodman WA, Levine AD, Massari JV, Sugiyama H, McCormick TS, Cooper KD. IL-6 signaling in psoriasis prevents immune suppression by regulatory T cells. J Immunol 183:3170-3176, 2009. |
| [75] | Victor FC, Gottlieb AB. TNF-alpha and apoptosis: implications for the pathogenesis and treatment of psoriasis. J Drugs Dermatol 1:264-275, 2002. |
| [76] | Pories WJ, Mansur EG. In: Prasad AS, D'Oberleas, Editors. 'Trace elements in human health and diseases. New York Acad Press, P115, 1976. |
| [77] | Day C, McCollum EV. Effect of acute dilatory zinc deficiency in rat. Proc Exp Bio Med 45:282-287, 1940. |
| [78] | Portnoy K, Molokhia M. Zinc and copper in psoriasis. Br J Dermatol 86:205-209, 1972. |
| [79] | Prasad AS: Discovery of human zinc deficiency and studies in an experimental human model. Am J Clin Nutr 53(2): 403-412, 1991. |
| [80] | Marreiro Ddo N, Fisberg M and Cozzolion SM: Zinc nutritional status and its relationships with hyperinsulinemia in obese children and adolescents. Biol Trace Elem Res 100(2): 137-149, 2004. |
| [81] | Canatan H, Bakan I, Akbulut M, Halifeoglu I, Cikim G, Baydas G and Kilic N: Relationship among levels of leptin and zinc, copper, and zinc/copper ratio in plasma of patients with essential hypertension and healthy normotensive subjects. Biol Trace Elem Res 100(2): 117-123, 2004. |
| [82] | Chen MD, Song YM and Lin PY: Zinc may be a mediator of leptin production in humans. Life Sci 66(22): 2143-2149, 2000. |
| [83] | Tungtrongchitr R, Pongpaew P, Phonrat B, Tungtrongchitr A, Viroonudomphol D, Vudhivai N and Schelp FP: Serum copper, zinc, ceruloplasmin and superoxide dismutase in Thai overweight and obese. J Med Assoc Thai 86(6): 543-551, 2003. |
| [84] | Tallman DL and Taylor CG: Effects of dietary fat and zinc on adiposity, serum leptin and adipose fatty acid composition in C57BL/6J mice. J Nutr Biochem 14(1): 17-23, 2003. |
| [85] | Di Toro, Marotta A, Todisco N, Ponticiello E, Collini R, Di Lascio R and Perrone L: Unchanged iron and copper and increased zinc in the blood of obese children after two hypocaloric diets. Biol Trace Elem Res 57(2): 97-104, 1997. |
| [86] | Yuko S, Yasuo K, Sakamoto S. Increased Plasma Levels of Zinc in Obese Adult Females on a Weight-loss Program Based on a Hypocaloric Balanced Diet. In Vivo 19: 1035-1038, 2005. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML