-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Condensed Matter Physics
p-ISSN: 2163-1115 e-ISSN: 2163-1123
2024; 13(1): 9-20
doi:10.5923/j.ajcmp.20241301.02
Received: May 7, 2024; Accepted: May 31, 2024; Published: Jun. 7, 2024

Effects of Stirring Speed of Precursor Solution on the Structural, Optical and Morphological Properties of ZnO:Al:Ga Co-Doped Nanoparticles Synthesized via a Facile Sol-Gel Technique
Kiprotich Bett, Sharon Kiprotich
Department of Physical and Biological Sciences, Murang’a University of Technology, Murang’a, Kenya
Correspondence to: Sharon Kiprotich, Department of Physical and Biological Sciences, Murang’a University of Technology, Murang’a, Kenya.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Solar energy offers solutions to the growing demand of energy due to rising population but available silicon-based solar cells are expensive also zinc oxide (ZnO)-based solar cells suffer low efficiency. Therefore the precise control over the material properties is necessary for efficient solar cell technologies, and the properties of ZnO:Al:Ga co-doped nanoparticles are mostly determined by the stirring speed. In this work, we examine the effects of stirring speed on the material properties of ZnO:Al:Ga nanoparticles that have been synthesized for possible use in solar cell technology. Using the sol-gel method technique in conjunction with characterization methods such as FTIR (Fourier-transform infrared spectroscopy), SEM (scanning electron microscopy), XRD (X-ray diffraction) and (UV-Vis) Ultraviolet -visible spectroscopy. This study investigate how the speed of stirring affects the structural, optical, morphological, elemental composition properties of synthesized ZnO:Al: Ga co-doped nanoparticles for possible solar cells. The study revealed that synthesized nanoparticles have enhanced best crystallinity at 1000 rpm, homogeneous doping as shown by SEM micrographs, increased surface area and optimized optical properties that are good for improving solar cell efficiency. This evidence by UV-Vis absorbance at wavelength of 378 nm equivalent to 3.29 eV band gaps which is relatively lower than the standard ZnO’s bulk of 3.37 eV. Low energy band-gap of the material is associated with high performance of solar cells. Also XRD reveals stirring speed of 1000 rpm, lattice parameters which are close to standard lattice parameters’ of Zinc Oxide. EDX (Energy Dispersive X-ray Spectroscopy) hexagonal wurtize spectrum confirmed sol-gel technique as simple and inexpensive method of synthesizing nanoparticles as supported by XRD results. In addition, under FTIR, 1000 rpm showed more intense stretching vibrations modes between 400-500cm-1that exhibits M-O chemical-bonding in nanoparticles that corresponds to chemical functional composition of Zn-O. These findings pave a way for the logical engineering and design of ZnO:Al:Ga nanoparticles. Furthermore, it explore1000 rpm as the best stirring speed suitable for synthesizing ZnO:Al:Ga nanoparticles.
Keywords: ZnO:Al:Ga co-doped nanoparticles, Stirring speed, Sol-gel method, Material properties, Optical properties
Cite this paper: Kiprotich Bett, Sharon Kiprotich, Effects of Stirring Speed of Precursor Solution on the Structural, Optical and Morphological Properties of ZnO:Al:Ga Co-Doped Nanoparticles Synthesized via a Facile Sol-Gel Technique, American Journal of Condensed Matter Physics, Vol. 13 No. 1, 2024, pp. 9-20. doi: 10.5923/j.ajcmp.20241301.02.
Article Outline
1. Introduction
- The world’s growing population demands more sustainable, adequate or sufficient energy for various production of consumer goods for instance industries. Most people uses the traditional sources of energy such as fossils fuels which are not friendly to the environment but adversely affects the climate. Solar energy is potential solution to the arising problem of high demand of energy due to its clean nature and friendly to the environment. With its clean and renewable nature, solar energy has great promise to both reduce environmental pollution and meet the world's energy needs (Lewis &Nocera, 2006). Zinc oxide (ZnO) solar cells are one of the most popular photovoltaic technologies because of their abundance, sustainability, and unique optoelectronic properties (Liang et al., 2015). Specifically, co-doped ZnO:Al:Ga nanoparticles with gallium (Ga) and aluminum (Al) show improved optical and electrical conductivity, which makes them attractive candidates for efficient solar cell applications (Afre et al., 2010).Sol-gel synthesis of ZnO:Al:Ga nanoparticles provides a flexible way to modify their material properties to suit solar cell technology needs (OTHMANE et al., 2018). To maximize their performance in photovoltaic systems, control over the material properties such as particle size, crystallinity, and doping must be achieved (Zhou et al., 2018). The stirring speed during the creation of the precursor solution stands out among the other synthesis factors as crucial in determining the final properties of the prepared nanoparticles (Gatou et al., 2023).With an emphasis on their applicability to solar cell applications, this study examine the impact of stirring speed on the material properties of ZnO:Al:Ga co-doped nanoparticles produced using the sol-gel method followed by characterization using XRD (X-ray diffraction) and (UV-Vis) Ultraviolet -visible spectroscopy, FTIR (Fourier-transform infrared spectroscopy), SEM (scanning electron microscopy). The study aim to clarify the ideal stirring conditions that facilitate the synthesis of nanoparticles with improved, morphology, structural, elemental, chemical-bonding and optical properties, all of which can further their potential for effective solar energy conversion. In addition, the research helps in growing knowledge on nanoparticle nanotechnology and facilitates the sensible design of ZnO-based materials for use in sustainable solar energy applications which is renewable with no negative effects to the environment or climate. Study and optimization of the growth parameters are key in engineering ZnO:Al:Ga nanoparticles to posses the desired material properties. Effects of pH, growth temperature, reaction time and Ga:Al mole doping concentrations have been reported in the literature (Ungula et. al 2024 and Gakuru et. al 2024). However, research on the effects of stirring speed of the precursor solution on the structural, morphological and optical properties of the ZnO:Al:Ga NPs is scanty. Therefore, this research explores to add to the body of knowledge on the effects of this parameter.
2. Methodology
- MaterialsZinc acetate, aluminum nitrate, gallium nitrate, sodium hydroxide pellets, Ethanol, deionized water. All the chemicals used were of analytical grade and used as purchased without any alteration.ProcedureTo examine the effects of stirring speed of precursor solution on the material properties of ZnO:Al:Ga co-doped nanoparticles for solar cell applications, ZnO:Al:Ga co-doped nanoparticles was prepared from Zinc acetate dehydrate, Aluminum nitrate, gallium nitrate, sodium hydroxide, ethanol and deionized water. In the beaker 2.1951 g of zinc acetate is dissolve with 50 mL of ethanol until formation of clear solution, add drop wise 25 mL of 1M sodium hydroxide, once mixture of zinc acetate, ethanol and sodium hydroxide is formed under constant growth temperature of 75°C, pH value 9 and varying stirring speed of values: 1, 1.5, 2, 2.5, 3, 3.5 and 4 x 103 r.p.m. Thereafter, a solution containing 0.187565 g of aluminum nitrate, 0.12787g of gallium nitrate added to the precursor solution and left until duration of one hour is over. The suspension formed of ZnO:Al:Ga was removed from hot plate and allowed to age. The ZnO:Al:Ga nanoparticles then washed with deionized water through decantation. The washed sample was dried for 60 minutes at 120°C in the ovenand annealed at 600°C for 1 hour. The ZnO:Al:Ga co-doped nanoparticles samples were taken for characterization using X-ray diffraction (XRD) patterns in the 2θ range, using Cu/Kα radiation (λ = 1.54056 Å) from an X-ray diffractometer (Model ARL EQUINOX 100 X-Ray Diffractometer), Scanning electron microscopy (SEM) micrographs and Energy Dispersive X-ray Spectroscopy (EDS) Model Phenom XLG2, UV- Spectrophotometer Model UV-1800 SHIMADZU and Fourier transform infrared (FTIR) transmission spectra FTIR Spectrophotometer Model IRspirit SHIMADZU.
3. Results and Analysis
- XRD AnalysisZnO:Al:Ga co-doped nanoparticles have favorable electrical and optical properties that make them promising for use in solar cell applications (Chakraborti et al., 2008). To maximize their performance, it is imperative to understand how synthesis factors like stirring speed affect their material properties. Figure 1(i) shows all synthesized samples, XRD examination showed distinctive peaks compatible with the hexagonal wurtzite structure of ZnO nanoparticleswhich correspond to the earlier research (Ungula et al., 2017) that matches well with JCPDS No. 79–0208. This consistency attests to the synthesis's success at various stirring rates using sol-gel method.
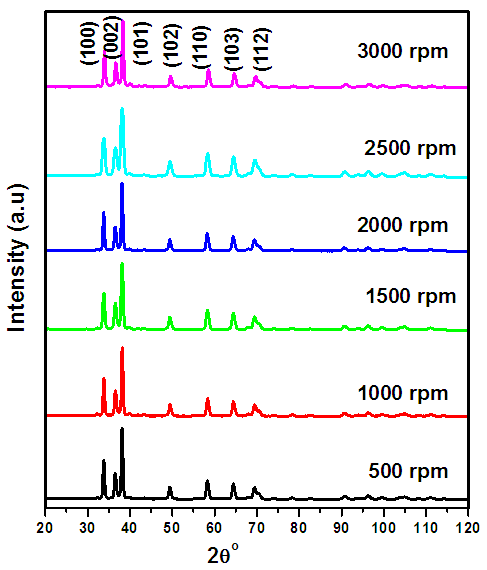 | Figure 1(i). XRD pattern between 20° – 120° for all synthesized samples of ZnO:Al:Ga co-doped nanoparticles under varying stirring speed |
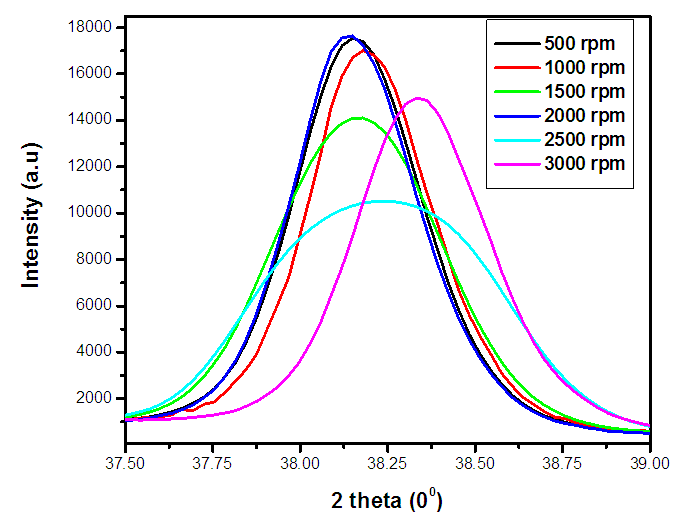 | Figure 1(ii). Magnified XRD pattern between 37.5° – 39.0° for the synthesized ZnO:Al:Ga co-doped nanoparticles under varying stirring speed |
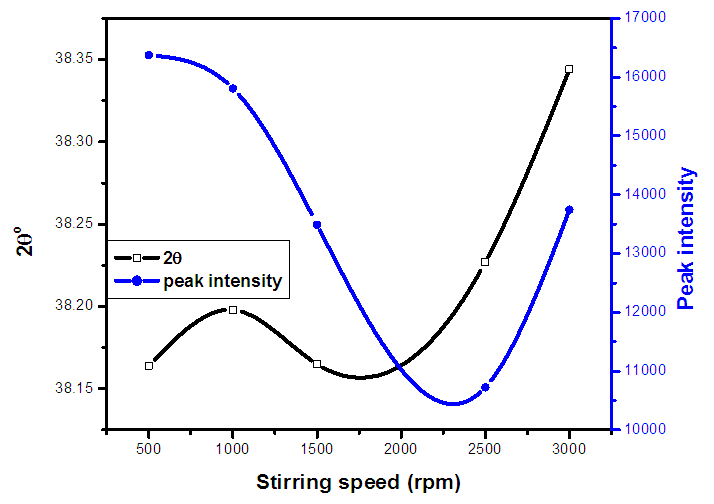 | Figure 1(iii). A graph of most intense peak for the synthesized ZnO:Al:Ga co-doped nanoparticles under varying stirring speed |
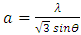 and
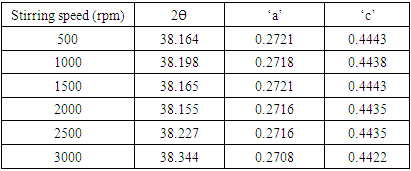
and 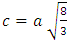 where ‘a’ and ‘c’ are the lattice parameters. The prepared ZnO:Al:Ga nanoparticles showed ‘a’ range between 0.2708 – 0.2721 nm and ‘c’ to be 0.4443 – 0.4422 nm as shown in table 1.
where ‘a’ and ‘c’ are the lattice parameters. The prepared ZnO:Al:Ga nanoparticles showed ‘a’ range between 0.2708 – 0.2721 nm and ‘c’ to be 0.4443 – 0.4422 nm as shown in table 1.
|
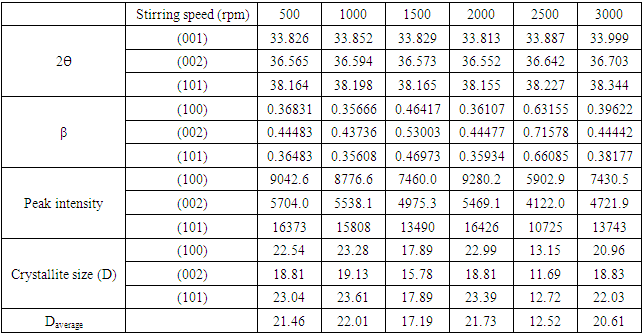
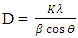 (Gherbiet al., 2022) where D is crystalline size, K is constant (0.89), λ is 0.154056 nm, mean wavelength of CuKα1 radiation, β is full width half maxima and θ is Bragg’s angle in radians, the average crystallite size analysis, as presented in Table 2 were as follows21.46, 22.01, 17.19, 21.73,12.52 and 20.61nm for 500 rpm, 1000 rpm, 1500 rpm, 2000 rpm, 2500 rpm and 3000 rpm respectively. The obtained lattice parameters and crystallite size of synthesized ZnO:Al:Ga nanoparticles showed minimal changes in the bulk’s ZnO standard lattice parameters similar to the research done by Geetha (2020). This affirms the purity of synthesized nanoparticles.
(Gherbiet al., 2022) where D is crystalline size, K is constant (0.89), λ is 0.154056 nm, mean wavelength of CuKα1 radiation, β is full width half maxima and θ is Bragg’s angle in radians, the average crystallite size analysis, as presented in Table 2 were as follows21.46, 22.01, 17.19, 21.73,12.52 and 20.61nm for 500 rpm, 1000 rpm, 1500 rpm, 2000 rpm, 2500 rpm and 3000 rpm respectively. The obtained lattice parameters and crystallite size of synthesized ZnO:Al:Ga nanoparticles showed minimal changes in the bulk’s ZnO standard lattice parameters similar to the research done by Geetha (2020). This affirms the purity of synthesized nanoparticles.
|
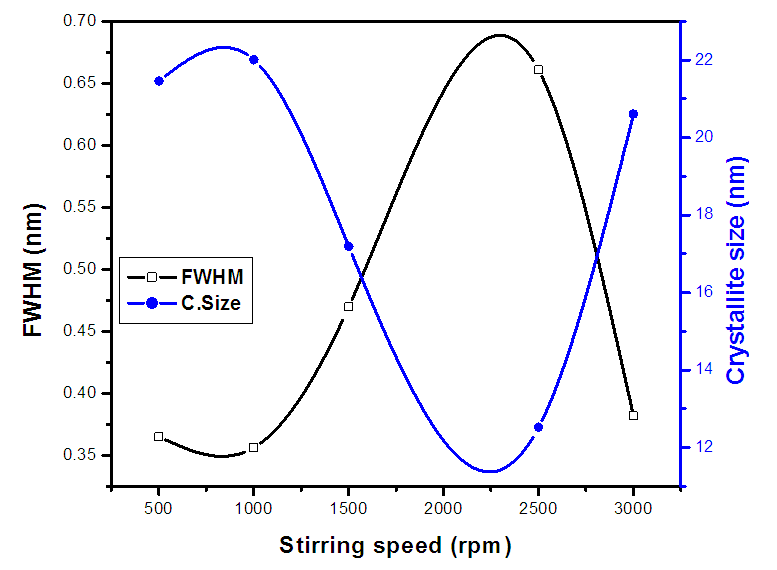 | Figure 1(iv). The graph of FWHM and crystallite sizeof synthesized ZnO:Al:Ga co-doped nanoparticles for various stirring speeds |
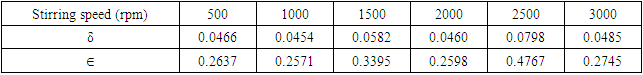
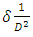 (Ungula et al., 2024) where δ is the length of dislocation density and D is the crystalline size; was applied in order to calculate the dislocation density, as table 3 illustrates the results. Also, table 3 shows the micro-strain
(Ungula et al., 2024) where δ is the length of dislocation density and D is the crystalline size; was applied in order to calculate the dislocation density, as table 3 illustrates the results. Also, table 3 shows the micro-strain 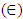 values, which were computed using the equation,
values, which were computed using the equation, 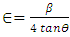 (Kiprotich et al., 2022) where ϴ is the diffraction angle and β is the full width at half maximum.
(Kiprotich et al., 2022) where ϴ is the diffraction angle and β is the full width at half maximum.
|
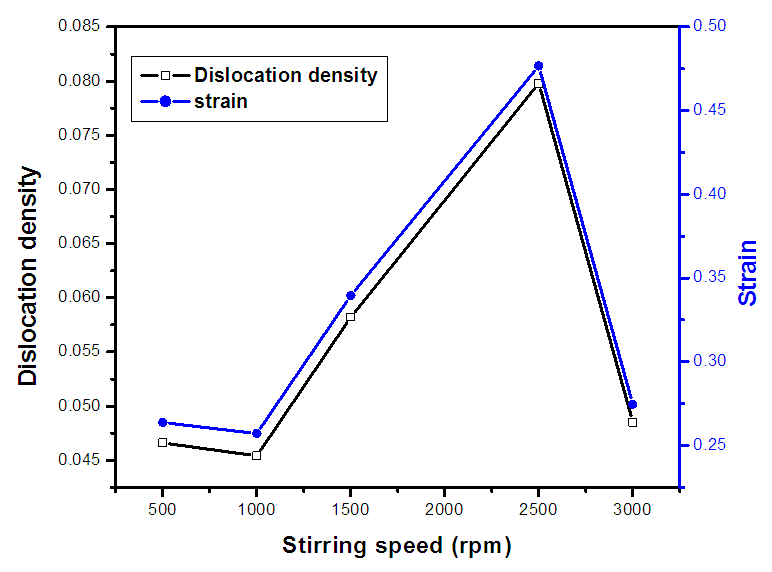 | Figure 1(v). The graph of dislocation density and strain of prepared ZnO:Al:Ga co-doped nanoparticles for various stirring speeds |
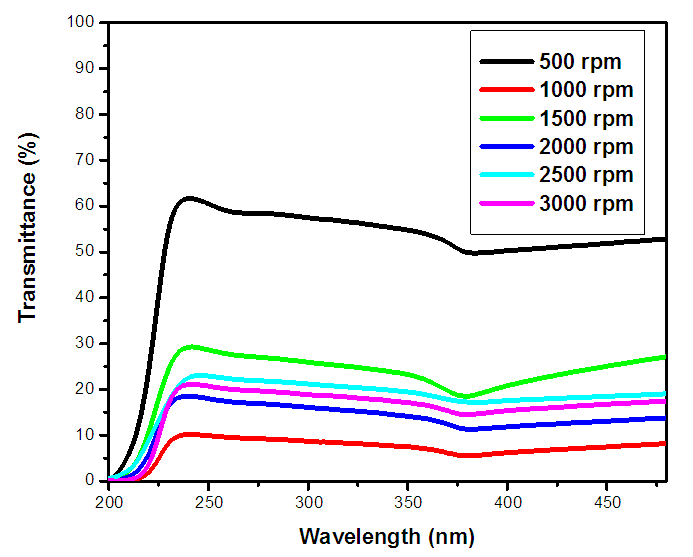 | Figure 2(i). UV-Vis spectrum showing transmittance (%) against wavelength (nm) of as prepared ZnO:Al:Ga co-doped nanoparticles for varying stirring speed |
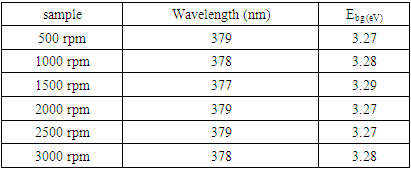
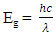 where h is the Plank’s constant c is the velocity of light and λ is the absorbance peak. The ZnO:Al:Ga co-doped nanoparticles' resultant energy band gaps are listed in Table 4 for each stirring speed. The energy band-gapsfindings, which are around 3.27 and 3.29 eV, support patterns seen in earlier studies by Droepenu et al. (2022), who also found that stirring speed had a significant impact on band gap energies in zinc oxide nanoparticles. The controlof stirring speeds at optimum enhance good nanoparticles with light absorption and electron transport.
where h is the Plank’s constant c is the velocity of light and λ is the absorbance peak. The ZnO:Al:Ga co-doped nanoparticles' resultant energy band gaps are listed in Table 4 for each stirring speed. The energy band-gapsfindings, which are around 3.27 and 3.29 eV, support patterns seen in earlier studies by Droepenu et al. (2022), who also found that stirring speed had a significant impact on band gap energies in zinc oxide nanoparticles. The controlof stirring speeds at optimum enhance good nanoparticles with light absorption and electron transport.
|
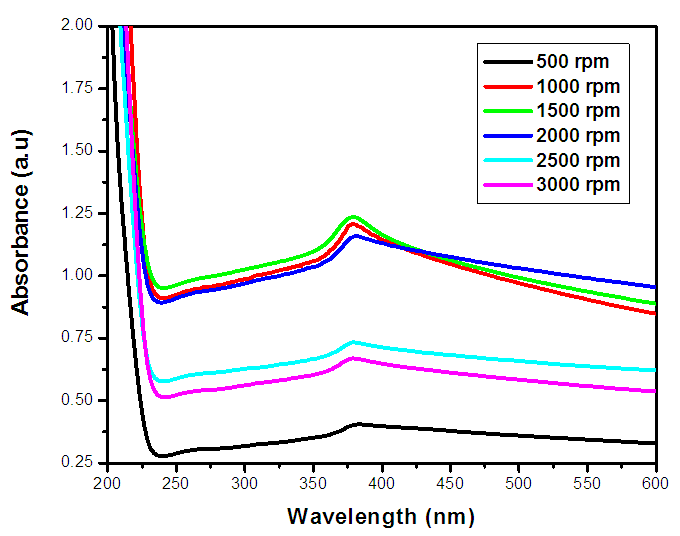 | Figure 2(ii). UV-Vis spectrum absorbance (a.u) against wavelength (nm) of ZnO:Al:Ga co-doped nanoparticles with varying stirring speed |
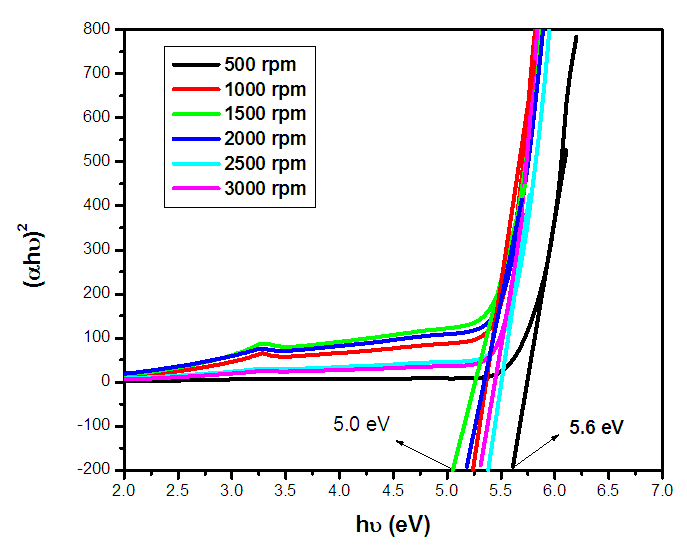 | Figure 2(iii). Tauc’s plot of ZnO:Al:Ga co-doped nanoparticles with varying stirring speed |
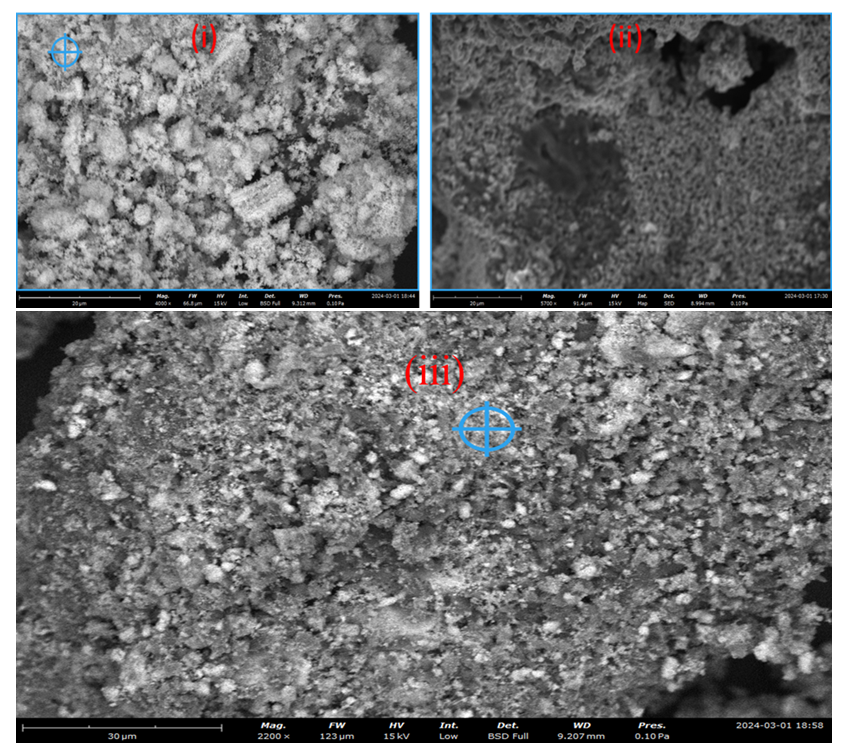 | Figure 3(i). SEM micrographs of synthesized ZnO:Al:Ga co-doped nanoparticles under varying stirring speed (i) 500 rpm, (ii) 2500 rpm (iii) 3000 rpm |
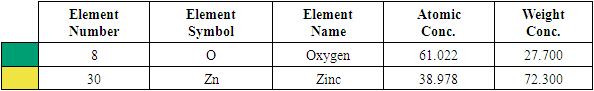
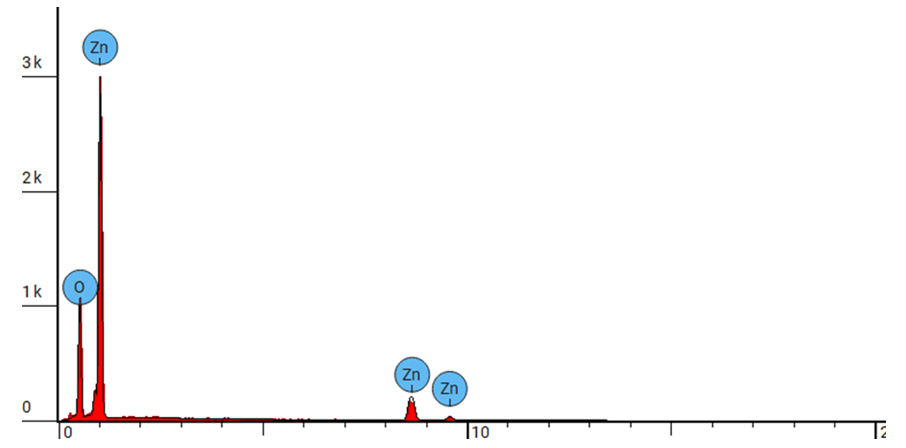 | Figure 3(ii). Showing EDS elemental spectrum pattern synthesized ZnO:Al:Ga co-doped nanoparticles with stirring speed 500 rpm |
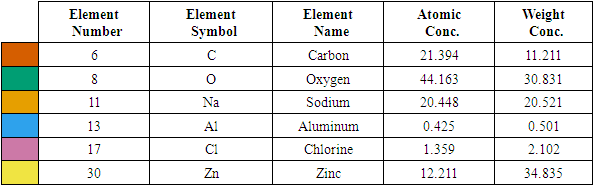
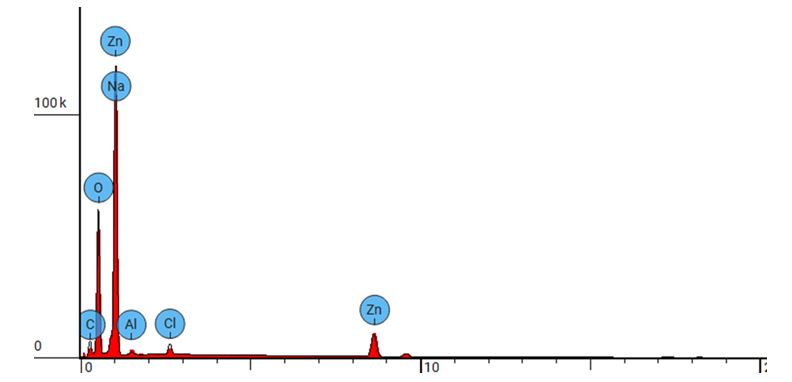 | Figure 3(iii). Showing EDS elemental spectrum pattern synthesized ZnO:Al:Ga co-doped nanoparticles with stirring speed 2500 rpm |
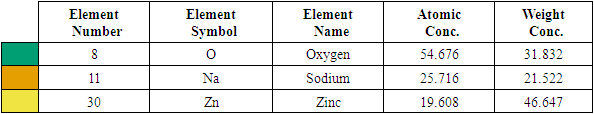
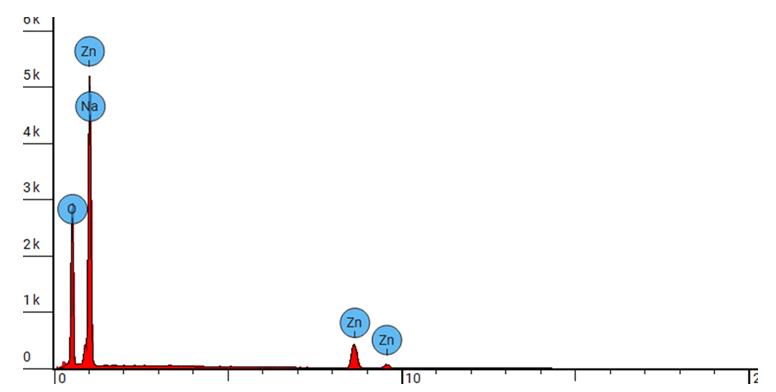 | Figure 3(iv). Showing EDS elemental spectrum pattern synthesized ZnO:Al:Ga co-doped nanoparticles with stirring speed 3000 rpm |
|
|
|
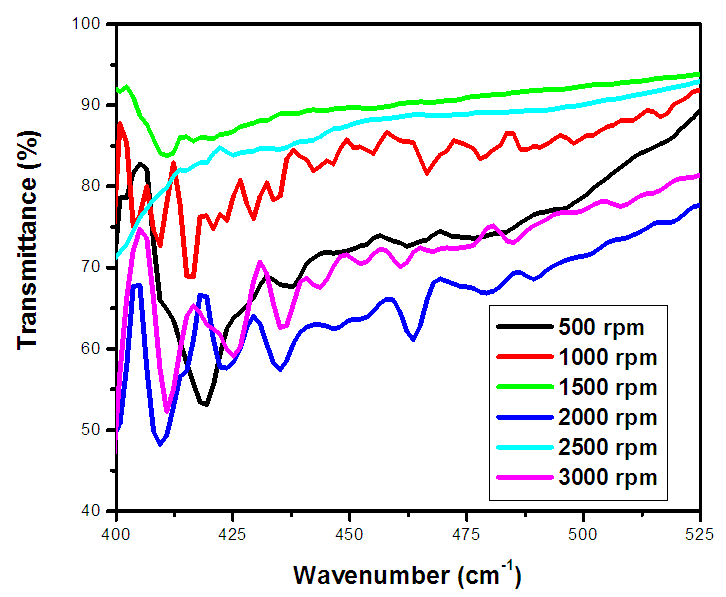 | Figure 4. FTIR spectrum % transmittance against wavenumber (cm-1) of ZnO:Al:Ga co-doped nanoparticles under varying stirring speed |
4. Conclusions
- ZnO:Al:Ga co-doped nanoparticles synthesized and characterized for solar cell applications at different stirring speeds have revealed a plethora of information that is essential for the advancement of renewable energy technology. The identification of a hexagonal wurtzite phase by XRD analysis offers a strong basis for comprehending the structural properties of the nanoparticles. This affirms that sol-gel technique is successful method of synthesis with desirable qualities of nanoparticles. Also, the resulting crystallite size of 21.46 - 20.61 nm showed improved nanoparticles.The optical properties are clarified by UV-Vis spectroscopy, which is necessary for effective light absorption and transmittance. The absorbance spectra of ZnO:Al:Ga co-doped nanoparticles registered wavelength range 377 - 379 nm equivalent to the energy band-gaps 3.27 and 3.29 eV respectively. This corresponds to the standard ZnO’s energy band-gap but contrary to the tauc’s plot estimation. SEM imaging demonstrated uniform distribution within the nanoparticles but that shape varies with speed, with higher speeds of 3500rpm producing smaller nanoparticles while lower speed of 500rpm showed large morphological particle grain-like sizes. EDS examination reveals stirring speed-induced contaminants and validates the existence of key elements. The lower speed showed less incorporation of imperfections while higher speeds showed contamination of nanoparticles. Surface functionalization and chemical composition was better understood with the use of FTIR analysis which confirmed M-O chemical- bonding as evidenced by vibrations modes between 400 – 500 cm-1. The findings of the study showed stirring speed of 1000 rpm as good stirring velocity of the precursor. The speed of 1000 rpm was accompanied with the minimal imperfections and impurities as shown by the values of dislocation density and strain. The highest crystallite size at 1000rpm also support. Furthermore the band-gap of wavelength 378 nm from the UV-vis absorbance spectrum with the FTIR showing more stretching vibrations modes with the region of 400 – 500 cm-1. All these finding supports 1000 rpm as the effective stirring speed for synthesizing nanoparticle for possible solar cells with improved device performance, both of which support the long-term growth of clean energy sources.
ACKNOWLEDGEMENTS
- This research has been successful through the financial support received from African AI Research grant Award: DSA and Deep Learning Indaba. The authors wish to thank this team and Murang’a University of Technology for giving us access to various synthesis and characterization techniques for the research.
Declaration of Conflict of Interest
- The authors have no conflict of interest to declare.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML