-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Condensed Matter Physics
p-ISSN: 2163-1115 e-ISSN: 2163-1123
2017; 7(4): 87-92
doi:10.5923/j.ajcmp.20170704.02

Curing Parameters and Mechanical Properties of NR/SBR Blends
1City for Scientific Research and Technological Applications, Borg Al-Arab, Alexandria, Egypt
2Physics Department, Faculty of Science, Cairo University, Cairo, Egypt
Correspondence to: A. S. Doma, City for Scientific Research and Technological Applications, Borg Al-Arab, Alexandria, Egypt.
| Email: |  |
Copyright © 2017 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this paper, different rubber compounds were prepared by usingSMR-20 type of natural rubber (NR) and SBR-1502 type of styrene-butadiene rubber. The effect of blend ratio on the curing parameters, mechanical and electrical properties of NR/SBR blends have been studied. It was found that the increment of NR percentages increases the values of mooney viscosity (M.V.), low torque (ML), maximum torque (MH), tensile strength (TS), modulus at 300% (M300) and dc conductivity. Moreover, the values of optimum cure time (T90), scorch time (Tsc), elongation at break (Eb), hardness and specific gravity (Sp. Gr.) have been decreased. The increase of the rheometric parameters upon increasing NR content could be attributed to the increase of the cross-linking density. The marked increase in the mechanical parameters (TS and M300) could be attributed to the increase of Eb upon increasing the NR content is due to the uncoiling process of NR chains compared with that of SBR. The blending of SBR raised the value of specific gravity to 0.94 with respect to NR of lower value of 0.92 which satisfies the law of mixturing.
Keywords: Natural rubber, Styrene-butadiene rubber, Tensile strength, Elongation at break, Dc conductivity
Cite this paper: A. S. Doma, H. H. Hassan, Curing Parameters and Mechanical Properties of NR/SBR Blends, American Journal of Condensed Matter Physics, Vol. 7 No. 4, 2017, pp. 87-92. doi: 10.5923/j.ajcmp.20170704.02.
Article Outline
1. Introduction
- Polymer blending has attracted much attention as an easy and cost-effective method of developing polymeric materials that have versatility for commercial applications. In other words, the properties of the blends can be manipulated according to their end use by correct selection of the component polymers. One obvious advantage is that it requires a lower cost relative to the production of new polymers. Meanwhile, the market pressure is so high that producers of plastics need to provide better and more economic materials with superior combinations of properties as a replacement for the traditional metals and polymers. It is also possible to produce a range of materials with properties completely different from those of the blend constituents. Since, ultimately, the properties of a polymer blend will depend on the final morphology, various research groups have recently undertaken extensive studies of the miscibility and phase behaviour of polymer blends. In practice, the physical properties of interest are found either by miscible pairs or by a heterogeneous system, depending on the type of application. Natural rubber has been studied and reported on extensively because of its superior performance in tire applications. Elastomer Blends are used for many purposes such as lowering the compound cost. NR and SBR have been blended for these reasons [1,2]. A lot of studies have demonstrated that the mechanical properties of such blends can be significantly improved by adding a suitable compatibilizer [3,4]. NR blending has been widely studied and reported as good compatible blends that provide desirable mechanical properties [5-9]. Kim [10] investigated the mechanical properties of NR blended with SBR. Normally, rubbers are vulcanized by systems based on sulphur or peroxide [11]. The common feature of these systems is that they all require activation energy in the form of heat. The extremely high temperatures have the disadvantage that the final properties of the finished product may be affected in one way or another like those obtained by sulphur curing. The type of crosslinks formed in this method give rise to better mechanical properties at higher temperature [12,13]. The crosslinks can be introduced between polymer chains by using different types of vulcanizing agents. Sulphur and organic peroxides are the two commonly used vulcanizing agents. A mixture of Sulphur and peroxide produces mixed crosslinks. Raw rubber or non-polar (SBR) has poor phyisco-chemical properties. To improve these properties, some ingredients such accelerators, activators, antioxidants and softeners should be added to the raw rubber in small quantities. These small quantities could affect the physical properties of the blends [14]. In the study of Reffaee et al. [15], some attempts on electrical and mechanical properties of NBR, SBR and NBR/SBR blends were carried out without the addition of carbon black. In the present study, six different NR/SBR formulations of compound were produced, and mechanical properties were studied by tensile strength, hardness and specific gravity. On the other hand, vulcanization characteristics and dc conductivity were also studied. The present work examines, in more detail, the properties of some industrial rubber blends with changing the blend ratio in the rubber matrix. The physical properties were studied as a function of the blend ratio.
2. Experimental Procedures
2.1. Materials and Formulations
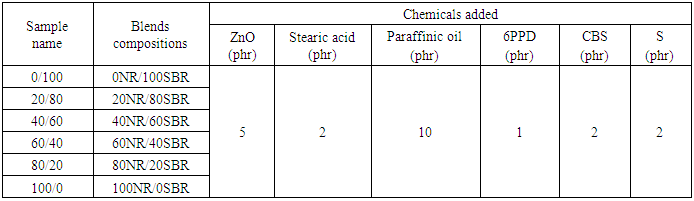
- Natural Rubber (NR) (SMR-20, specific gravity 0.934 (g/cm3); ash content 1%) and SBR (SBR-1502, Sp.Gr. 0.945, styrene content 23.5%) were donated from Transportation and Engineering Co., (TRENCO, Alexandria, Egypt). Sulphur, stearic acid and zinc oxide were used in commercial grade without purification and locally manufactured by ADWIC Co. Egypt. The preparation of six different formulations of NR/SBR blends was carried out according to ASTM D 3185–99. All rubber ingredients were accurately weighed, and mixing was carried out on a laboratory mill at Alexandria Tire Tread Company, with the following dimensions: outside diameter 460 mm, working distance 250 mm, speed of the slow roll 16 rpm, gear ratio 2. The mill has the facility of rolling temperature control. Before the tests, all rubber blends were vulcanized in curing hot press at 143± 2°C and 15 minutes by means of standard dies in accordance with (ASTM D-3191). The compositions of NR/SBR blends for vulcanization step were carried out with blend ratios of (SBR/NBR) as (0/100, 20/80, 40/60, 60/40, 80/20 and 100/0), and are listed in Table 1.
2.2. Preparation of NR/SBR Investigated Samples
- The mixing operation was executed on two stages. The first one called master batch consists of rubbers, activators, antioxidant / antiozonants and processed oil. The addition of 10 phr paraffinic oil into the rubber compound serves as plasticizer or softener in order to decreases the shore hardness of the final blend (Table 1). The second stage is called the final batch. It consists of the previous master batch, curing agent (Sulphur), retarder and accelerators. These materials are added at the end of process to prevent pre-vulcanization which may occur due to the elevated temperatures, the test specimens were die cut from test slabs.
|
2.3. Rheometric Measurements
- Vulcanizing parameters were measured using MDR-2000 Version 1.00 England with temperature 100-200°C according to standard method (ASTM D-5289).
2.4. Mechanical Properties
- The mechanical properties of vulcanized blends were described by measuring tensile strength, modulus at 300%, elongation at break and hardness, while specific gravity was measured as physico-mechanical properties for blends. The Mooney viscosity was measured using (MV-2000, England) with rotor speed 2 rpm and calibrated temperature 100-200°C. The tensile of blends were measured on a Monsanto Tensometer (capacity 10 KN and deformation rate of 60 mm min-1) according to (ASTM D-412) at room temperature. Samples were prepared by cutting three individual dumbbell shape specimens from the vulcanized sheets. The minimum thickness
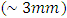 was determined using a dial-gauge. The hardness measurements were performed by a Durometer in Shore A system according to (ASTM D-2240). A system was performed by hand-time at which the reading was taken for 15 sec. Sp. Gr. were determined using (E. Mettler, Zurich, Switzerland) and the used empirical specific gravimetric equation is given as follows:
was determined using a dial-gauge. The hardness measurements were performed by a Durometer in Shore A system according to (ASTM D-2240). A system was performed by hand-time at which the reading was taken for 15 sec. Sp. Gr. were determined using (E. Mettler, Zurich, Switzerland) and the used empirical specific gravimetric equation is given as follows: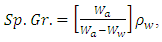 | (1) |
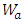 is weight of the sample in air,
is weight of the sample in air, 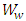 is weight of the sample in water and
is weight of the sample in water and 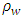 is water density.
is water density.2.5. DC Conductivity Measurements
- The rubber specimens used for electrical conductivity measurements are in the form of discs 1.6 cm diameter and about 0.3 cm thickness. All samples were coated with silver paste which showed ohmic contacts with rubber [16]. The sample holder consists of two parallel plates of brass 1cm dia., the lower being supported with a light pressure spring. The two electrodes were isolated from each other using Teflon.
3. Results and Discussion
3.1. Vulcanization Characteristics of NR/SBR Rubber Blends
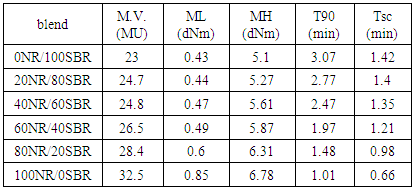
- M.V., ML, MH, Tsc and T90 change significantly with blend composition as given in Table-2. In all blend compositions, a comparatively high optimum torque is obtained when the concentration of NR in the blend is increases. It is already reported that the MH is dependent on crosslink density and chain entanglement [17]. As a result of the comparatively high molecular weight of SBR, the long chain tends to coil (see Table-3). The different portions of each SBR chain entangle with chain of NR and its own chains [18]. High extent of entanglement causes an increase in M.V., ML and MH. The dependence of T90 values on the blend ratio is also presented in Table-2. The decreasing trend may be attributed to the good acceleration of crosslinking reaction. This is also due to the existence of proteins content and other substances such as lipids which could act as natural accelerators for the vulcanization process [19]. In other words, the incorporation of NR in the NR/SBR blend causes an increase of the rate of vulcanization. This is because of the increase of reactive sites on the rubber molecules for the crosslinking reaction. Table-2 shows the dependence of Tsc on the blend ratio of NR and SBR. This is again due to the increasing of the reactive sites of curing process.
|
|
3.2. Mechanical Properties for NR/SBR Rubber Blends
- The effect of the blend ratio on the mechanical properties of NR/SBR blends is presented in Fig.2. The dependence of tensile strength (TS), elongation at break (Eb) and modulus at 300% on the blend ration of NR and SBR is shown in Fig. (2a-c). All figures exhibit that T.S, Eb and M300 increase with the increasing of NR composition in the blends. The presence of non-rubber components in NR seems to play a major role on increasing the crosslinking density [19] which results in a substantial increase in TS and M300. Fig. 2-d shows the dependence of hardness on the blend ratio of NR and SBR. It can be seen from this figure that for the single rubber SBR exhibits hardness decrement compared with NR/SBR blends in various percentage of each one. The hardness of the blend also rises with increasing ratio of NR in the blends. SBR has copolymer of butadiene and styrene group which acts as a harder block to increase the degree of hardness of the SBR [20]. Fig. 2-e shows the variation of the specific gravity with blend ratio for NR/SBR blends. It is clear that the law of mixing could be applied. Since the specific gravity for the vulcanisates, of SBR=0.94 and that of NR=0.92, it is expected that the blends from these rubbers will have intermediate value between these two values. In other words, the increase of NR content in the blend tends to reach the extreme value of specific gravity of NR.
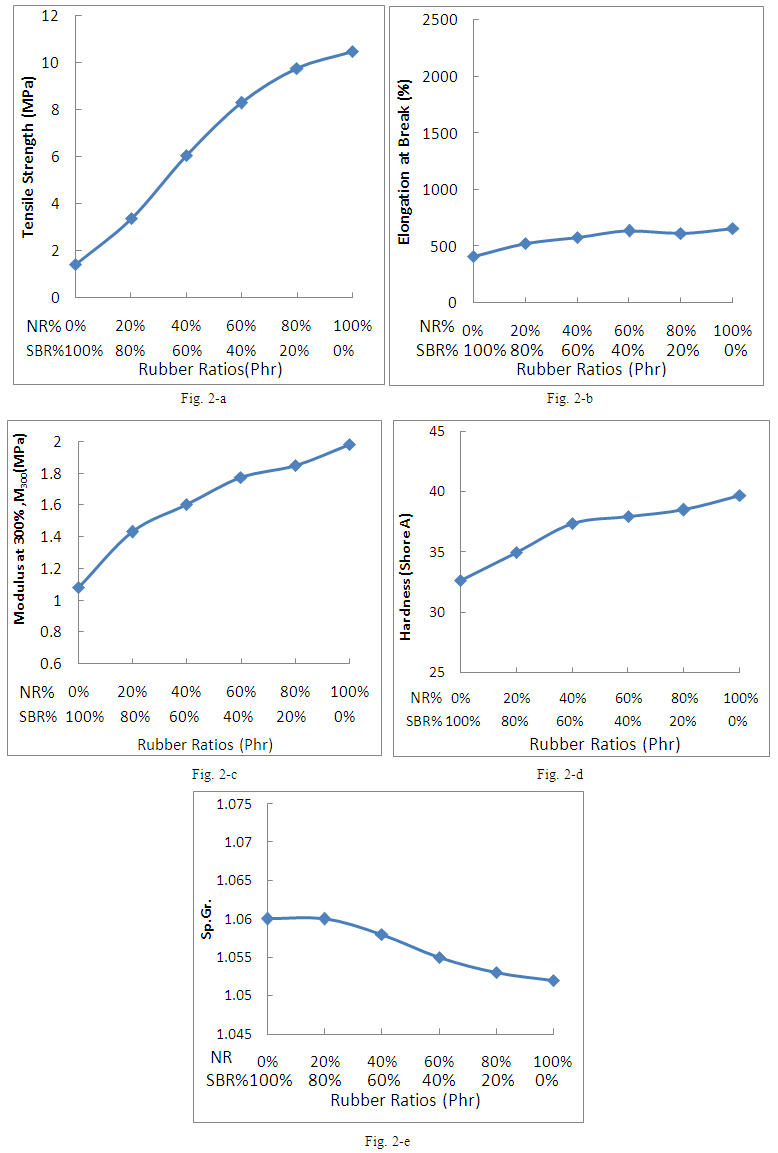 | Figure 2. Effect of the blend ratio on: a) TS, b) Eb, c) M300, d) Hardness and e) Sp.Gr. of NR/SBR Blends |
3.3. Electrical Properties for NR/SBR Rubber Blends
- Fig.-3 shows the variation of dc conductivity upon the blend ratios. It is clear that the electrical conductivity of SBR is lower than that of NR. The increase in conductivity with increasing NR content is likely to be due to the polar non-rubber species in NR. The values of
 for the intermediate blends confirm the low of mixturing between the conductivity of NR and that of SBR.
for the intermediate blends confirm the low of mixturing between the conductivity of NR and that of SBR.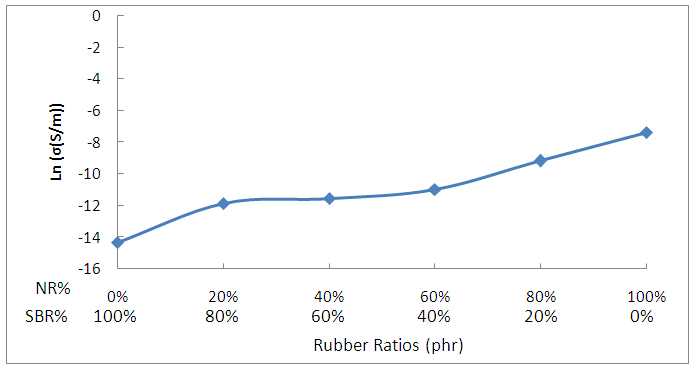 | Figure 3. Variation of dc conductivity upon the blend ratios |
4. Conclusions
- From the results of this paper, it may be concluded that the increase of NR content in NR/SBR blends results in a marked increase of M.V., MH, ML, TS, Eb and M300. The increase of the rheometric parameters upon increasing NR content could be attributed to the increase of the crosslinking density. It is noticed that T90 decreases by about 67% upon the addition of 100 phr of NR, while Tsc decreases by about 54%. The marked increase in the mechanical parameters (TS and M300) could be attributed to the increase of NR content in the blend which has also increased the hardness value with respect to NR chains compared to that of SBR. The blending of SBR raised the value of specific gravity to 0.94 with respect to NR vulcanisates of lower value (0.92) which satisfies the law of mixturing. Furthermore, the marked increase of dc conductivity is due to the increase of polar non-rubber species in NR.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML