-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Condensed Matter Physics
p-ISSN: 2163-1115 e-ISSN: 2163-1123
2017; 7(4): 81-86
doi:10.5923/j.ajcmp.20170704.01

Water Treatment Improvement by Ultrasonic Approach
A. Bakhtiari, T. Berberashvili, P. Kervalishvili
Engineering Physics Department, Georgian Technical University, Tbilisi, Georgia
Correspondence to: T. Berberashvili, P. Kervalishvili, Engineering Physics Department, Georgian Technical University, Tbilisi, Georgia.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Water as the most prominent substance on the earth, needs treatment to use. On the other hand, different impurities with different physical or chemical specifications should be purified by specific additive materials and methods. Recently, Nanomaterials as additives instead of common materials have been used to eliminate water impurities what made water treatment more efficient because of Nano size extraordinary physical potentials, but they also can pollute water that their elimination would be more difficult and needs specific methods. Then, Nanoparticles are one of the most important impurities what needs precise treatment. There are some different common processes to demolish Nano impurities, but ultrasound as mechanical wave in aqueous area not only is capable to eliminate them from water but also it is the most powerful and efficient method to effect on nanoparticles.
Keywords: Nanoparticles, Ultrasound, Water Purification, Ultra sonification
Cite this paper: A. Bakhtiari, T. Berberashvili, P. Kervalishvili, Water Treatment Improvement by Ultrasonic Approach, American Journal of Condensed Matter Physics, Vol. 7 No. 4, 2017, pp. 81-86. doi: 10.5923/j.ajcmp.20170704.01.
Article Outline
1. Introduction
- Water is the most important enablers of life on the earth. Recently, water quality has been associated with the development index of society. Realizing the molecular nature of contamination in drinking water, significant progress has been made to utilize the chemistry of nanomaterials for water purification [1, 2].Nanotechnology, the engineering and art of manipulating matter at the nanoscale (1-100 nm), offers the potential of novel nanomaterials for treatment of surface water, groundwater and wastewater contaminated by toxic metal ions, organic and inorganic solutes, and microorganisms. Due to their unique activity toward recalcitrant contaminants and application flexibility, many nanomaterials (nanostructured catalytic membranes, Nano sorbents, Nano catalysts and bioactive nanoparticles) are under active research and development. Among all nanoparticles available at the market, a few of them show the tremendous role in water purification, such as Au, Ag, zero-valent iron, Fe3O4, and TiO2 nanoparticles. Ag nanoparticles have generally been used for the removal of pathogens from water and heavy metals from many years. Recently, nZVI nanoparticles have been used mainly for the degrading toxic organic pollutants and removal of heavy metals. They have been found to be useful for the removal of As (III) and As (V) from water, which is a significant work in water purification. Fe3O4 nanoparticles have been used as non-absorbent for the removal of pollutants, and these nanoparticles have been easily removed by applying magnetic field (Fig. 1). Further, TiO2 nanoparticles have effectively been used for the degradation of pesticides present in water, and due to their catalytic property, they have been used for the inactivation of bacteria present in water. Hence, nanoparticles have tremendous application in water purifications [3].
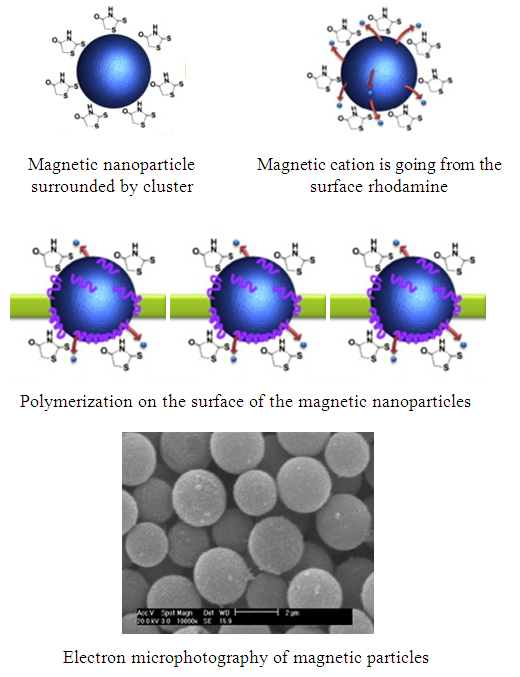 | Figure 1. Scheme of usage of magnetic nanoparticles for water cleaning |
2. Method of Ultrasonic Treatment
- Ultrasonic waves as useful instrument for water purification is well known [7, 8]. Effective application of the ultrasonic purification process requires consideration of a number of parameters, while time, temperature and chemical nature of the particle remain important in ultrasonic as they are in other purification technologies, there are other factors which must be considered to maximize the effectiveness of the process. Especially important are those variables which affect the intensity of ultrasonic cavitation in the liquid. For maximizing the cavitation very important is to precisely manage the temperature which is the most important single parameter to be considered in maximizing cavitation intensity. Changes in temperature result in changes in viscosity, the solubility of gas in the liquid, the diffusion rate of dissolved gasses in the liquid, and vapour pressure, all of which affect cavitation intensity. In pure water, the cavitation effect is maximized at approximately 70°C. For most effective purification, the cleaning liquid must contain as little dissolved gas as possible. Gas dissolved in the liquid is released during the bubble growth phase of cavitation and prevents its violent imploding, which is required for the desired ultrasonic effect. In water at a temperature about 70°C, optimum cleaning is often seen at higher or lower temperatures which depends on concentration of impurity particles in the specific volume of the water. During the negative pressure portion of the sound wave, the liquid is torn apart and cavitation bubbles start to form. As a negative pressure develops within the bubble, gasses dissolved in the cavitating liquid start to diffuse across the boundary into the bubble. As negative pressure is reduced due to the passing of the rarefaction portion of the sound wave and atmospheric pressure is reached, the cavitation bubble starts to collapse due to its own surface tension. During the compression portion of the sound wave, any gas which diffused into the bubble is compressed and finally starts to diffuse across the boundary again to re-enter the liquid. This process, however, is never complete as long as the bubble contains gas since the diffusion out of the bubble does not start until the bubble is compressed. In addition, once the bubble is compressed, the boundary surface available for diffusion is reduced. As a result, cavitation bubbles formed in liquids containing gas do not collapse all the way to implosion but rather well in a small pocket of compressed gas in the liquid. This phenomenon can be useful in degassing liquids. The small gas bubbles group together until they finally become sufficiently buoyant to come to the surface of the liquid. At slower pulse rates, more rapid degassing of liquids occurs as coalescing bubbles of air are given an opportunity to rise to the surface of the liquid during the time the ultrasonic energy is off. At more rapid pulse rates the cleaning process may be enhanced as repeated high energy "bursts" of ultrasonic energy occur each time the energy source is turned on. In sweep operation which is very dependent on the ultrasonic amplitude, the frequency of the output of the ultrasonic generator is modulated around a central frequency which may itself be adjustable. In order to well understand the process of degassing, the liquid (water) it is useful to go through the process of condensation of water vapour onto aerosol particles, which is happening during the ultrasonic cavitation. The ambient atmosphere often becomes slightly water supersaturated [9]. This typically occurs as air masses become elevated when crossing higher orographic obstacles, for example accessing a mountain from the direction of the sea. Other mechanisms include convective cooling during changes in daylight. A supersaturated air mass exceeding 100% relative humidity will hold a few percent more water in the gaseous state for a period of time than expected in the equilibrium state. Usually there are two main routes for the return to equilibrium conditions. The first is dilution by dry air masses – in this case no condensation of water will be observed. The second mechanism is condensation on ultrafine water-soluble particles. Simply put, water vapour forms brine at the particle surface (e.g. NaCl). Because of the water vapour, pressure above such droplet surfaces becomes depleted (according to Raoul’s law). As described by Fick’s law water diffusion remains active in the direction of the “sink” (the brine), as long as there is a vapour pressure gradient between the particle acting as the condensation nuclei and the surrounding supersaturated air mass. This process comes to a halt after some time because of increasing dilution of the brine vapour pressure gradient no longer exists to stimulate further water vapour condensation. Because of the release of condensation heat during diffusion to and from condensing nucleus the whole process might become very complex. Qualitatively, well dissolvable particle cores will serve as very efficient condensation nuclei. In essence, the particles will become incorporated into the water cycle and have a chance to become precipitated. For the assessment of the role of dispersed nano-engineered particles within the water cycle, we have to identify those which can be involved in such water condensation processes. As already mentioned above, dissolvable material would fit perfectly. In contrast, hydrophobic particles as individuals can only act as condensation nuclei when a certain water supersaturation is present. The well-known Kelvin equation describes such a situation of condensing water vapour without any dissolution process [10, 11]. Originally set only for pure water droplets this equation at least qualitatively gives an estimate for the ruling principle:
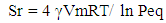 | (1) |
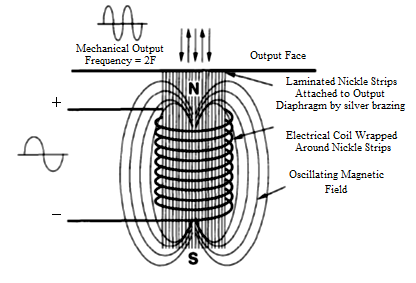 | Figure 2. Scheme of magnetostrictive transducer |
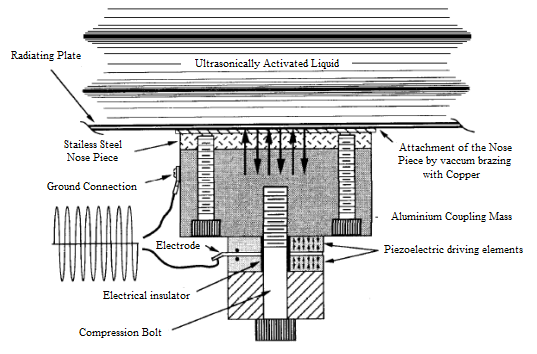 | Figure 3. Scheme of piezoelectric transducer |
3. Drinking Water Treatment
- Drinking water treatment in most municipalities is performing by a huge treatment plant based on screening, coagulation, flocculation, filtration and disinfection [19].Basically, coagulation and flocculation are used to separate the suspended solids portion from the water. Suspended solids in water have a negative charge and since they have the same type of surface charge, they repel each other when they come close together then suspended solids will remain in suspension. Therefore, they will not clump together and settle out of the water, unless proper coagulation and flocculation is used. Coagulation and flocculation occurs in successive steps, allowing particles collision and growth to floc and then followed by sedimentation. To release suspended solids negative charges, coagulant with charges opposite those of the suspended solids are added to the water to be neutralized then the small suspended particles are capable of sticking together. Then, coagulant makes submicroscopic microflocs that in flocculation they collide together and make bonds to produce larger visible pinflocs. Flocs size continues to build larger and once they reach optimum size, strength and weight then it will be ready for sedimentation. As a matter of fact, there are different flocculation process according to different rate that depend on collision frequency induce by the relative motion. Relative motion caused by Brownian movement is called perikinetic flocculation and if it is caused by velocity gradient is called orthokinetic that flocculation by external forces categorized in the second one [20].Generally, coagulation and flocculation remove turbidity, colour, pathogens, algae, phosphates, bad smell and bad taste factors and some others then it is an important step of water treatment that its improvement means more efficient treatment. Hence, coagulants should be added to water in flash mixer for flocculation in flocculation basin. Flocculation improvement depends on different factors like PH, temperature, coagulant efficiency, but high-energy and rapid-mix to properly disperse coagulant and promote particle collisions is needed to achieve good coagulation what is followed by flocculation process but with different mixing velocity and energy to prevent floc from tearing apart. Anyway, flash and effective dispersion of coagulant improve collision of particles and then micoflocs formation that will improve flocculation what finally will improve sedimentation as more efficient water treatment [21].Refer to coagulation efficiency as an important factor for flocculation then sedimentation, nowadays though different nanoparticles usage make all these processes more efficient, but method of ultrasonic treatment gives the possibilities to increase the size of nanoparticles moving in the water [22]. That means better mixing and 20% lower scattering intensity (Fig 4) then more homogenous solution. On the other hand, flocs growing during flocculation as a significant process needs precise control that could be achieved via accurate mechanism like ultra-sonication. Therefore, all of them conduct coagulation improvement, more efficient flocculation and sedimentation then less plant construction that means plant that is more feasible.
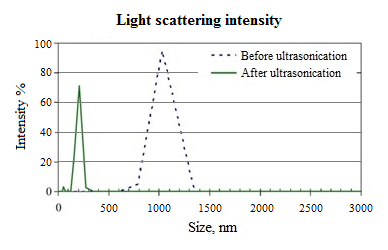 | Figure 4. Improvement of ultra-sonication [18] |
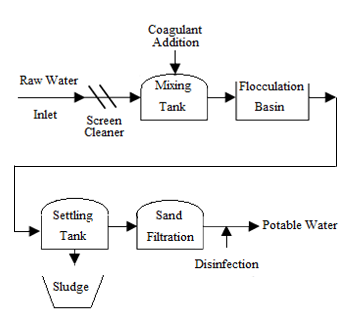 | Figure 5. Basic model of water treatment |
4. Conclusions
- Ultra-sonication is an effective method of water treatment from different nanoparticles used for cleaning the water from different toxic and pathogenic substances. Along with sound waves parameters (frequency, harmonic modes etc.) the effectiveness of ultrasonic treatment is very depend of general power and density of ultrasound flow.Development of construction of transducing systems with flexible electromagnetic and mechanical properties will give the possibilities to build new precise instruments and ultrasonic sources allow purification of water from Nano sized particles of different origin as well as from their clusters and conglomerates.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML