S. Thirumaran, J. Earnest Jayakumar
Department of Physics (DDE), Annamalai University, Annamalainagar
Correspondence to: S. Thirumaran, Department of Physics (DDE), Annamalai University, Annamalainagar.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Abstract
The present study exploring about the possible intermolecular interactions existing between the ternary mixtures of 2-propanol + acetophenone + substituted benzenes at varying temperatures. For this, densities, viscosities and speed of sound for the ternary mixtures of substituted benzenes such as chlorobenzene, bromobenzene, nitrobenzene and toluene with 2-propanol and acetophenone were measured as a function of mole fraction at 303.15, 308.15 and 313.15K at normal atmospheric pressure. The binary solvent mixture prepared with 2-propanol and acetophenone taken in the ratio of 1:3 on molefraction basis. The substituted benzenes were added as solutes in the binary solvent at different molar concentrations. From the above observed experimental measurements, the related excess parameters such as excess adiabatic compressibility (βE), excess intermolecular free length (LfE), excess free volume (VfE), excess internal pressure (πiE), excess Gibb’s energy (∆GE), excess viscosity (ηE) and interaction parameter (d) were meticulously calculated. These excess values were found to be positive as well as negative throughout the whole range of composition for all the four liquid systems and are critically discussed in the light of strong and weak molecular intermolecular interactions between unlike molecules.
Keywords:
Intermolecular free length, Free volume, Gibb’s Free energy, Interaction parameter, Internal pressure
Cite this paper: S. Thirumaran, J. Earnest Jayakumar, Excess Thermodynamic Studies of Ternary Liquid Mixtures of Substituted Benzenes in Aqueous Mixed Solvent Systems at 303.15, 308.15 and 313.15 K, American Journal of Condensed Matter Physics, Vol. 5 No. 2, 2015, pp. 41-50. doi: 10.5923/j.ajcmp.20150502.01.
1. Introduction
Ultrasonic velocity of a liquid is fundamentally related to the binding forces between the atoms or the molecules and has been adequately employed in understanding the nature of molecular interaction in pure liquids [1]. The variation of ultrasonic velocity and related parameters throw much light upon the structural changes associated with the liquid mixtures having weakly interacting components [2] as well as strongly interacting components. The study of molecular association in organic ternary mixtures having alcohol as one of the components is of particular interest, since alcohols are strongly self-associated liquid having a three dimensional network of hydrogen bond and can be associated with any other group having some degree of polar attractions. Accurate knowledge of thermodynamic mixing properties such as adiabatic compressibility, intermolecular free length, free volume, internal pressure and molar volume and their excess values for mixtures of protic, non-protic and associated liquids has a great importance in theoretical and applied areas of research. Alcohols [3] and aromatic ketones such as acetophenone [4] exist as associated structures in liquid state. Mixing of ketones with alcohols provide interesting properties due to specific interactions arising from charge-transfer, dipole-dipole, donor–acceptor and hydrogen bond formation forces may be observed. Acetophenone is typical aromatic ketone has been used in perfumery and fragrances. The 2-propanol, a secondary alkanol is widely used as coupling and dispersive agent in the chemical pharmaceuticals and household industries and as carrier and extraction solvent for natural products. Though, a number of workers have studied the behaviours of substituted benzenes with primary alcohols [5], only a feeble literature survey available about the combined effect of ketones with secondary alcohols interacting with substituted benzenes. Hence, the attempt has been made to carry out the present study of molecular interactions of ternary liquids mixtures of substituted benzenes with binary solvent mixtures of acetophenone, an aromatic ketone and 2-propanol, a secondary alcohol added in the ratio of 1:3 at different temperatures. The present ternary liquid systems taken up for investigation at 303.15, 308.15 and 313.15 K are (i) cholorobenzene+2-propanol+acetophenone, (ii) bromobenzene+2-propanol+acetophenone, (iii) nitrobenzene + 2-propanol + acetophenone and (iv) toluene + 2-propanal + acetophenone.
2. Materials and Methods
All the chemicals used in present investigation were of analytical grade, were purchased from Merck, and were kept in sealed dark bottle dried over molecular sieves for 2 to3 weeks prior to their use. Binary mixtures were prepared by mass in air-tight, stoppered glass bottles using an analytical balance (AX-180, Shimadzu, Japan) with a least count of 0.0001g. Binary solvent mixtures were prepared with 2-propanol and acetophenone respectively in the fixed ratio of 1:3 by molefraction. Substituted benzenes such as cholorobenzene, bromobenzene, nitrobenzene, and toluene were added as solutes at different molar concentration. The densities of pure and experimental liquids were measured with a 5ml capillary specific gravity bottle. An Oswald U-tube viscometer of 10ml capacity with sufficient long efflux time was used for viscosity measurements. An electronic digital stopwatch (Racer-10) with a readability of ±0.01s was used for flow time measurement. At least three repetition of each data point obtained were reproducible to ± 0.05s and the results were averaged. All measurements were carried out in a thermostatic water bath (Ragaa Industries-Chennai) controlled to ±0.1K. Speed of sound was measured by using a 2 MHz ultrasonic interferometer (Mittal Enterprises, New Delhi) with the accuracy of ±2 ms-1. The purity of organic chemicals used in the present investigation were first tested by measuring its corresponding refractive index and later with density values also eventually compared with earlier literature values.
3. Results and Discussion
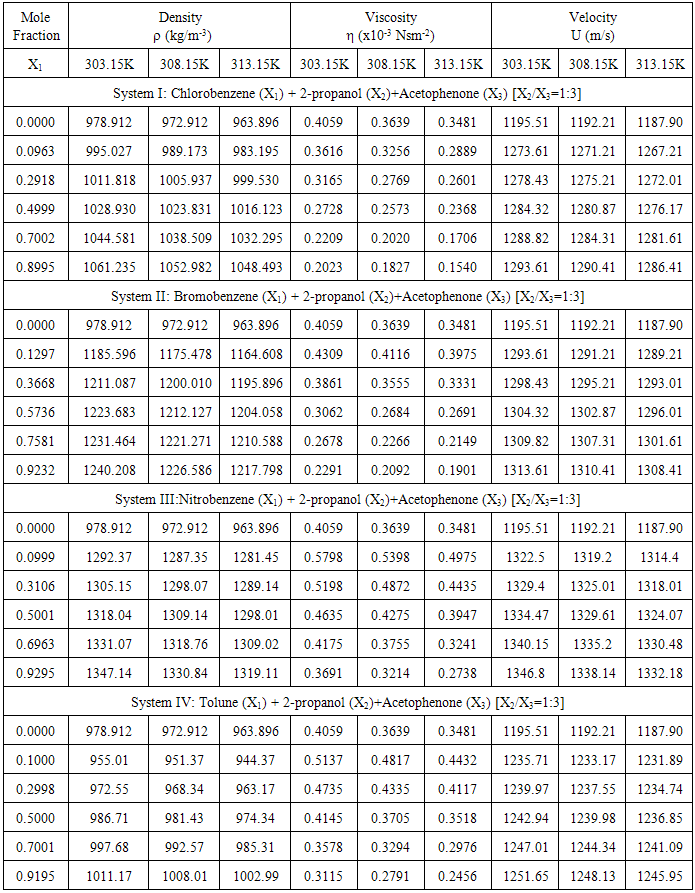
In the present study all the four ternary liquid systems, the density (ρ) and the ultrasonic velocity (U) increases with increasing molar concentrations of solutes, such as chlorobenzene, bromobenzene, nitrobenzene, and toluene, whereas the viscosity (η) shows a decreasing trend. These are tabulated in Table-1. The variation of ultrasonic velocity in a mixture depends on the increase (or) decrease of intermolecular free length after mixing the components. According to Eyring and Kincaid for sound projection [6], ultrasonic velocity should decrease, if the intermolecular free length increase and vice-versa, which is ascertained in the present investigation for all the four liquid systems. Further, it is interesting to note that density, viscosity and ultrasonic velocity values decrease with increase of temperature.Table 1. Density (ρ), Viscosity (η) and Ultrasonic velocity (U) at 303.15, 308.15 and 313.15K
 |
| |
|
The thermodynamic excess properties of organic liquid mixtures depend on the chemical structure, size and shape of their constituent molecules. The excess functions which are a measure of deviation from ideal behaviour are found to be highly sensitive to intermolecular interactions between component molecules of the mixtures. The sign and magnitude of these functions depend on the strength of interaction between dissimilar molecules in the mixture.The perusal of Table-2 reveals the values of excess adiabatic compressibility (βE) for all the four liquid systems. The negative values of βE are associated with a structure-forming tendency, while positive values are of structure-breaking tendency due to hetero-molecular interaction between the component molecules of the mixtures. The positive values of excess adiabatic compressibility which indicates the loosely packed molecules in the mixtures due to their shape and size. From Table-2, it is evident that the excess adiabatic compressibility values are exibit positive trends in Systems I & II and negative trends in systems III & IV. One can notice from the fig-1 in the former systems, the positive deviations are decreasing and in the latter systems the negative values are increasing with the increase of molefraction of solutes (substituted benzenes). It may seen that the negative values of the E increase with increasing concentrations of substituted benzenes in systems III & IV as well as increase of temperature. It may be due to in acetophenone, the carbonyl group is highly polar and hence has a high percentage of ionic character [7] and there exists is a negative charge on the carbonyl oxygen atom of acetophenone. Hence, the increasing trend of negative values will make a strong interactions with substituted benzenes such as chlorobenzene, bromobenzene, nitrobenzene, and toluene due to dipole-dipole interactive forces. In substituted benzenes, the bromine, chlorine and nitrogen all having a tendency to act as an electron acceptors. The decrease in excess adiabatic compressibility in systems I & II may be as attributed to the internal interactions between -electrons of C = O bond and -electrons of the benzene ring [8]. A plausible explanation for decreasing as the oxygen atom of carbonyl group of acetophenone being strongly electronegative would act as a good electron acceptor towards the -electrons of the aromatic ring, forming donor-acceptor complexes [9]. The formation of donar-acceptor complexes seems to be an indication of weak molecular interaction between unlike molecules resulting from the breaking up of the hydrogen bonds of substituted benzenes that makes the compressibility to decrease. The evaluated values in Table-2 reporting the trends of excess free length (LfE) for all the four ternary liquid systems. The LfE values are positive in systems I & II and negative in Systems III & IV, out of which the former two systems are found to be decreased with increasing molar concentration of substituted benzenes and temperature, whereas the latter two systems are found to be increased with same. According to Kannappan et al. [10], the negative values of LfE indicate that sound waves cover long distances due to decrease in intermolecular free length describing the dominant nature of hydrogen bond interaction between unlike molecules. Fort et.all [11] suggested that the positive excess values of free length should be attributed to the dispersive forces and negative excess values due to charge transfer and hydrogen bond formation. The Fig-2 depicts the variation of excess free length with mole fraction, which shows an increasing trend of negative excess values of intermolecular free length (LfE) with the increasing molar concentration of substituted benzenes as well as increase of temperature. This increasing trend may be interpreted as aromatic ketones such as acetophenone which is being a highly polar molecule (μ = 2.96) and it may enhance the polarities of the substituted benzenes.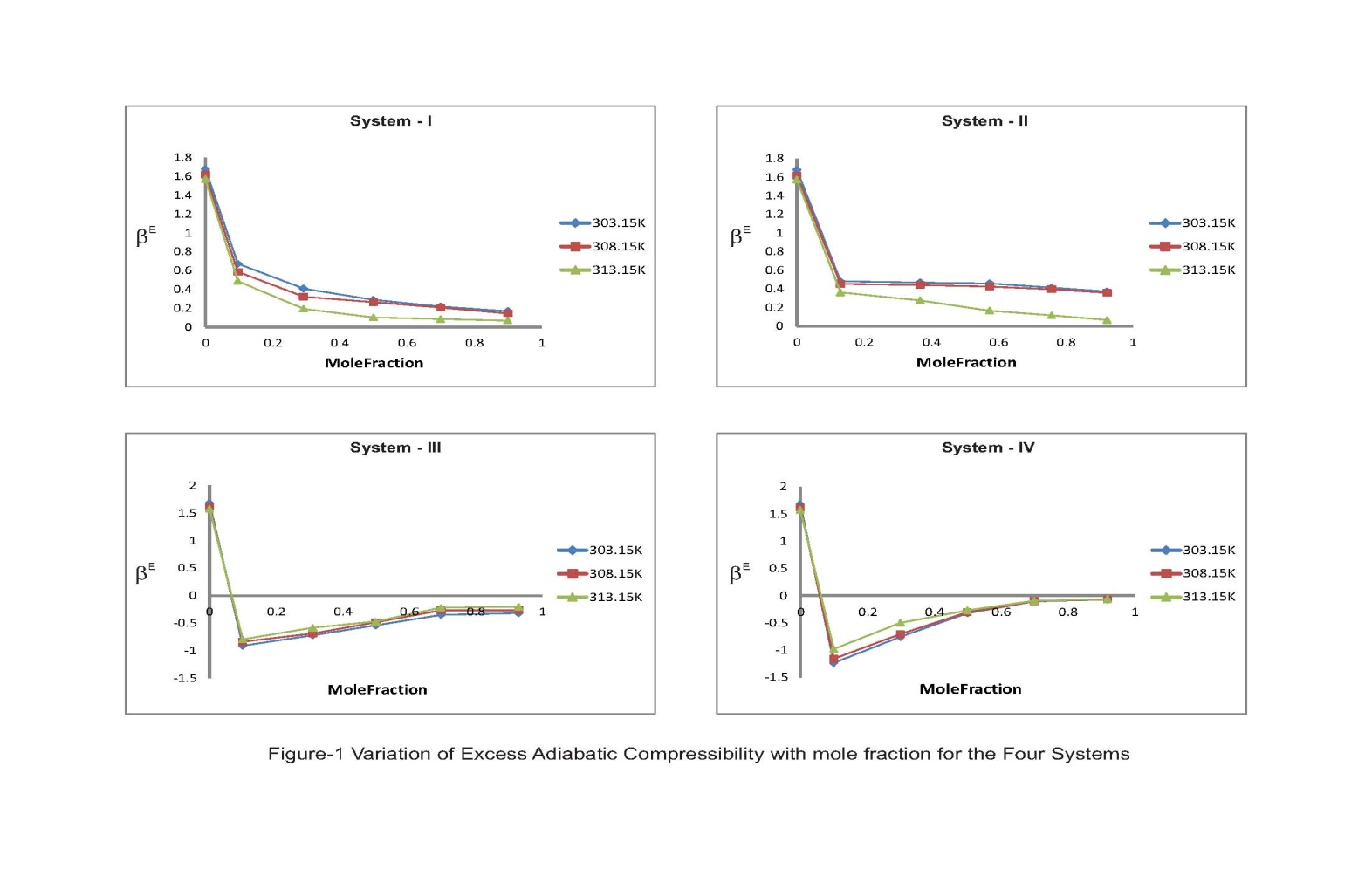 | Figure 1. Variation of Excess Adiabatic Compressibility with mole fraction for the Four Systems |
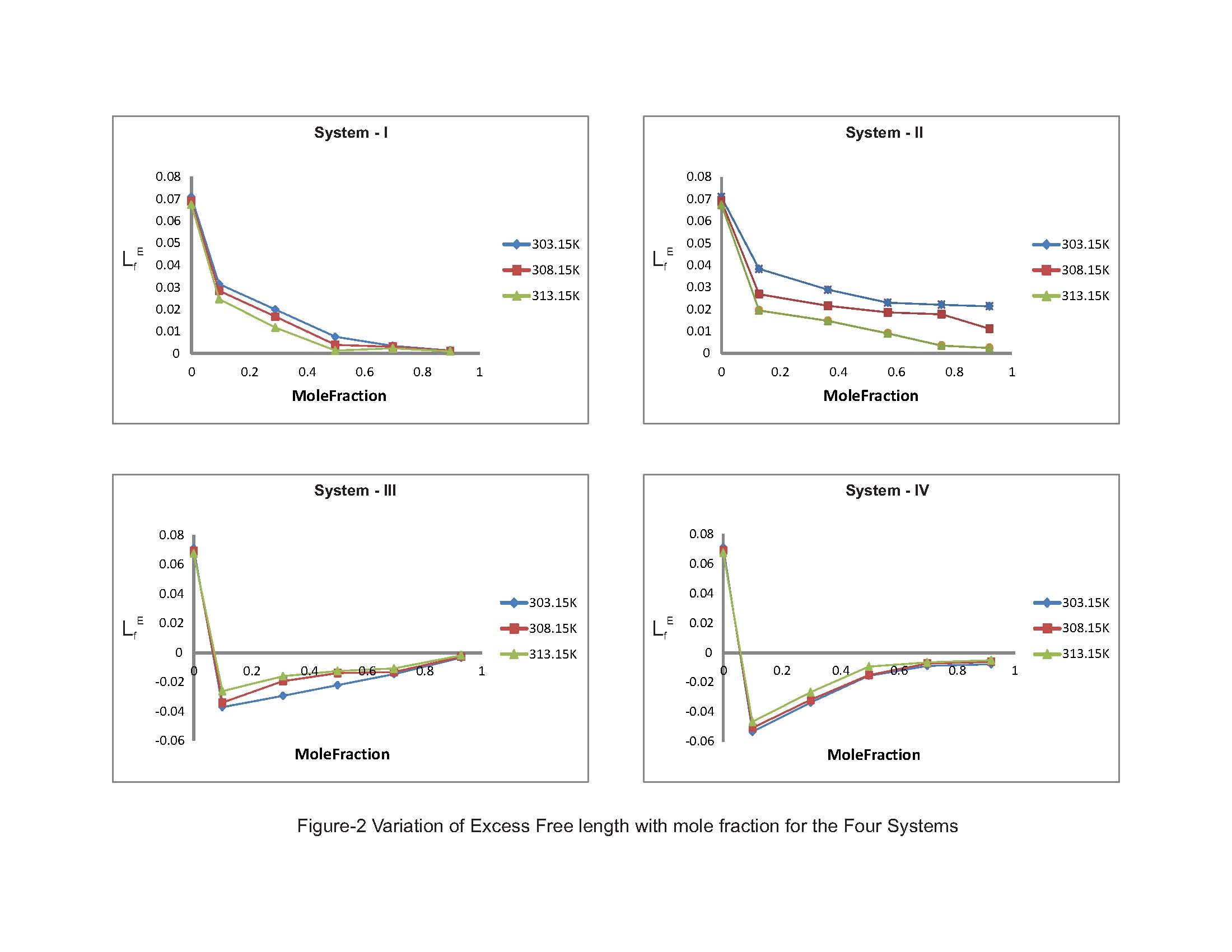 | Figure 2. Variation of Excess Free length with mole fraction for the Four Systems |
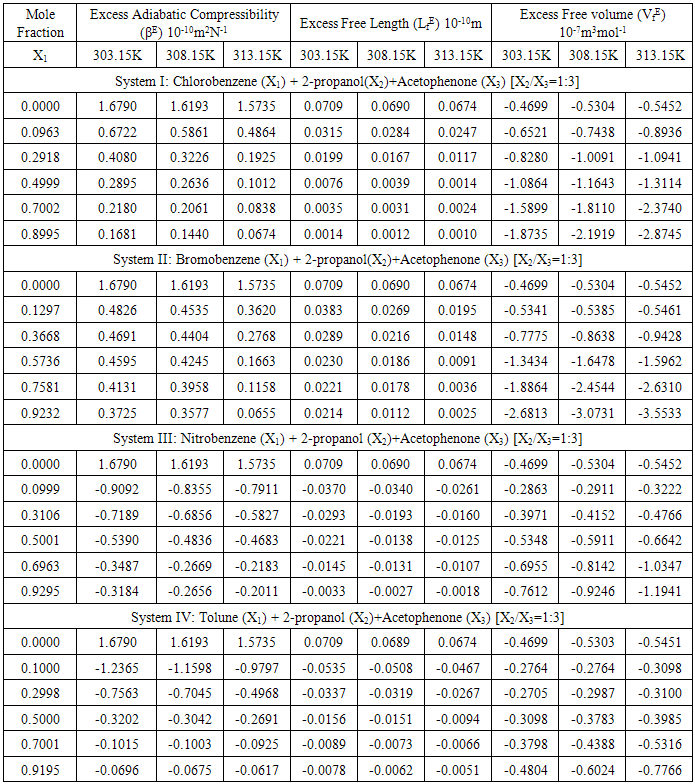
Table 2. Excess Adiabatic compressibility (βE), Excess Free Length (LfE), and Excess Freevolume (VfE) at 303.15, 308.15 and 313.15K
 |
| |
|
The π - electron density in derivatives of benzene ring depends upon that is attached to it. The heteromolecular interaction between component molecules necessarily depends upon the net π - electron density in the ring. Moreover; these interactions seemed to depend on relative orientation of the two groups in the ring. As the separation between these two groups increases, the intermolecular interaction is expected to increase. Hence, an increase in excess free length (Lf) is noticed in Systems III & IV. The perusal of Table-2 provides a qualitative picture of excess free volume (VfE) values for all the four ternary liquid systems. The excess values for all the four ternary liquid systems are negative. These negative deviations found to be decreased on increasing the molar concentration of substituted benzenes (solutes) as well as elevation of temperature for the systems I & II however a reverse trend is observed for the systems III & IV. Adgaonkar et al. [12] advocated positive values of VfE indicating the existence of weak molecular interactions in the liquid mixtures. In the present study of our binary solvent, 2-propanol, a secondary alcohol also a polar one which is in association with acetophenone forms hydrogen bonding. Grace selvarani et al. [13] had investigated about the molecular association which is due to the hydroxyl group of alkanols and acetophenone. Accordingly, aromatic ketones such as acetophenone are more associated with alcohols than the aliphatic one. Since alkanols are liquids which are associated through hydrogen bonding and in the pure state, they exhibit equilibrium between multimer and monomer species. When the substituted benzenes such as nitrobenzene is mixed with 2-propanol, the NO2 group can interact with OH group. The aromatic derivatives will have interaction between the π-electrons cloud and the hydroxyl group. Though the interaction is of a minor intensity compared with hydrogen bonding, but this may lead to formation of intermolecular complexes [14]. In the liquid mixtures, the study of internal pressure may give some suitable information regarding the nature and strength of the forces existing between the molecules. In fact, the internal pressure is a broader concept and it is a measure of the totality of forces of the dispersion, ionic and dipolar interaction that contribute to be overall cohesion of the liquid systems. In the present study the Fig-4 with composition dependence with excess internal pressure depicts the values πEi are negative in all the four liquid systems are decreasing on increasing the molar concentration of substituted benzenes. This indicate that dipolar forces are operating between the unlike molecules and responsible for decreasing behaviour of excess internal pressure values. Hence the weakening of cohesive forces results due to making up of the structure of the solvent. 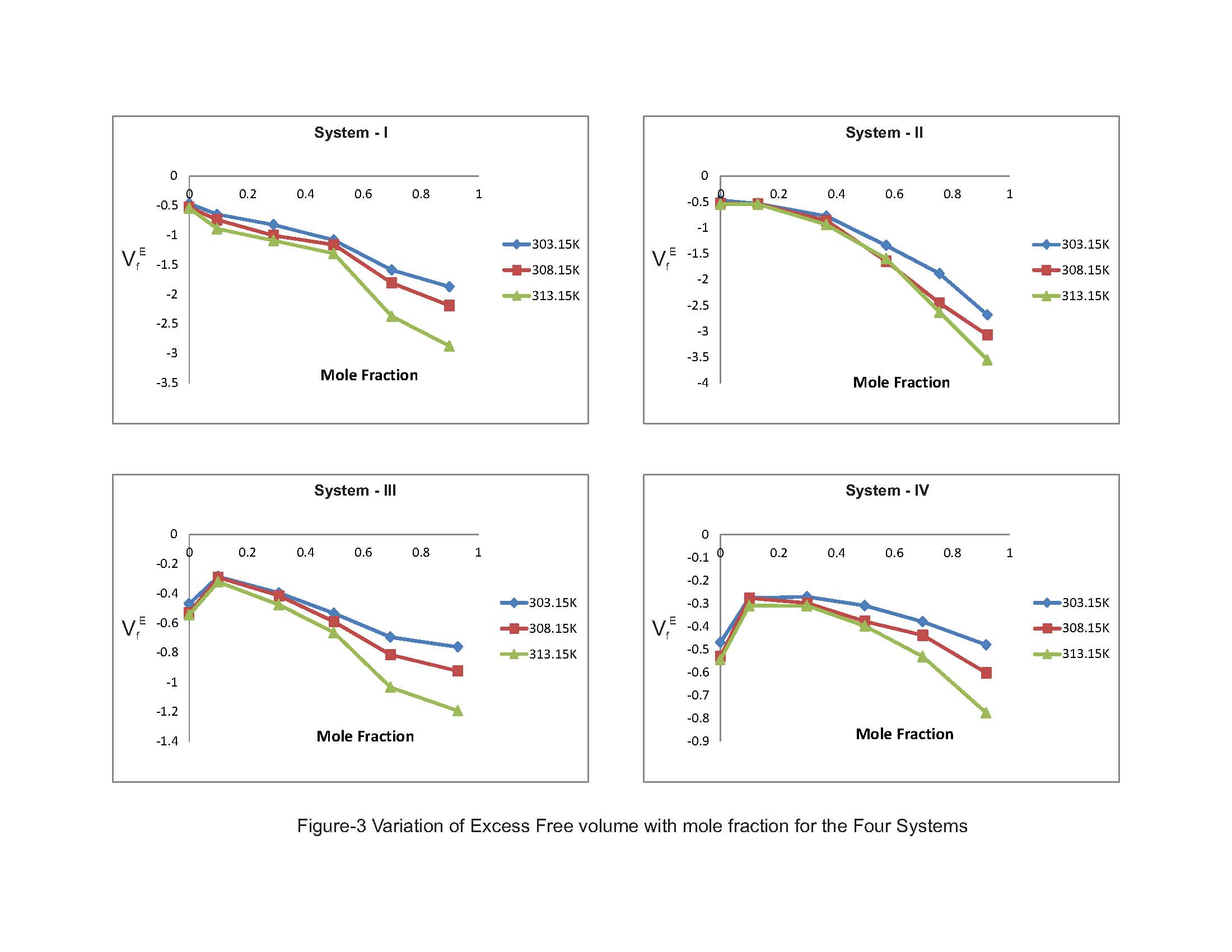 | Figure 3. Variation of Excess Free volume with mole fraction for the Four Systems |
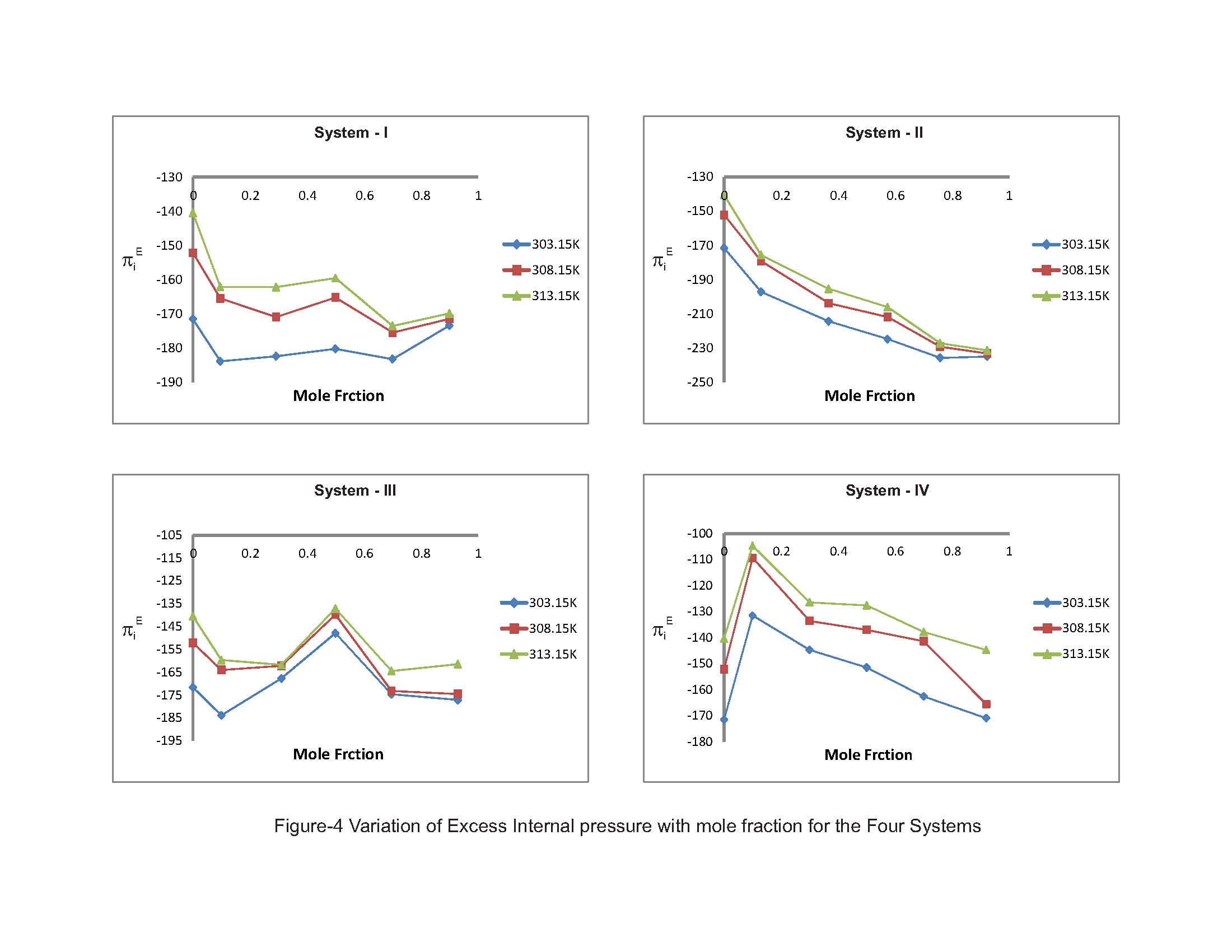 | Figure 4. Variation of Excess Internal pressure with mole fraction for the Four Systems |
The evaluated values in Table-3 shows the variation of excess Gibbs energy ∆GE with mole fraction of for the four ternary liquid systems. The values of ∆GE are all negative in all the liquid systems concerned and increase with molar concentration of substituted benzenes as well as temperature. According to Read et al. [15], the positive values of excess Gibbs energy values may be attributed to specific interactions like hydrogen bonding and charge transfer, while negative ∆GE values may be to the dominance of dispersion forces [16]. Our present investigation, from the perusal of Fig-5, observes that the increasing trend negative values of ∆GE. This suggests that the strength of interaction gets weakened with further addition of substituted benzenes. It is also learnt from the present investigation the increasing behaviour of negative deviation over the elevation of temperature also weakens the strength of the molecular association as it causes rupture of more and more hydrogen bonded and hetero-association between unlike molecules leading to weakening of strength of molecular interaction in the liquid mixtures. 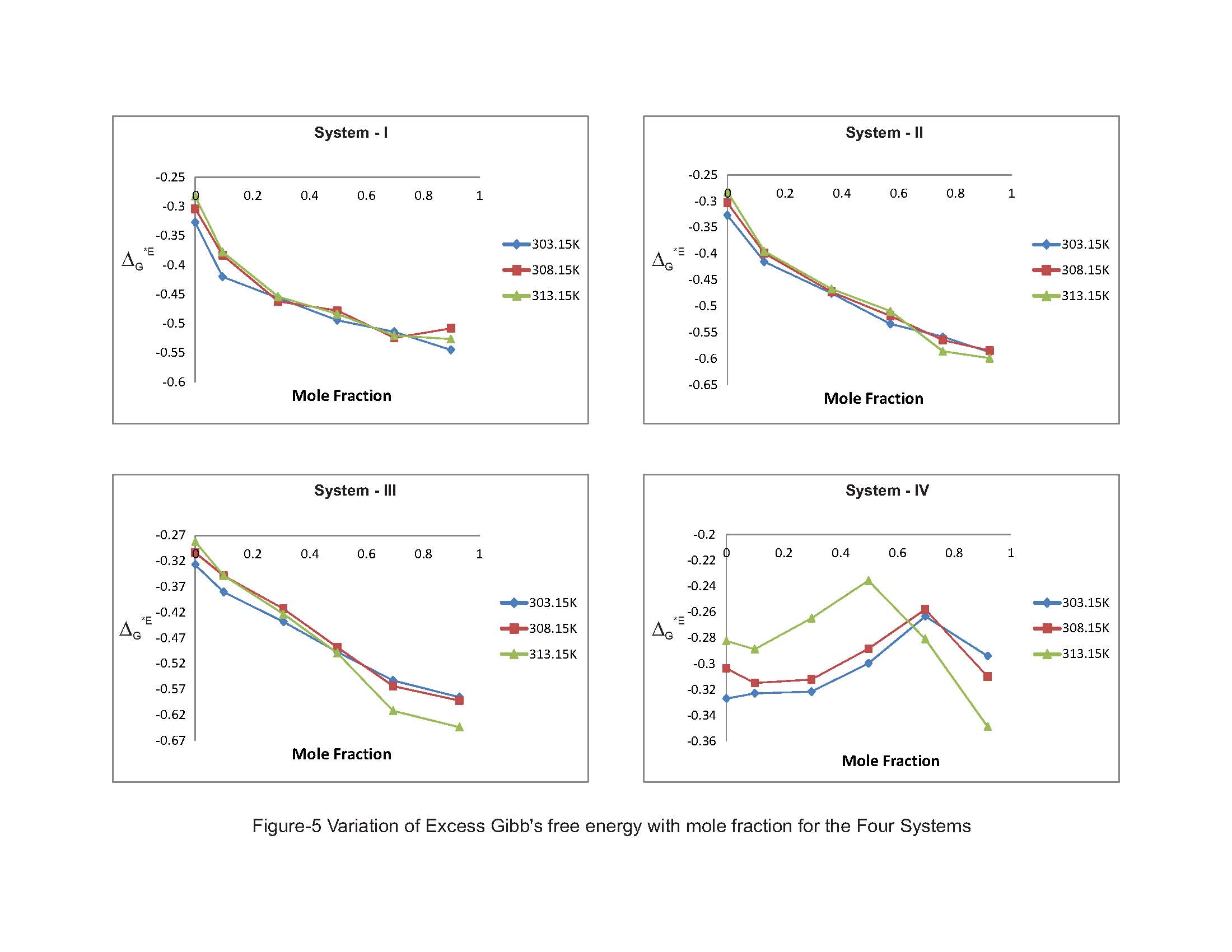 | Figure 5. Variation of Excess Gibb’s free energy with mole fraction for the Four Systems |
 | Figure 6. Variation of Excess Viscosity with mole fraction for the Four Systems |
Table 3. Excess Internal Pressure (πi), Excess Gibb,s Free Energy (∆G*E) , and Excess Molar Volume (VmE) at 303.15, 308.15 and 313.15K
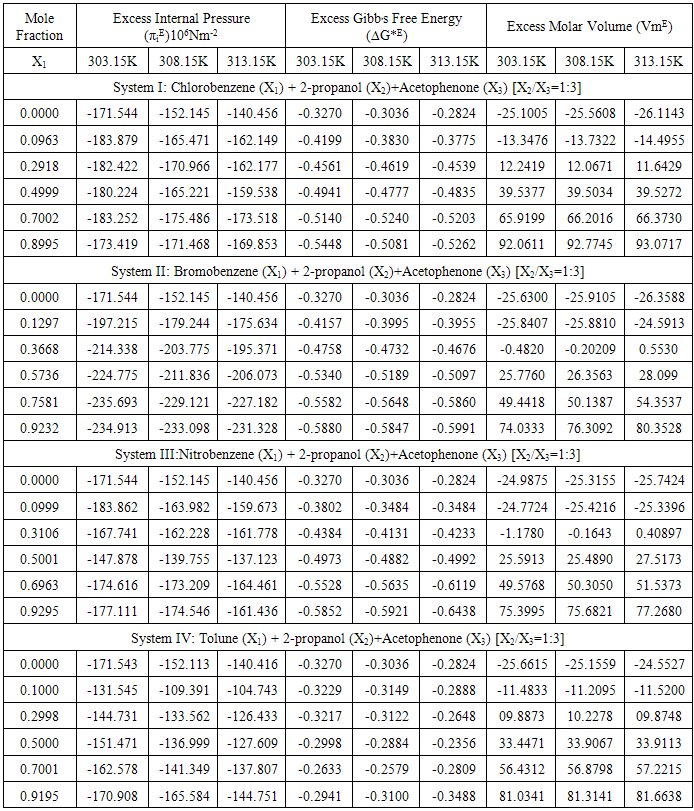 |
| |
|
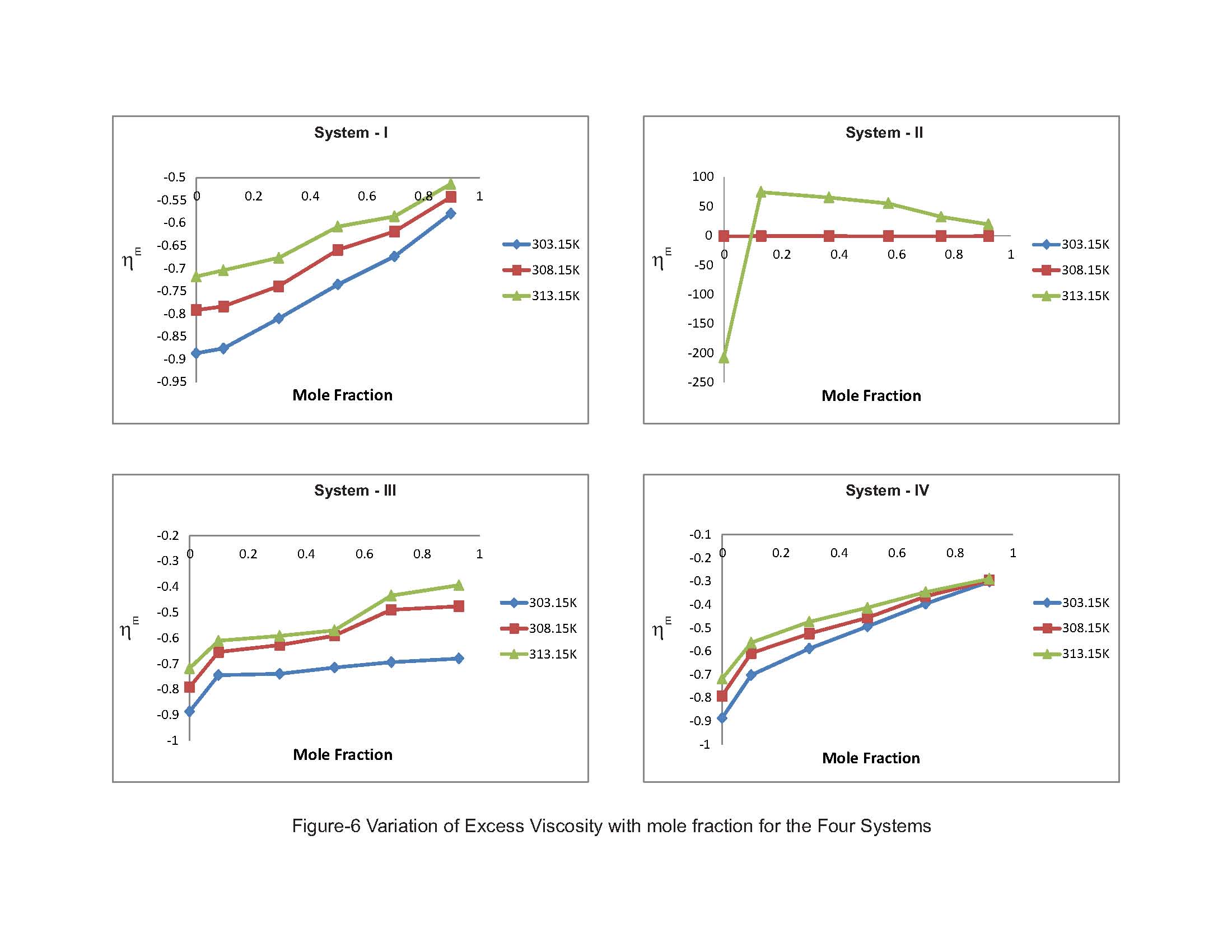
The other vital parameter, excess viscosity, which indicates strength of molecular interactions in the mixtures has been reported from the Table-4. The evaluated values of excess viscosity (ηE) are negative in all the liquid systems and increase with the further addition of substituted benzenes as well as elevation of temperature. Fort et al. [11] for systems, where dispersion, induction, and dipolar forces which are operated by the values of excess viscosity are found to be negative, whereas the existence of specific interactions leading to the formation of complexes in liquid mixtures tends to make excess viscosity positive. The presence of negative values advocates the existence of weak dipolar forces in the liquid mixtures. The increasing negative values of ηE also suggests that the dominance of dispersive interaction resulting from the breaking up of the hydrogen bonds of substituted benzenes. Further, the increasing negative deviations over the elevation of temperature in all the four liquid systems clearly suggesting the strength of interaction gets weakened as thermal dispersion forces are highly operative in the liquid mixtures. The perusal of Table-3 exhibits the excess molar volume (VmE) are all positive deviations over the entire range of concentration range of the substituted benzenes (solutes). According to Awwad et al. [17] measured excess molar volume obtained in the present study can be qualitatively explained as arising to due to differences in the sizes of the component molecules and interactions between them. Table 4. Excess Ultrasonic Velocity (UE), Excess Viscosity (ηE) and Interaction Parameter (d) at 303.15, 308.15 and 313.15K
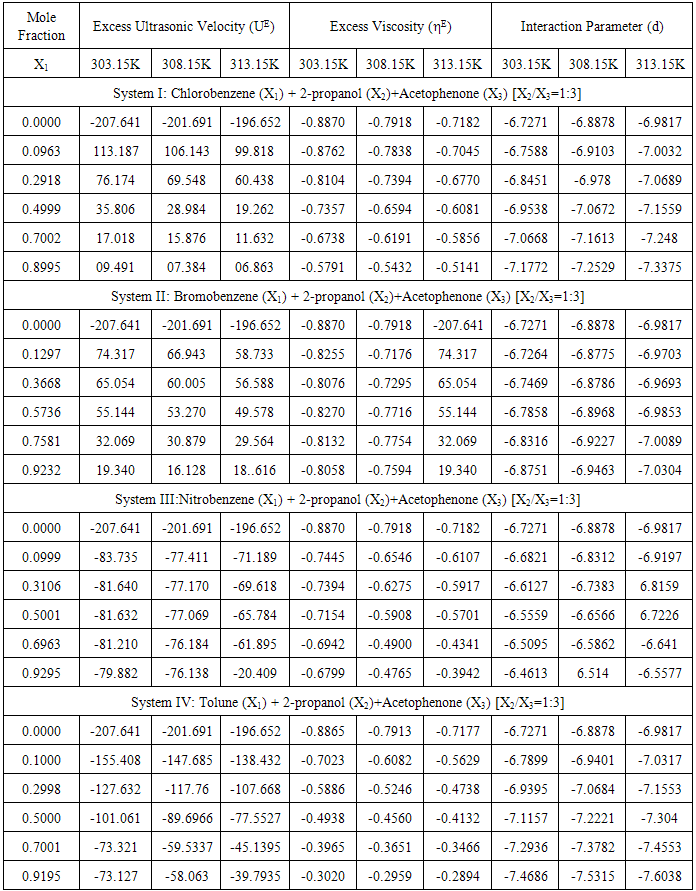 |
| |
|
Differences are mainly due to positive contributions arising from changes of free volume in the real mixtures, comprising alkanol monomers and multimers and acetophenone and substituted benzene molecules. The Table-3 sees that the excess molar volume (VmE) values are exhibiting positive deviations in all the four liquid systems. The positive deviations are found to be decreased with the increase of molar concentration of substituted benzenes and temperature in Systems I & II. However, for the systems III & IV, a reverse trend is observed. The decreasing trend of negative values of VmE with further addition of substituted benzenes in systems I & II clearly predicting the existence of weak dipolar forces.From the evaluation of above excess parameters, it is so far more obvious that although generally a weak molecular interaction was prevailing, the nitrobenzene (system-III) and the toluene systems (system–IV) however exhibit a slight deviation towards the stronger interaction side. The reason for towards the stronger interaction may be interpreted as in the nitrobenzene, which is relatively complex molecule and its polarity arisen out of C-N and N-O bonds. As far as nitro-group is concerned, it rotates freely along the C-N axis which is likely to give more flexibility to the interaction arising due to the two highly polar N-O bonds. This more flexible tendency of this substituted benzene makes the molecular association in a stronger interaction way. Moreover, in the toluene liquid system, the electron withdrawing capacity of –NO2 group which acts as an electron-acceptor towards the π-electrons of toluene ring. This is due to the fact that –CH3 group of toluene is an electron-donating group through induction, enhances the π-electron density of the toluene ring. Such an enhancement of π-electron density in the toluene leading to existence of stronger interaction in this liquid system.The existence of weak intermolecular interaction between unlike molecules further confirmed by evaluating the other interaction parameter (d) in the Gruenberg and Nissan equation which is a measure of strength of interaction between the mixing components. d-values were said to indicate various type of interactions [18]. Large and positive d-values indicate strong specific interaction, small positive values indicate weak specific interaction and large negative values indicate no specific interaction. It is evident from Table-4; the d-values are negative and decrease with increasing of mole fraction of substituted benzenes (solutes) and temperature, except in System-III, where a reverse trend is noticed The decreasing behaviour of d-values reiterates weaker interaction between the unlike molecules, and also their decreasing behaviour over the rise of temperature clearly indicating the weakening of intermolecular interaction due to thermal dispersion forces.
4. Conclusions
The evaluated excess values in the present investigation suggesting a weak molecular interaction in all the four liquid systems. However, nitrobenzene and toluene liquid systems (Systems III & IV) exhibiting a deviation towards a stronger interaction mode comparing other liquid systems. The present study further observes that strength of the molecular interactions gets weakened on further addition of substituted benzenes as well as rise of temperature. It is noticed that weak dipolar and cohesive forces which are highly operative in the liquid mixtures. Moreover the elevation of temperature in the present investigation too plays a vital role in weakening of molecular interactions.
References
| [1] | Isht Vibhu. Amit Misra Manisha Guptha and J.P. Shukla: Ultrasonic and infrared study of molecular interactions in ternary mixtures of 1-naphthol and 2-naphthol with 2-propanone in benzene. Ind. Academy of Science. 62(5), 2004, 1147-1158. |
| [2] | Arul and Palaniappan L: Estimation of sound velocity in ternary liquid mixtures of 2-propanol in cyclohexane with Toluene. Acoustic. Soci. Ind. 28, 2000, 393-395. |
| [3] | Kincaid, J.F. and H. Eyring:A partition function for liquid mercury. J of Chem.Phys. 5,1937,587. |
| [4] | Mohammed Almasi and Hossein Iloukhani: Densities, Viscosities, and Refractive Indices for Binary Mixtures of Acetophenone and 2-Alkanols. J.Chem.Eng.Data. 55,2010, 1416-1420. |
| [5] | Thirumaran, S and Indhu, K: Ultrasonic studies in ternary liquid mixtures of substituted benzenes in toluene with pyridine at different temperatures. Rasayan. J. Chem. 2(3), 2009, 760-768. |
| [6] | Erying. H.J and Kincaid. J.F: Free volume and free angle ratios of molecules in liquids. J.Chem .phys. 6,1938, 620-629. |
| [7] | Pauling L.J: The nature of chemical bonds, 2nd Edn Cornell University Press.115,1948. |
| [8] | Galka. J. L. Suski. P. Tomczyx: Ultrasonic velocity and compressibility of used bismuth + bismuth halide solutions. J. Chem. Thermodynamics. 9,1977,673-681. |
| [9] | Thirumaran, S and Savithri, S: Ultrasonic investigation in ternary liquid mixtures of substituted benzenes with acetophenone at different temperatures. J. Ind. Chem. Soc. 87,2010,279-287. |
| [10] | Kannappan AN. and Palani R: Studies on molecular interaction in ternary liquid mixtures by ultrasonic velocity measurements. Ind. J. Phys. 70B,1996.59-65. |
| [11] | Fort R.T. and Moore W.R: Adiabatic compressibilities in binary liquid mixtures. Trans. Farad. Soc., 61,1965, 2102. |
| [12] | Adgaonkar. C.S. and Agnihotri: Theoritical evaluation of ultrasonic velocity in binary liquid mixtures. Ultrasonics. 27,1989,248-251. |
| [13] | Grace Selvarani. J. Poongodhi and Sabesan: J.Pure.Appl. Ultrasonics 12,.1990,15-7. |
| [14] | Prigogine. I: The moleculer of solutions North Holland Publisher Co. Amsterdam. (1957). |
| [15] | Reed. T.M. and Taylor, T.E: Viscosities of liquid mixtures. J. Phys. Chem.63, 1959, 58-62. |
| [16] | Qin. A.W. D.E. Haffmann and P. Munk:Excess volumes of mixtures of alkanes with carbonyl compounds. J. Chem. Eng. Data. 37,1992,55-61. |
| [17] | Awwad. A. Kanbrur. F.I. Albos E.I: Excess volumes of (an n-alkanoal + N-formylmorpholine). J. Chem. Thermodyn. 16,1984, 733-737. |
| [18] | Syamala, V.K. Sivakumar and Venkateswaralu P: Volumetric, ultrasonic and viscometric studies of binary mixtures of dimethyl sulphoxide with chloro and nitro substituted aromatic hydrocarbons at T=303K, J. Chem. Thermodyn.16, 2006,1153-1159. |







 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML


