-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Condensed Matter Physics
p-ISSN: 2163-1115 e-ISSN: 2163-1123
2013; 3(2): 21-24
doi:10.5923/j.ajcmp.20130302.01
State of Transition Element Atoms in the Solid Solutions of Lanthanum Ortoferrites Containing Calcium and Strontium
Natalia V. Chezhina, Anna V. Fedorova, Stepan M. Musatov
Sankt-Petersburg State University, Universitetskii pr. 26, Sankt-Petersburg, 198504, Russia
Correspondence to: Natalia V. Chezhina, Sankt-Petersburg State University, Universitetskii pr. 26, Sankt-Petersburg, 198504, Russia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
With the help of magnetic dilution method lanthanum ortoferrites containing calcium and strontium were studied. The clusters of iron atoms with competing antiferro- and ferromagnetic superexchange interactions were found, which do not disintegrate at the infinite dilution of the solid solutions. They necessarily contain the atoms of bivalent doping element and accompanying vacancies in the oxygen sublattice. By the method of Mossbauer spectroscopy a small fraction of FeIV was found in calcium containing solid solutions, whereas only FeIII is present in strontium containing solutions in two different surroundings by heavy metal atoms (La,Sr). Clustering seems to be the reason for the stability of perovskite structure of doped lanthanum ortoferrite.
Keywords: Magnetic Dilution, Effective Magnetic Moment, Clusters, Superexchange
Cite this paper: Natalia V. Chezhina, Anna V. Fedorova, Stepan M. Musatov, State of Transition Element Atoms in the Solid Solutions of Lanthanum Ortoferrites Containing Calcium and Strontium, American Journal of Condensed Matter Physics, Vol. 3 No. 2, 2013, pp. 21-24. doi: 10.5923/j.ajcmp.20130302.01.
Article Outline
1. Introduction
- Rare earth ortoferrites, RFeO3 (where R is a rare earth element) attracted considerable recent attention of the researchers in the context of the possibilities of their application as highly efficient magnetic materials. Doped lanthanum ortoferrites, La1-xAxFeO3, (where A is an alkaline earth element) occupy a significant place among oxide semiconductors. They find use in the field of nanoelectronics, as flexible constant magnets etc[1],[2],[3]. In spite of a large number of works devoted to obtaining materials with particular characteristics, the mechanisms of the influence of diamagnetic substituents on functional properties of the materials remains obscure up to the present. Complex oxides doped with bivalent elements – calcium and strontium attract particular interest of the researchers. Introduction of alkaline earth elements in the perovskite structure may initiate a transfer of iron atoms to various valence states and is the reason for oxygen conductivity in the complex oxides based on lanthanum ortoferrite[4],[5],[6]. Recent studies show that not only the nature of the doping diamagnetic element but also its concentration has a profound effect on the physical and chemical properties of these materials. Currently the attempts are made to exert an influence on the properties of doped ortoferrites by substituting aluminium for a fraction of iron atoms[7],[8]. In this case the substitution of AlIII for FeIII is not associated with FeIII transfer to other valence states, however it results in the changes in the properties of these compounds, a decrease in the electronic and oxygen conductivity among them. The authors of[7],[8] studied Mossbauer spectra of lanthanum ortoferrites containing alkaline earth elements and found the transfer of iron atoms to various valence states – FeIV and even FeV, the latter being extremely doubtful.The study of magnetically concentrated systems such as lanthanum ortoferrites fails to trace the influence of the nature of diamagnetic elements on the state of iron atoms and physico-chemical characteristics of materials based on La1-xAxFeO3 , since in the perovskite structure short and long order superexchange interactions are superimposed thus disguising the state of a paramagnetic atom and excluding the possibility to determine the interactions between the nearest atoms. This problem may be solved with the help of magnetic dilution method based on the study of magnetic properties of the solid solutions of isomorphic substitution. Examination of temperature and concentration dependences of magnetic properties of diluted solid solutions allows the state of a paramagnetic atom and the character of super exchange interactions between magnetic centres to be determined to closer limits of accuracy.The aim of this work was to study the state of iron atoms in the solid solutions xLa0,67Ca0,33FeO3 – (1-x)LaAlO3 and xLa0,67Sr0,33FeO3 – (1-x)LaAlO3 (х=0.01 – 0.15) and to determine the influence of the nature of a substituting diamagnetic element on the state of iron atoms and the character of super exchange interactions.
2. Experimental
- Solid solutions xLa0,67Ca0,33FeO3 – (1-x)LaAlO3 and xLa0,67Sr0,33FeO3 – (1-x)LaAlO3, where x =0,01, 0,02, 0,03, 0,04, 0,05, 0,06, 0,08, 0.15, were obtained by ceramic procedure. The starting compounds: special pure grade lanthanum and iron (III) oxides, analytical pure grade strontium and calcium carbonates, aluminium oxide obtained by thermal decomposition of analytical pure grade aluminium nitrate hexahydrate were homogenized in an agate mortar for 1 h. The quantities of the starting substances were calculated by the solid state reaction: 0,5La2O3+0,33xАCO3+0,5(1-х)Al2O3+ 0,5хFe2O3 = xLa0,67А0,33FeO3–(1-x)LaAlO3 + 0,33хCO2,where A is an alkaline earth element (calcium or strontium). The obtained mixtures were pressed into pellets and sintered at 1450oC in air for 50 h.The X-ray patterns were recorded on a DRON-3 diffractometer using FeKα emission. All the solid solutions were single phase and had the structure of cubic lanthanum aluminate. We determined the content of iron atoms in the solid solutions after sintering by the method of atom absorption spectroscopy. The error of the chemical analysis did not exceed 3% from x in the solid solution formula.Magnetic susceptibility of the solid solutions under study was measured by Faraday method in the temperature range 77-400 K. The error of relative measurements was 1%. Diamagnetic corrections for calculating the paramagnetic component of magnetic susceptibility were introduced with respect to the susceptibility of diamagnetic LaAlO3 matrix measured over the same temperature range.We studied the state of iron atoms by the method of Mossbauer spectroscopy. The Mossbauer examination of samples employed a WISSEL spectrometer. The measurements were performed in the absorption geometry at room temperature with 57Cо/Rh source. The isomer shift (IS) values are referenced relative to α-Fe. The experimental spectra were folded and fitted by Lorentz functions using the computer program MossFit.
3. Results and Discussion
- For the solid solutions under study the temperature and concentration dependencies of paramagnetic component of magnetic susceptibility calculated per one mole of iron atoms were plotted. The plots of inverse paramagnetic component vs temperature are linear over the whole temperature interval; χFe for both systems obeys Curie-Weiss low with small negative Weiss constants. Of utmost interest are the isotherms χFe – х plotted for 16 temperatures measured and their comparison with the LaFeO3-LaAlO3 system [9] (Figure 1). It is seen from figure 1 that in the region of х>0.04 both isotherms for calcium and strontium containing solid solutions almost coincide being located higher than the dependencies for LaFexAl1-xO3. This can point to the fact that the character of long order exchange interactions is the same for both systems, but differ significantly from the exchange in the system containing no bivalent doping elements. However at a greater dilution both isotherms are moving apart. For calcium containing solid solutions the dependencies χFe – х lie higher and for the solid solutions doped with strontium – lower than for the solid solutions LaFexAl1-xO3.
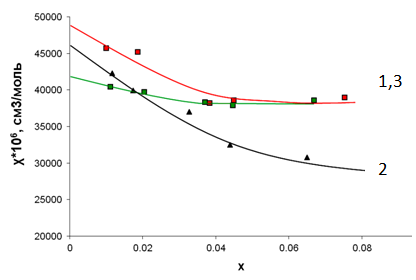 | Figure 1. Plots of paramagnetic component of magnetic susceptibility vs x for the solid solutions La1-0.33xCa0.33xFexAl1-xO3 -1; LaFexAl1-xO3 -2; La1-0.33xCa(Sr)0.33xFexAl1-xO3 -3 |
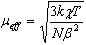 were extrapolated to the infinite dilution of the solid solutions (χFe, μeff, x→0) (Figure 2.). We emphasize that the effective magnetic moment at the infinite dilution of the solid LaFexAl1-xO3 solution is 5.92 BM , does not depend on temperature, and accurately fits FeIII - 3d5, 6A1g[10]. The effective magnetic moments for the La1-0.33xCa(Sr)0.33xFexAl1-xO3 solid solutions under study slightly increase as the temperature increases and appear to be higher (6.0-6.22 BM) for the solid solutions doped with calcium and lower than 5.92 BM (5.35-5.68 BM) for strontium containing solid solutions.
were extrapolated to the infinite dilution of the solid solutions (χFe, μeff, x→0) (Figure 2.). We emphasize that the effective magnetic moment at the infinite dilution of the solid LaFexAl1-xO3 solution is 5.92 BM , does not depend on temperature, and accurately fits FeIII - 3d5, 6A1g[10]. The effective magnetic moments for the La1-0.33xCa(Sr)0.33xFexAl1-xO3 solid solutions under study slightly increase as the temperature increases and appear to be higher (6.0-6.22 BM) for the solid solutions doped with calcium and lower than 5.92 BM (5.35-5.68 BM) for strontium containing solid solutions.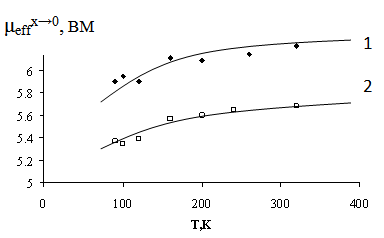 | Figure 2. Plots of effective magnetic moments at infinite dilution (μeffx→0) of the solid solutions La1-0.33xCa0.33xFexAl1-xO3 -1; La1-0.33xCa(Sr)0.33xFexAl1-xO3 -2 |
| |||||||||||||||||||||||||||
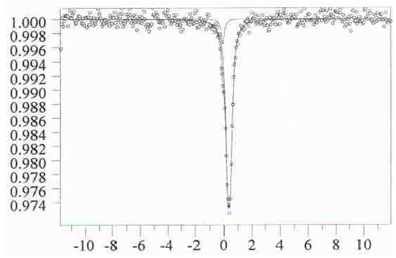 | Figure 3. Mossbauer spectrum of 0,15La0,67Ca0,33FeO3 –0,85LaAlO3 |
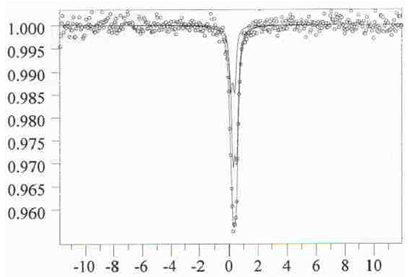 | Figure 4. Mossbauer spectrum of 0,15La0,67Sr0,33FeO3 –0,85LaAlO3 |
4. Conclusions
- The study of magnetic dilution of lanthanum ortoferrites doped with alkaline earth elements showed that the introduction of heterovalent doping elements into the sublattice of lanthanum results in an increase in iron atoms clustering in the solid solution in comparison with the LaFexAl1-xO3 system. The sizes and the exchange interactions in the clusters stable even at the infinite dilution depend on the nature of the doping element. Introduction of relatively small calcium atoms (compared to strontium) results in a fraction of iron (IV) appearing in the solid solution, which seem to stabilize the structure, whereas in the strontium containing solid solutions only two kinds of FeIII surrounding with heavy metal atoms show themselves in the Mossbauer spectra. The clusters include also the atoms of a doping element and accompanying vacancies in the oxygen sublattice.
ACKNOWLEDGEMENTS
- The authors are grateful to professor Valentin G. Semenov and Dr. Vitalii V. Panchuk for their invaluable help in recording the Mossbauer spectra and discussing their results.
References
| [1] | P. Ciambelli, S. Cimino, L. Lisi, «La, Ca and Fe oxide perovskites: preparation, characterization and catalytic properties for methane combustion», Applied Catalysis B: Environmental 33, p. 193–203, 2011. |
| [2] | Gina Pecchi, Particio Reyes, «Effect of prepapation method on the catalytic activity of La1-xCaxFeO3 perovskite-type oxides», Catalysis Today 133-135, pp. 420-427, 2008. |
| [3] | K.H. Ahn, X.W. Wu, «Magnetic properties and colossal magnetoresistance of La(Ca)MnO3 materials doped with Fe», Physical review B: vol.54, no. 21, pp.299-302, 1996/ |
| [4] | M.A. Ahmed, S.I. El-Dek, «Extraordinary role of Ca2+ ions on the magnetization of LaFeO3 orthoferrite», Materials Science and Engineering B 128, pp. 30–33, 2006. |
| [5] | Martin L.W., Chu Y.-H., Ramesh R., «Advances in the growth and characterization of magnetic, ferroelectric, and multiferroic oxide thin films», Materials Science and Engineering R 68, pp. 89-133, 2010. |
| [6] | J.M. Hudspeth, G.A.Stewart, A.J.Studer, D.J.Goossens, «Crystal and magnetic structures in perovskite-related La1-xCaxFeO3-δ (x.0.2, 0.33)», Journal of Physics and Chemistry of Solids 72, pp. 1543–1547, 2011. |
| [7] | A.A. Yaremchenko, M.V. Patrakeev, V.V. Kharton, F.M.B. Marques, I.A. Leonidov, V.L. Kozhevnikov, «Oxygen ionic and electronic conductivity of La0.3Sr0.7Fe(Al)O3−δ perovskites», Solid State Sciences 6 , pp. 357–366, 2004. |
| [8] | Jaroslav Cihlar, David DelFavero, Jaroslav Cihlar Jr., Anton.ın Buchal, Jan Van herle, «Synthesis, structure and oxygen thermally programmed desorption spectra of La1−xCaxAlyFe1−yO3−δ perovskites», Journal of the European Ceramic Society 26, pp. 2999–3004, 2006. |
| [9] | B.Ya.Brach, B.N.Dudkin, N.V.Chezhina. «Magnetic properties of the solid solutions with perovskite structure containing 3d-elements in the trivalent state», Zh. Neorg. Khim., vol. 24, no.8, pp. 2064-2067, 1979. |
| [10] | Kalinnikov V.T., Rakitin Yu.V. Introduction to Magnetochemistry. Method of Static Magnetic Susceptibility in Chemistry. Moscow: Nauka, 1980. 302 p. |
| [11] | Shannon K.D., Prewitt C.T. «Effective ionic radii», Acta Crystal (B), vol. 25, pp. 925-946, 1969 |
| [12] | Natalia V.Chezhina, Anna V.Fedorova, «Atomic states and interatomic interactions in perovskite-like oxides: XXIV. Influence of yttrium atoms on magnetic properties of lanthanum manganites doped with strontium», Russian Journal of General Chemistry, vol. 80, no. 2, pp. 203-206, 2010. |
| [13] | Natalia V.Chezhina, Anna V.Fedorova, «Atom states and interatomic interactions in perovskite-like oxides: XXVI. Short order in magnetoresistive lanthanum manganites doped with various diamagnetic elements», Russian Journal of General Chemistry, vol. 80, no. 5, pp. 909-914, 2010. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML