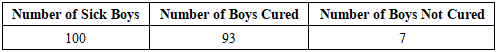-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biomedical Engineering
p-ISSN: 2163-1050 e-ISSN: 2163-1077
2026; 14(1): 1-8
doi:10.5923/j.ajbe.20261401.01
Received: Dec. 23, 2025; Accepted: Jan. 17, 2026; Published: Jan. 28, 2026

Phytochemical Analysis of Molecules, Antimicrobial Activities of Secamone linearifolia Klack, and Its Use as a Remedy for Erectile Dysfunction (Male Impotence)
Zafilaza Armand, Tora Martine
University of Antsiranana Madagascar, Faculty of Sciences, Department of Fundamental and Applied Biochemistry Option of Biotechnologie et Microbiologie
Correspondence to: Zafilaza Armand, University of Antsiranana Madagascar, Faculty of Sciences, Department of Fundamental and Applied Biochemistry Option of Biotechnologie et Microbiologie.
| Email: |  |
Copyright © 2026 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

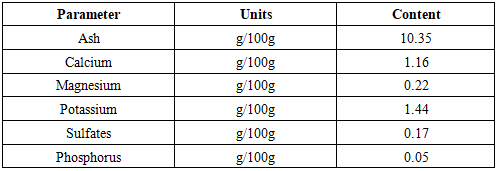
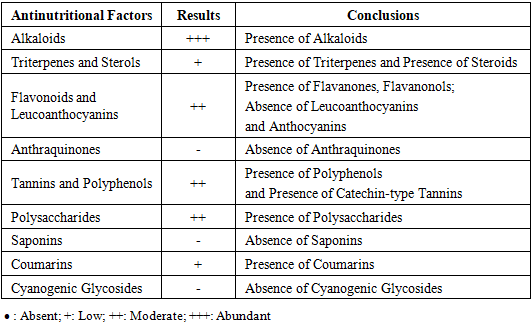
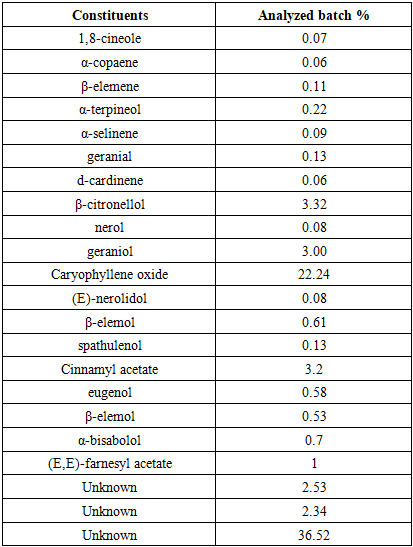
In the DI.A.N.A region of Madagascar, the Khaty and Cola practice, which utilizes the plant Secamone linearifolia, is prevalent among young men seeking stimulant effects for sexual activity, despite reported adverse effects including temporary impotence. This study aimed to characterize the phytochemical profile of S. linearifolia, document its traditional preparation by local healers, and evaluate its therapeutic efficacy. Phytochemical screening of leaves and lianas identified alkaloids, flavonoids, polyphenols, tannins, polysaccharides, and coumarins. Gas chromatography analysis revealed caryophyllene oxide as the major volatile constituent (22.24%), with lower proportions of β-citronellol (3.32%), cinnamyl acetate (3.2%), and geraniol (3%); 36.52% of the composition remains uncharacterized. Mineral analysis by atomic absorption spectrophotometry indicated notable concentrations of potassium (1.44 g/100 g), calcium (1.16 g/100 g), and magnesium (0.22 g/100 g). The plant extract exhibited in vitro antimicrobial activity against Gram-positive and Gram-negative bacteria as well as Candida albicans. Ethnobotanical surveys indicate that traditional practitioners use a preparation of approximately 10 g of the whole plant to treat sexual disorders. Interviews conducted with males aged 18–40 reported a 93% perceived cure rate, with the treatment described as effective when the healer's protocol is strictly adhered to. Secamone linearifolia is an endangered Malagasy endemic. These findings underscore its potential value as a source of compounds for addressing erectile dysfunction and highlight the imperative for its conservation and further bioprospecting.
Keywords: Secamone linearifolia Klack, Moasy, Traditional Practitioner, Phytochemical Screening, Ethnomedical
Cite this paper: Zafilaza Armand, Tora Martine, Phytochemical Analysis of Molecules, Antimicrobial Activities of Secamone linearifolia Klack, and Its Use as a Remedy for Erectile Dysfunction (Male Impotence), American Journal of Biomedical Engineering, Vol. 14 No. 1, 2026, pp. 1-8. doi: 10.5923/j.ajbe.20261401.01.
Article Outline
1. Introduction
- The research is conducted in the northern part of Madagascar, in the Antsiranana province, DI.A.N.A region (Diego Suarez - Ambilobe - Nosy Be - Ambanja). The region is widely recognized for its ecotourism development, and many protected areas have been established. Nearly 75% of the country's exports are found in this region, including vanilla, cocoa, coffee, and especially various fishery products. The DI.A.N.A region generates 80% of the country's foreign exchange earnings, making it the lung of the Malagasy economy. This region also hosts the cultivation of Khaty over hectares of land. It is another financial source for the local population. Khaty is exported throughout Madagascar. The plant Secamone linearifolia Klack is found in the Ambanja District of the Sambirano region. This is a district very rich in fauna and flora. It is home to the Tsaratanana massif, which comprises several protected areas with a humid bioclimatic, tropical sub-humid, rainy, and warm climate. The plant grows at altitudes of 0-499 m, 500-999m, and 1000-1499m [1,2].
1.1. Problem Statement
- Currently, many young Malagasy people are affected by various types of sexual illnesses. One that is very prevalent in the DIANA region is male impotence or erectile dysfunction. Almost the majority of young people in this region are affected by this condition. Many causes can lead to sexual impotence, whether physical or psychological. There are types of diseases that cause male impotence, such as: diabetes, hypertension, cholesterol, conditions like Parkinson's disease, multiple sclerosis, or even prostate cancer. However, in this region, erectile dysfunction occurs too early, between the ages of 20 and 35. In principle, the percentage of male impotence by age group is: 5% between 40 and 60 years; 10% between 60 and 70 years; 15% between 70 and 80 years; 40% beyond 80 years. In this region, the "Khaty and Cola" phenomenon is very popular, as young people use it as a sexual stimulant during intercourse. Therefore, male impotence is caused by Khaty, Cola, and other chemical products.
1.2. Research Objectives
- The objectives are:1. What are the different types of molecules present in the plant?2. What are the methods used by the traditional healer during this treatment?3. And an ethnobotanical survey on the effectiveness of this treatment.
1.3. Erectile Dysfunction (Male Impotence)
- Erectile dysfunction is also referred to as erection problems, and sometimes as sexual impotence (although this term is used less and less today). It is an exclusively male sexual dysfunction, defined as the persistent or recurrent inability to achieve or maintain an erection sufficient for satisfactory sexual performance. The causes can vary, and depending on the situation, treatments are available to improve erectile function. Erection problems have different causes: a physical condition, psychological issues, or the use of certain medications. Often, these factors are combined, and the disorders have multiple origins. Physically-based erectile disorders often appear gradually. They primarily affect men over 50, as age is a major risk factor due to the progressive decline in testosterone levels and the onset of health problems that impact erectile function. However, in our research, the condition (erectile dysfunction) is manifesting in young men between 20 and 40 years old (in the Ambanja District). The rates of the disease increase as the consumption of Khaty and Cola increases. Therefore, Khat and Kola play a significant role in the cause and origin of this disease [3,4,5,6].
1.4. Chemical Constituents of Khat (Catha edulis) and Kola (Cola nitida)
- Khat leaves contain alkaloids, tannins, terpenoids, flavonoids, and sterols. Phenylalkylamines are responsible for the stimulating and euphoric effects of khat. They are composed of: Cathinone, Cathine, Norephedrine, Amphetamine, as well as Calcium and Vitamin C. However, Kola nuts contain caffeine, phenolic compounds, amino acids (Threonine, Valine, Isoleucine, Lysine, and Tryptophan), xanthines, kolatin and kolatein, carbohydrates, proteins, and lipids [7,8,9,10].
1.5. Study Area
1.5.1. Description of the Region
- The plant Secamone linearifolia Klack is found in the Ambanja District of the Sambirano region. This is a district very rich in fauna and flora. It is home to the Tsaratanana massif, which comprises several protected areas with a humid bioclimatic, tropical sub-humid, rainy, and warm climate. The plant grows at altitudes of 0-499 m, 500-999m, and 1000-1499m [1,2].
1.5.2. Classification of Secamone linearifolia Klack
- The genus Secamone is a genus of plants in the Apocynaceae family, which includes about a hundred species spread across Madagascar, Africa, Asia, and Australia. It belongs to the Asclepiadoideae subfamily. The family contains 220 genera and 1300 species; 23 genera (10 of which are endemic) and 146 species (140 of which are endemic). Secamone linearifolia Klack is a 5-meter liana with latex, opposite leaves, and an ovoid fruit [1,2].It is endemic to Madagascar.Kingdom: PlantaeSubkingdom: TracheophytaDivision: MagnoliopsidaClass: EquisetopsidaSubclass: MagnoliopsidaSuperorder: AsteranaeOrder: GentianalesFamily: ApocynaceaeSubfamily: SecamonoidaeaGenus: SecamoneSpecies: Secamone linearifolia KlackVernacular name: Vahy zato
2. Material and Methods
2.1. Work Method for the Ethnobotanical Study
- The work method involves conducting ethnobotanical surveys:• Direct interviews with boys aged 18 to 40 in the two municipalities: the Urban Municipality of Ambanja and the Rural Municipality of Antranokarany.• Interviews with a traditional healer on how to cure sick men.
2.2. Methods Used by the Healer to Treat Erectile Dysfunction
- The healer uses 10g of the whole plant (leaf + vine + root) to treat the illness. The Secamone linearifolia plant is boiled in a pot for 30 minutes. Afterwards, a steam bath is applied to the part being treated (the penis) for 5 to 10 minutes. Finally, one glass of the decoction (from the leaf + vine + root) is drunk three times a day: morning, noon, and evening. The treatment lasts for 15 days.
2.3. Ethno-medical Information
- The ethno-medical information was collected from traditional practitioners, commonly known as "healers" in the study area. This species was identified and confirmed at the herbarium of the identification center in Tsimbazaza. Its vernacular names are Vahy zato. Its leaves and vines are mixed for use in traditional medicine to treat a diseased penis and its pelvic muscles.
2.4. Mineral Analysis
- The powder is placed in a muffle furnace at 550°C to obtain a white ash containing the minerals. The mineral contents of Ca, Mg, K, and Na are determined by atomic absorption spectrophotometry. After moistening, 5 to 25 ml of concentrated hydrochloric acid are added. The suspension is then brought to a boil and filtered. The phosphorus content is determined by colorimetry or by spectrophotometry at 560 nm [11,12,13,14,15,16,17,18].
2.5. Quantitative Phytochemical Analysis
- The method of Harborne, J. B. (1998) [8] was adopted to perform quantitative phytochemical analyses to determine the concentrations of alkaloids, glycosides, phenols, saponins, flavonoids, and tannins in the leaves and vines of Secamone linearifolia. The different chemical groups were characterized according to the techniques described in the work of Harborne, J. B., & Baxter, H. (Eds.). (1993). The results are classified as follows: Presence: +; Absence: - [11,12,13,14,15,16,17,18].
2.5.1. Collection and Preparation of Plant Material
- The plant material is extracted from the leaves and vines. The plant materials were harvested in March in the dense forest of Ampondrabe. These organs were washed with running water and dried in the shade until a constant weight was achieved. The dried plant sample was ground into a fine powder using a grinder, packaged in glass jars, and stored at 4°C until analysis [11,12,13,14,15,16,17,18].
2.5.2. Detection of Sterols and Polyterpenes
- Sterols and polyterpenes were detected in the residues (R1) using the Lieberman reaction. An aliquot of the residue is dissolved while hot in 1 ml of acetic anhydride in a capsule, then transferred to a test tube into which 0.5 ml of concentrated H₂SO₄ is carefully poured. The appearance of a violet color, turning to blue and then to green, indicates a positive reaction [11,12,13,14,15,16,17,18].
2.5.3. Determination of Alkaloids
- Five grams of the ground sample were weighed into a 250 ml beaker, and then 200 ml of 20% acetic acid in ethanol were added. The mixture was covered and left to stand for 4 hours. The mixture was filtered, and the extract was concentrated in a water bath to evaporate a quarter of the initial volume. Concentrated ammonium solution was added drop by drop to the extract until complete precipitation occurred. The solution was decanted, and the precipitate was collected by filtration and then weighed [11,12,13,14,15,16,17,18].
2.5.4. Determination of Glycosides
- Five grams of the ground sample were soaked in 100 ml of distilled water for 3 hours in a beaker. The mixture was filtered using Whatman filter paper and a funnel. The filtrate (2 ml) was transferred into a test tube; 2 ml of 3,5-dinitrosalicylic acid were added and placed in a boiling water bath for 15 minutes. The test tube was removed and left to cool. Distilled water (5 ml) was added as a dilution factor. Absorbance and concentration were measured at 460 nm using a visible spectrophotometer [11,12,13,14,15,16,17,18].
2.5.5. Determination of Phenols
- A defatted sample was prepared as follows: two grams of the sample were defatted with 100 ml of diethyl ether using a Soxhlet apparatus for 2 hours. The defatted sample was brought to a boil with 50 ml of ether for 14 minutes. Five milliliters of the extract were pipetted into a 50 ml flask, and then 10 ml of distilled water were added. Two milliliters of an ammonium hydroxide solution and 5 ml of concentrated ethyl alcohol were also added. The sample was prepared up to the mark and left to react for 30 minutes for color development. The absorbance of the solution was measured using a visible spectrophotometer at a wavelength of 505 nm [11,12,13,14,15,16,17,18].
2.5.6. Determination of Saponins
- Twenty grams of the ground plant sample were dispersed in 200 ml of 20% ethanol. The suspension was heated in a water bath for 4 hours under continuous agitation at approximately 55°C. The mixture was filtered, and the residue was re-extracted with an additional 200 ml of 20% ethanol. The combined extracts were reduced to 40 ml in a water bath at approximately 90°C. The concentrate was transferred into a 250 ml separatory funnel, and 20 ml of diethyl ether were added and shaken vigorously. The aqueous layer was recovered while the ether layer was discarded. The purification process was repeated. 60 ml of normal butanol extracts were washed twice with 10 ml of 5% aqueous sodium chloride. The remaining solution was heated in a water bath. After evaporation, the sample was dried in an oven until a constant weight was achieved. The saponin content was calculated as a percentage [11,12,13,14,15,16,17,18].
2.5.7. Determination of Flavonoids
- Five grams of the ground plant sample were weighed into a 250 ml titration flask, and then 100 ml of 80% aqueous methanol were added at room temperature and stirred for 4 hours on an electric shaker. The entire solution was filtered through Whatman No. 1 filter paper (125 mm), and this process was repeated. The filtrate was then transferred into a crucible, evaporated to dryness in a water bath, and weighed [11,12,13,14,15,16,17,18].
2.5.8. Determination of Tannins
- Five hundred milligrams of the ground sample were weighed into a 100 ml bottle; 50 ml of distilled water were added and shaken for 1 hour on a shaker. The mixture was filtered into a 50 ml volumetric flask and made up to the mark. Then, 5 ml of the filtrate were pipetted into a tube and mixed with 3 ml of 0.1 M FeCl₃ in 0.1 N HCl and 0.008 M potassium ferrocyanide. The absorbance was measured using a visible spectrophotometer at a wavelength of 120 nm within 10 minutes. A control sample was prepared and read at the same wavelength. A standard was prepared using tannic acid to obtain 100 ppm and was measured [11,12,13,14,15,16,17,18].
2.5.9. Detection of Quinones
- Quinones were detected in the residues (R1 - R5) using Borntraëger's reagent. An aliquot of the residue dissolved in 5 ml of HCl diluted 1:5 is heated in a boiling water bath for 30 min, then extracted with 20 ml of CHCl₃ after cooling. To the organic phase, 0.5 ml of NH₄OH diluted to 50% is added. The appearance of a hue ranging from red to violet indicates a positive reaction [11,12,13,14,15,16,17,18].
2.5.10. Detection of Saponins
- Saponins were detected in the residues (R1 – R5) by the foam test, and their presence was confirmed by the blood test and by determining the optical density (OD). The residues are taken up in 5 ml of distilled water and then introduced into a test tube. The test tube is shaken vigorously. The formation of stable foam (height greater than 1 cm) persisting for 1 hour indicates the abundant presence of saponins. The blood test was performed on the aqueous extracts of R4 and R5. Then, a few drops of the aqueous extract are added to a test tube containing 2 ml of fresh animal blood dissolved in a physiological solution (0.9% aqueous NaCl solution). Observation of discoloration compared to a control tube indicates a positive test. Next, 1 ml of blood solution (1 ml of blood in 25 ml of isotonic solution) is placed into 3 test tubes. One of the tubes serves as a control. Into each of the two remaining tubes, 5 and 10 drops of the extracts to be tested are added respectively. After homogenization, the contents of the test tubes are centrifuged for 10 min at 2000 g, and then the OD of each collected supernatant is measured using a colorimeter (wavelength 420 nm) [11,12,13,14,15,16,17,18].
2.6. Detection of Reducing Sugars
- Reducing sugars were detected in the crude extracts (S1 - S5) using Fehling's reagent, and their presence was confirmed by the Tollens test. To perform the Fehling test, 5 ml of Fehling's solution are added to 5 ml of crude extract. The formation of a brick-red precipitate after 2-3 minutes of heating in a water bath at 70°C indicates a positive reaction. The detection of reducing sugars by the Tollens test involved adding 5 ml of Tollens reagent to 5 ml of crude extract. The formation of a silver mirror after a few minutes indicates a positive reaction [16,17,18,19,20].
2.7. Detection of Coumarins
- Coumarins were detected in the residues (R1 – R5) by the reaction on the lactone ring. 2 ml of the ethanolic solution obtained from each residue are placed into 2 test tubes. 0.5 ml of 10% NaOH is added to one of the test tubes, and then the test tubes are heated in a water bath until boiling. After cooling, 4 ml of distilled water are added to each test tube. If the liquid in the test tube where the alkaline solution was added is clear or clearer compared to the liquid in the control test tube (without alkaline solution), then the reaction is positive. When acidifying the clear solution with a few drops of concentrated HCl, it loses its yellow color, becomes cloudy, or a precipitate forms [17,18,19,20].
2.8. Detection of Proteins
- Proteins were detected in the residues (R1 – R5) by the biuret reaction. To an aliquot of residue dissolved in 2 ml of 20% aqueous NaOH in a test tube, 2-3 drops of a 2% aqueous CuSO₄ solution are added. The appearance of a violet color, sometimes with a reddish hue, indicates a positive reaction [11,12,13,14,15,16,17,18].
2.9. Detection of Alkaloids
- Alkaloids were detected in the residues (R1 – R5) with Dragendorff's and Burchard's reagents (precipitation reagents): 0.1 g of residue is taken up in 6 ml of 60% ethanol, then divided into 2 test tubes. In the first tube, 2 drops of Dragendorff's reagent are added. The appearance of an orange-red or brownish-red precipitate indicates a positive test. In the second tube, 2 drops of Burchard's reagent are added. The appearance of a brown precipitate indicates a positive test [11,12,13,14,15,16,17,18].
2.10. Molecular Analysis
- Separation of molecules in a mixture by gas chromatography (GC).The sample (a volatile liquid) is first introduced at the head of the column via a microsyringe which will pass through a soft disc, called a septum, into a small chamber upstream of the column called the injector. The injector is traversed by the carrier gas and heated to a temperature appropriate for the volatility of the sample. The injected quantities are in split mode 51/50° with 0.02% air percentage integration threshold. Then, once volatilized, the different compounds of the sample are carried by the carrier gas through the column; in this experiment, hydrogen gas is used at a constant pressure of 0.50 bar, and they separate from each other depending on their affinity with the stationary phase. The stationary phase is a non-volatile (or low volatility) liquid (gas-liquid chromatography). It will cause a chromatographic retention phenomenon with the different compounds, called solutes. The more affinity a compound has with the stationary phase, the longer it will take to exit the column. The raw experimental value is called the retention time; it is the time elapsed between the injection of the sample and the appearance of the maximum signal of the solute at the detector. To promote the transport of all compounds through the column (elution), the correct oven temperature must be determined. In the experiment, the oven temperature is from 50°C to 250°C at a rate of 5°C/min. The temperature must be slightly higher than the boiling point of the compounds so that they do not exit too early, which would result in their peaks overlapping with the void time peak. The work must be isothermal, meaning at a fixed temperature throughout the entire analysis, or with a temperature program that varies [11,12,13,14,15,16,17,18].At the column outlet, the compounds encounter an essential element called the detector; the column used is UB-WAX (30m x 0.32mm x 0.5µm). This element continuously assesses the quantity of each of the separated constituents within the carrier gas by measuring different physical properties of the gaseous mixture. The detector sends an electronic signal to a recorder (a sort of printer) which will draw the curve of each peak according to their intensity (a Gaussian-type curve) and the set of peaks is called a chromatogram. In the experiment, the detector used is FID (Flame Ionization Detector). Currently, software is increasingly and more advantageously replacing paper recorders for interpreting the signals sent by the detectors [11,12,13,14,15,16,17,18]
2.11. Antimicrobial activity
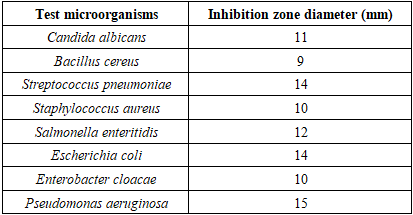
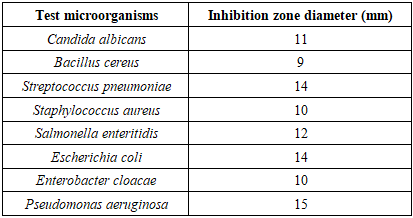
- In the antimicrobial activity test using Secamone linearifolia, many microbes are used. These include Gram-negative and Gram-positive bacteria, as well as pathogenic unicellular fungi. They were sourced from the Environmental Microbiology Laboratory (LME) of the National Center for Environmental Research (CNRE).To determine the antimicrobial activity, the agar diffusion method is used. A Petri dish containing an agar medium (culture inoculated in 5 ml of molten agar to cover the nutrient agar medium) is seeded over its entire surface with the strains of bacteria and fungi under study. The Secamone linearifolia extract is applied to filter paper discs, which are then placed on the surface of the agar. During incubation, the extract diffuses from the filter paper into the agar (incubation for 24 to 48 hours). The further away from the disc, the lower the concentration of the extract becomes. At a certain distance from the disc, the Minimum Inhibitory Concentration (MIC) is reached. Beyond this limit, the bacteria and fungi develop, whereas closer to the disc, growth is inhibited. A zone of inhibition is created, with a diameter proportional to the amount of antimicrobial agent deposited on the paper disc, the solubility of this agent, the diffusion coefficient, and the overall activity of the agent.Here is the list of microbes used during the test series, along with their characteristics [21,22,23,24,25,26].- Gram-negative Bacteria:a). Escherichia coli: Known as "coliform," this bacterium is a normal inhabitant of the intestines of humans and warm-blooded animals. It is a fecal coliform, used as an indicator of fecal contamination in water and food. It is tested for in butcher's meat, seafood products, and raw and fermented milk.The strains responsible for infections in humans are different from those present in the intestinal flora. They are notably involved in diarrheal syndromes in adults and young children in both developing and developed countries.They are classified in risk group 2 for biological agents, meaning they can cause disease in humans and pose a hazard to workers, but their spread in the community is unlikely. Furthermore, effective prophylaxis and treatment exist [21,22,23,24,25,26].b). Salmonella Enteritidis: This is an intestinal parasitic bacterium in vertebrates. Its pathogenic strain is responsible for gastroenteritis during individual or collective food poisoning. These illnesses, called salmonellosis, manifest as diarrhea, vomiting, and fever that appear 8 to 10 hours after ingesting contaminated food. This bacterium is also classified as a risk group 2 pathogen [21,22,23,24,25,26].- Gram-positive Bacteria:a) Bacillus cereus: Living in soil and water, this bacterium can survive in the environment in the form of spores. It can contaminate plant-based foods and numerous prepared dishes. It is one of the microbes that must be routinely tested for in raw, peeled, and cut vegetable products, ready-to-use products, sprouted seeds, and raw vegetable and fruit juices [21,22,23,24,25,26].b) Staphylococcus: Staphylococci are spherical cocci, found isolated in clusters or as diplococci. They are non-motile, generally facultative anaerobes, and they grow on nutrient agar or trypticase soy agar at 37°C and a pH ranging from 7.2 to 7.4. They are of human or animal origin (poultry, cattle, etc.) and are pathogenic, classified in risk group 2. Their pathogenicity occurs either:• Through virulence: Staphylococci produce surface proteins and enzymes, including free coagulase and thermonuclease, thus causing a wide variety of infections including boils, pneumonia, etc.• Through toxin production: Staphylococci produce various toxins, including enterotoxins causing individual or collective food poisoning due to contaminated food. This food intoxication manifests within 2 to 4 hours as nausea, abdominal pain, repeated vomiting, and diarrhea lasting 24 to 48 hours [21,22,23,24,25,26].c) Enterobacter cloacae: Enterococci are ovoid cocci, found isolated, in pairs (diplococci), in short chains or chains. They are non-motile and facultative anaerobes. They are cultured at 37°C and pH 7.2 on common culture media.They are ubiquitous organisms found in soil, fresh and marine waters, and on plants, living as commensals in the human and animal intestine. They can be pathogenic for humans and animals (risk group 2). They are responsible for various and serious opportunistic human infections such as endocarditis, septicemia, meningitis, and urinary tract infections [21,22,23,24,25,26].- Pathogenic Yeast Fungi:a) Candida albicans: Colonies of this yeast are white to creamy-beige and convex on Sabouraud agar. It produces blastospores and chlamydospores. It is tested for during the microbiological control of cosmetic products. It is pathogenic for humans (risk group 2) and animals, and is an agent of mycosis of the skin or mucous membranes [21,22,23,24,25,26].
3. Results of Analyses
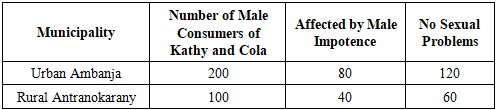
3.1. Ethnobotanical Study
- 200 male consumers of Kathy and Cola were surveyed in the Urban Municipality of Ambanja, and 100 boys were surveyed in the Rural Municipality of Antranokarany.
|
3.2. Treatment Results from Traditional Healers Using Secamone linearifolia
|
3.3. Mineral Analysis Results
|
3.4. Phytochemical Screening Results
|
3.5. Molecular Analysis Results
|
3.6. Antimicrobial Activity Results
|
3.7. Antimicrobial Activity Results
|
4. Discussion
- The traditional healer (Moasy) employs Secamone linearifolia as the foundational material for treating male impotence. Ethnobotanical survey data indicate a 93% reported cure rate (93 out of 100 patients), suggesting a high degree of therapeutic efficacy for erectile dysfunction associated with this plant-based treatment. This notable success rate, however, invites critical reflection on the practitioner's role: does the Moasy function as a genuine healer or a charlatan? To explore this further, follow-up research was conducted with a cohort of 15 patients who reported successful treatment. These individuals consistently described the cure as irreversible, with normal erectile function maintained over a follow-up period of two years post-treatment. A critical stipulation imposed by the Moasy is the strict prohibition against using any performance-enhancing or doping substances—whether natural stimulants like Khaty and Cola, or pharmaceutical agents—during sexual activity.From a scientific standpoint, the therapeutic efficacy is likely underpinned, at least in part, by the diverse phytochemical profile of Secamone linearifolia. Our analyses confirmed the presence of alkaloids, flavonoids, polyphenols, and other bioactive compounds with potential vasodilatory, anti-inflammatory, or neuro-modulatory properties relevant to erectile function. It is significant that this biochemical potential operates independently of the Moasy’s empirical knowledge, as the practitioner utilizes the plant effectively without awareness of its specific molecular constituents. Therefore, the treatment’s apparent success may arise from a confluence of the plant’s inherent pharmacological properties and the stringent behavioral protocol enforced by the healer, pointing to a complex biocultural mechanism of healing that intertwines biochemical action with ritual practice and taboo.
5. Conclusions
- The efficacy of treating the sexual disorder called "male impotence" or "erectile dysfunction" with the plant Secamone linearifolia is very tangible. The cure is irreversible, provided you follow the healer's (or Moasy's) instructions.The medicinal plant Secamone linearifolia is endemic to Madagascar and contains invaluable various types of molecules, such as Caryophyllene oxide (22.24%), β-citronellol (3.32%), and geraniol (3.00%).The plant analysis was only partially successful, as 36.52% of its composition consists of unidentified molecules.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML