-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biomedical Engineering
p-ISSN: 2163-1050 e-ISSN: 2163-1077
2015; 5(4): 130-135
doi:10.5923/j.ajbe.20150504.03

Age and Gender Related Variations of Pituitary Gland Size of Healthy Nepalese People Using Magnetic Resonance Imaging
Tika Ram Lamichhane1, Susheel Pangeni1, Sharma Paudel2, Hari Prasad Lamichhane1
1Central Department of Physics, Tribhuvan University, Kirtipur, Kathmandu, Nepal
2Institute of Medicine, Tribhuvan University Teaching Hospital, Maharajgunj, Kathmandu, Nepal
Correspondence to: Tika Ram Lamichhane, Central Department of Physics, Tribhuvan University, Kirtipur, Kathmandu, Nepal.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The pituitary gland is the master endocrine gland of the human body. Its size varies with age and in various pathological conditions including pituitary adenomas. Magnetic Resonance Imaging (MRI) is the standard tool for the imaging of pituitary gland without using any harmful ionizing radiations. The aim of the study was to obtain standard reference values for the anterior-posterior (AP), height, transverse dimensions and volume of pituitary gland of healthy population and to analyze the potential diagnostic values of dimensions of pituitary gland. These dimensions were measured using standard spin echo sequences with 6 mm thickness in 0.3T permanent magnet MRI. A group of 170 subjects were recruited at Institute of Medicine, Radiology and Imaging Department, Tribhuvan University Teaching Hospital (TUTH) during April 23, 2014 to June 20, 2014. These individuals demonstrated no evidence of abnormalities to the central nervous or endocrine systems prior to the study. The size and shape of a normal pituitary gland are affected by age and gender. The anterior – posterior, height, transverse dimension and volume of pituitary were observed to be 10.3 mm, 6.1 mm, 13.6 mm and 466.8 mm3 respectively. In this study, we had found that the size of the pituitary gland of the Nepalese people reflected the normal values as we expected in healthy people.
Keywords: Magnetic Resonance Imaging, Pituitary Gland, Pituitary Dimensions
Cite this paper: Tika Ram Lamichhane, Susheel Pangeni, Sharma Paudel, Hari Prasad Lamichhane, Age and Gender Related Variations of Pituitary Gland Size of Healthy Nepalese People Using Magnetic Resonance Imaging, American Journal of Biomedical Engineering, Vol. 5 No. 4, 2015, pp. 130-135. doi: 10.5923/j.ajbe.20150504.03.
Article Outline
1. Introduction
- Magnetic resonance (MR) occurs in the magnetic system that contains both magnetic moments and angular momentum [1]. At the resonance condition, the frequency of the applied magnetic signal matches with the natural frequency of the magnetic system. Living tissues contain ample amount of hydrogen atoms which has inherent magnetic moment that is the key tool in magnetic resonance imaging. When the tissue is placed in an intense static magnetic field, the protons precess around the magnetic field lines with certain frequency called Larmor frequency [2]. The Larmor frequency depends both on the magnetic moment of the proton and the applied magnetic field intensity. The natural Larmor frequency of the proton can be detected using another radio frequency signal in the perpendicular direction to the original magnetic field. This technique is called Magnetic Resonance. Hence by using MR, we can collect the information of protons and also the condition of the tissues. This atomic level information has the great influence on the medical applications [3].Magnetic Resonance Imaging (MRI) provides high resolution images. Unlike computed tomography (CT) scan and conventional radiographs, it does not use any harmful ionizing radiations. Hence, MRI is considered safe for human study because there is no known adverse effect from the strong magnetic field and the radio waves [4]. They are also extremely useful because of the wealth of information contained in the signal regarding both structural properties of the tissue and its bio-chemistry [5].The pituitary gland is the master endocrine gland of the human body [6]. It controls other glands and secretes important hormones. Evaluation of pituitary size and its shape are the most important factors for the diagnosis of its pathology. Pituitary adenomas especially the microadenomas are diagnosed mainly with the information of pituitary size and its configuration. So, the study of this gland helps us to find the morphological dimensions and their inter-relationship with age and sex. Various studies are being done for the evaluation of pituitary gland and have found that there is wide variation of pituitary size with age and gender [7, 8]. The pituitary gland volume changes depending on hormonal status. Generally, younger adults have larger glands [9]. Hormonally active individuals (puberty / pregnancy) have the largest glands. Younger has convex upper border with completely filled pituitary fossa, whereas older individuals will have a largely empty pituitary fossa. Reliable maximal Figures for the height of the pituitary gland [9]: children (less than 12 years) - 6mm (upper surface flat or slightly concave), puberty-10mm (upper surface convex, more in females), young adults male- 8mm and female-9mm, and pregnancy-12mm.However, such studies are lacking in our country. To the best of our knowledge, there were currently no useful reference data for the normal range of pituitary gland volumes in Nepalese people [10]. The aim of this study is to explore a compact insight to quantitative analysis of pituitary gland of Nepalese people using magnetic resonance imaging (MRI) techniques.
2. Materials and Methods
- The prospective cross sectional study of the pituitary glands of healthy Nepalese people was carried out during the period of about two months from April 23, 2014 to June 20, 2014. The data were collected from 170 subjects (99 male and 71 female) who underwent MRI examination of head at the Radiology and Imaging Department, TUTH, Kathmandu, Nepal. TUTH is 500 bedded tertiary care central referral hospital and deals with various kinds of diseases. Ethical clearance was sought from and approved by TU, Institute of Medicine (IOM), Research Department and Institutional Review Board [11]. Oral consent was taken from patient for the use of their result in the study. The brain MRI were included in this study if the participants were Nepalese (males and females), aged between 10 to 70 years and provided at least an oral consent. The brain MRI were excluded in this study if the participants were non-Nepalese citizen and described as abnormal with (a) evidence of space occupying lesions e.g. brain tumor, (b) cerebral haemorrhage, (c) infarctions, (d) head injuries, (e) features of raised intracranial pressure, (f) any kind of pituitary abnormality and (g) any previous intra cranial surgeries [12].All the MRI examinations were performed with 0.3T permanent magnet “Airis Vento, Hitachi Medical Systems, Japan”. Standard head coil was used for acquiring the images. The antero-posterior dimension, vertical height and transverse dimension of pituitary gland were measured and volume of pituitary gland was calculated. Superior surface of pituitary gland was also evaluated. Antero-posterior dimension and vertical height were measured in T1 weighted (repetition time/echo time (TR/TE) of 364/15ms) sagittal images with slice thickness of 6 mm. Superior surface of pituitary gland was also evaluated in T1 sagittal images as convex, flat or concave. Transverse dimension was measured in T2 weighted (TR/TE of 4500/100ms) axial images of 6 mm thickness. Though, measurement of pituitary gland size is most accurate in T1 weighted thin sections of 3mm thickness in sagittal and coronal planes [13], we could not use such images as in routine brain imaging. Only 6 mm thickness images were acquired and T1 weighted coronal images were not routinely acquired in the department. The measurements were done in the workstation using electronic callipers calibrated to 0.1 mm. The maximum anterior-posterior diameter was measured as the longitudinal distance defined by a line connecting two corners ‘a’ and ‘b’ of the T1 sagittal section. The maximum height of pituitary was measured as the vertical distance of the pituitary defined by a line connecting two points ‘c’ and ‘d’ of the T1 sagittal section [Figure-1].
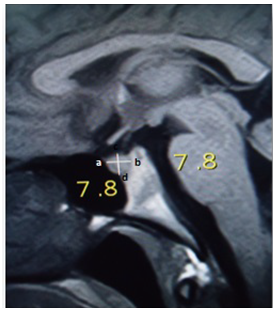 | Figure 1. Measurement of pituitary antero-posterior diameter and height in T1 weighted sagittal MRI (TR/TE of 364/15ms) |
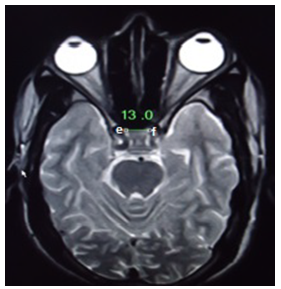 | Figure 2. Measurement of pituitary transverse diameter in T2 weighted sagittal MRI (TR/TE of 4500/100ms) |

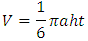 where a, h and t are the anterior-posterior, height and transverse dimensions of pituitary gland respectively [14]. All the collected data were analysed statistically with Origin Program 6.1, SPSS 20 and Microsoft Excel to calculate mean and standard deviations. The standard error (SE) of a set of independent observations x1, x2 ......... xN for their mean value
where a, h and t are the anterior-posterior, height and transverse dimensions of pituitary gland respectively [14]. All the collected data were analysed statistically with Origin Program 6.1, SPSS 20 and Microsoft Excel to calculate mean and standard deviations. The standard error (SE) of a set of independent observations x1, x2 ......... xN for their mean value 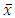 is given by
is given by 
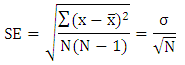 where σ is the standard deviation (SD) of the measured data [15].
where σ is the standard deviation (SD) of the measured data [15].3. Result and Discussion
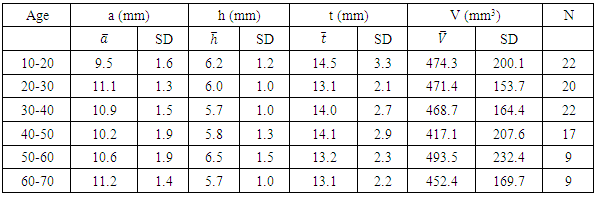
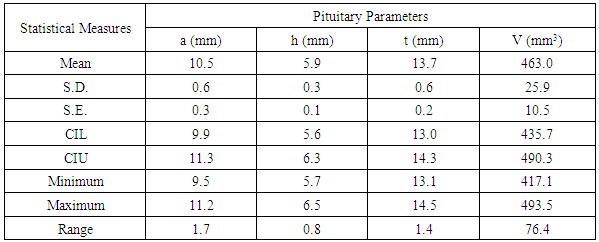
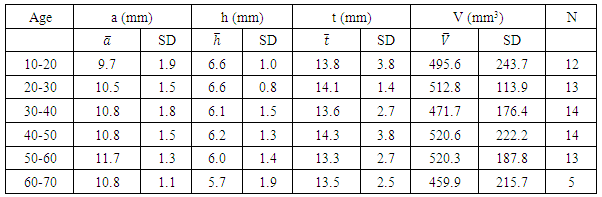
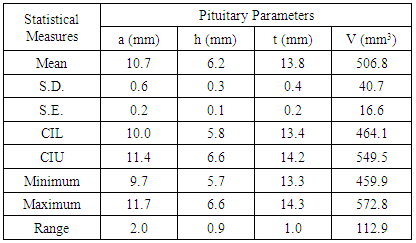
- We measured pituitary dimensions of 170 healthy Nepalese individuals with the subjects of different geographical regions of the country. Out of 170 individuals, 99 were male and 71 were female. Ages of the individuals ranged from 10 year to 70 years.The mean values of the pituitary parameters and the respective statistical measures for the overall age groups for male and female are listed in the Table 2 and Table 4. The mean value of anterior-posterior dimension in the age group (10-20) of male was 9.5 ± 1.6 mm and female was 9.7 ± 1.9 mm. In addition, the pituitary height in the age group (10-20) was 6.2 ± 1.2 mm for male and 6.6 ± 1.0 mm for female.
|
|
|
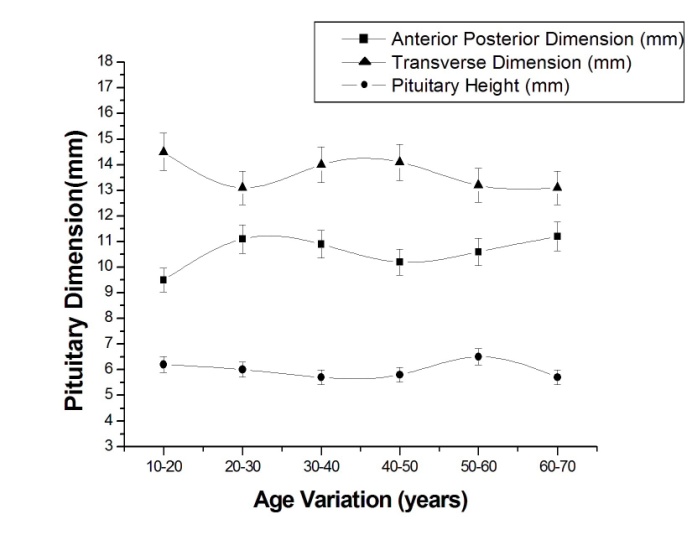 | Figure 3. Plot of average pituitary height, anterior-posterior and transverse dimension of pituitary in male with 5% level of significance |
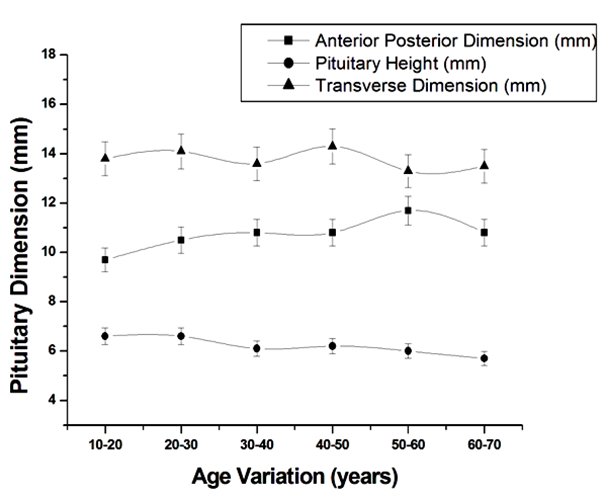 | Figure 4. Plot of average pituitary height, anterior-posterior and transverse dimension of pituitary in female |
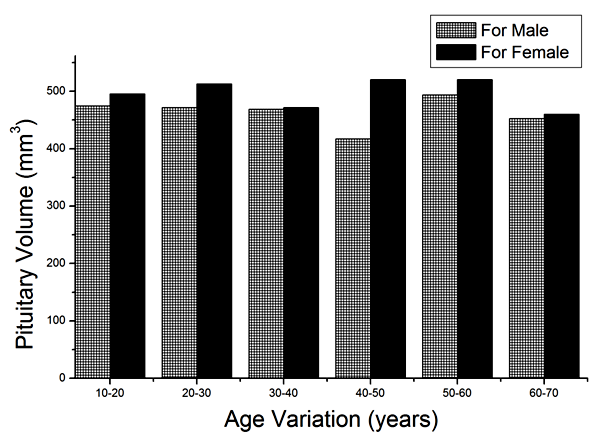 | Figure 5. Variation of pituitary volume with sex |
4. Conclusions
- Pituitary gland dimensions of healthy population were measured by using spin echo Magnetic Resonance Imaging (MRI) sequence with 6 mm thickness. The volume of the pituitary gland was calculated using tri-axial ellipsoid method. This volumetric measurement had moderate accuracy and smaller discrepancy. The anterior-posterior, height, transverse dimension and volume of pituitary were observed to be 10.3 mm, 6.1 mm, 13.6 mm and 466.8 mm3 respectively. In this study, we had found that the size of the pituitary gland of the Nepalese people reflected the normal values as we expected in healthy people.In addition, the results of the present study demonstrated that the pituitary gland height exhibited an increasing trend with age in healthy children. The increase in pituitary gland height was moderate in adolescent females, but was slower in males. The growth tendency was different between the pituitary gland height and volume, and the pituitary gland volume performed significantly better than height with regard to the detection rate. More precise evaluation required an association with neuro-imaging and clinical functional abnormalities of the pituitary gland. Further evaluation in higher field strength magnets with thinner slices in larger sample size is recommended before implementing the results in clinical practice.
ACKNOWLEDGEMENTS
- We are grateful to all the subjects who voluntarily participated in this research and to Ram Bahadur Chand for his valuable suggestions. We are also grateful to Central Department of Physics (CDP-TU) and Radiology and Imaging Department, TUTH, Kathmandu, Nepal for providing us instruments as well as warm research environment.
References
| [1] | Brown and Robert W., Magnetic Resonance Imaging: Physical Principles and Sequence Design, John Wiley & Sons, (2014). |
| [2] | Bushong, Stewart C. and Geoffrey Clarke, Magnetic Resonance Imaging: Physical and Biological Principles, Elsevier Health Sciences, (2013). |
| [3] | Budinger, Thomas F. and Paul C. Lauterbur, Nuclear Magnetic Resonance Technology for Medical Studies, Science 226.4672, 288-298 (1984). |
| [4] | Zrazhevskiy, Pavel, Mark Sena, and Xiaohu Gao, Designing Multifunctional Quantum Dots for Bioimaging, Detection, and Drug Delivery, Chemical Society Reviews 39.11, 4326-4354, (2010). |
| [5] | Gunderman and Richard B., Essential Radiology: Clinical Presentation, Pathophysiology, Imaging, Thieme, (2006). |
| [6] | Lehn and Jean-Marie, Toward Self-organization and Complex Matter, Science 295.5564, (2002): 2400-2403. |
| [7] | Tsunoda, A., O. Okuda, and K. Sato. MR Height of the Pituitary Gland as a Function of Age and Sex: Especially Physiological Hypertrophy in Adolescence and in Climacterium, American Journal of Neuroradiology 18.3, 551-554, (1997). |
| [8] | Ikram and Muhammad Faisal, Pituitary Height on Magnetic Resonance Imaging Observation of Age and Sex Related Changes, Pituitary, (2008). |
| [9] | Janssen, Y. J. H., J. Doornbos and F. Roelfsema, Changes in Muscle Volume, Strength, and Bioenergetics during Recombinant Human Growth Hormone (GH) Therapy in Adults with GH Deficiency 1, The Journal of Clinical Endocrinology and Metabolism 84.1, 279-284, (1999). |
| [10] | Subedi, Kalloo Sharma, and Pragya Sharma, Development of Radiology in Nepal: Gearing Up for Mountainous Challenges, Journal of the American College of Radiology 10.4, 291-295, (2013). |
| [11] | Mahat and Agya, Assessment of Graduate Public Health Education in Nepal and Perceived Needs of Faculty and Students, Human Resource Health 11.1, 16, (2013). |
| [12] | A. D. Elster, Modern Imaging of the Pituitary, Radiology, (1993). |
| [13] | Knosp and Engelbert, Pituitary Adenomas with Invasion of the Cavernous Sinus Space: a Magnetic Resonance Imaging Classification Compared with Surgical Findings, Neurosurgery 33.4, 610-618, (1993). |
| [14] | F. W. J. Olver, D. W. Lozier, R. F. Boisvert and C. W. Clark, Handbook of Mathematical Functions, Cambridge University Press, (2010). |
| [15] | Everitt B.S., The Cambridge Dictionary of Statistics, CUP, (2003). |
| [16] | Mangieri P., Suzuki K., Ferreira M., Domingues L. and Casulari L.A., Evaluation of Pituitary and Thyroid Hormones in Patients with Subarachnoid Hemorrhage due to Ruptured Intracranial Aneurysm, Arq Neuropsiquiatr 61, 14-9, (2003). |
| [17] | Gutmann, Rebecca J. and David H. Gutmann, 27 Predisposition Syndromes: Neurofibromatosis 2 and Tuberous Sclerosis Complex-Principles and Practice of Neuro-Oncology: A Multidisciplinary Approach, (2010). |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML