-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biomedical Engineering
p-ISSN: 2163-1050 e-ISSN: 2163-1077
2015; 5(1): 6-14
doi:10.5923/j.ajbe.20150501.02
Error Grid Analysis of Reference and Predicted Blood Glucose Level Values as Obtained from the Normal and Prediabetic Human Volunteers
Md Koushik Chowdhury, Anuj Srivastava, Neeraj Sharma, Shiru Sharma
School of Biomedical Engineering, Indian Institute of Technology, (Banaras Hindu University), Varanasi, India
Correspondence to: Md Koushik Chowdhury, School of Biomedical Engineering, Indian Institute of Technology, (Banaras Hindu University), Varanasi, India.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
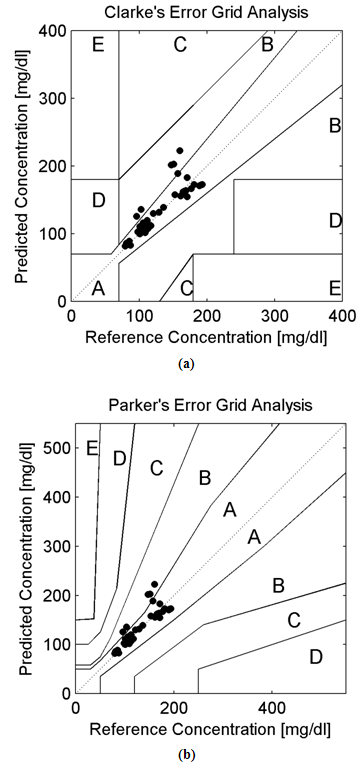
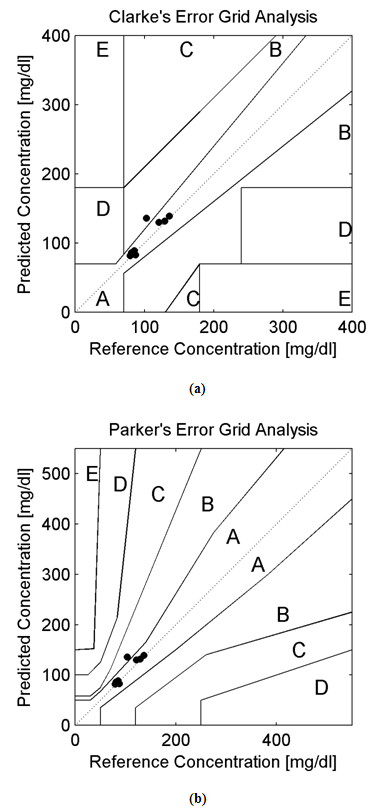
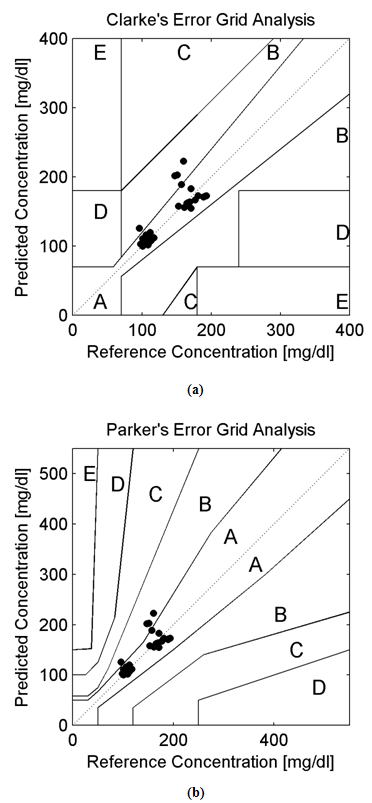
Background:In this research paper, we represent a new noninvasive blood glucose level determining technology based on Amplitude Modulated Ultrasound and Infrared techniques. The successful advent of a noninvasive blood glucose determining technology will be helpful for patients with abnormal episodes of elevated Blood Glucose Levels. Noninvasive device will increase patient’s compliances along with firm control over elevated Blood Glucose Levels (BGL). Moreover, it will reduce diabetes related medical emergency and burden from the shoulders of healthcare professionals. Research Design:A total of 10 adult human volunteers (02 Normal and 08 Prediabetic) had been engaged in this experimental pilot study. Main objective of these experiments are to analyze and compare the blood glucose levels as obtained from the established invasive (Accu-chek Active invasive blood glucose monitoring system from Roche Diagnostics) and indigenously developed noninvasive BGL determining technology (Amplitude Modulated Ultrasound and infrared Unit) respectively. The blood glucose levels after overnight fasting and 02 hour after meal had been observed in Normal and Prediabetic volunteers. Again following the next day, blood glucose level at fasting stage and 02 hour after 75gm/100ml glucose solution consumption had been monitored in those Normal and Prediabetic volunteers. Moreover, the invasive (reference) and noninvasive (predicted) blood glucose levels as obtained had been plotted over Clarke and Parkes Error Grids for evaluating indigenously developed technique performances. Results:The experimental findings reveal that Normal volunteers fasting blood glucose level exists more or less between (80-110) mg/dl. Similarly in separate pilot studies, their Blood Glucose Level varies more or less between (130-140) mg/dl after 02 hour of meal consumption and 75gm/100ml of glucose solution consumption respectively. But in case of Prediabetic volunteers, their fasting blood glucose level exists more or less above 110 mg/dl. Likewise in separate experimental studies, their Blood Glucose Level ranges more or less between (140-199) mg/dl after 02 hour of meal consumption and 02 hour after 75gm/100ml glucose solution consumption respectively. Moreover, the invasive (reference) and noninvasive (predicted) blood glucose levels of all the volunteers (normal and prediabetics) occupies medically significant and acceptable A and B zones in Clarke and Parkes Error Grids Analysis respectively.Conclusions:Experimental observations indicates the potential and prospective capability of our indigenously developed noninvasive Blood Glucose Level determining technique (Amplitude Modulated Ultrasound and Infrared unit) as revealed from the pilot studies over Normal and Prediabetic volunteers.
Keywords: Clarke and Parkes Error Grid Analysis, Invasive, Noninvasive, Blood Glucose, Amplitude Modulated Ultrasound, Infrared Techniques
Cite this paper: Md Koushik Chowdhury, Anuj Srivastava, Neeraj Sharma, Shiru Sharma, Error Grid Analysis of Reference and Predicted Blood Glucose Level Values as Obtained from the Normal and Prediabetic Human Volunteers, American Journal of Biomedical Engineering, Vol. 5 No. 1, 2015, pp. 6-14. doi: 10.5923/j.ajbe.20150501.02.
Article Outline
1. Introduction
- Individual with elevated blood glucose level but not as much high as compared with diabetic patients are generally suffering from the IGT (Impaired Glucose Tolerance) or IFG (Impaired Fasting Glucose) related symptoms [1-3]. IGT refers to the medical condition in which blood glucose level elevates within the range between (140-199) mg/dl even two hours after food consumption [1-3]. Similarly, the IFG refers to the medical situation where blood glucose level elevates above 110mg/dl even after overnight fasting time period [1-3]. The global populations resembling such typical symptoms are termed as prediabetics [1-3]. Consequently; the individuals with Prediabetic symptoms are severely prone to develop Type II Diabetes in near future [1-3]. Moreover, the IGT resembles Type II Diabetes symptoms adjunct with obesity, age progression, incapability of the human bodies to utilize insulin secreted form beta cells of the pancreas [1-3]. Complete change in lifestyle pattern, increased physical activity, controlled diet with low glycemic index food consumption checks the progression of IGT towards Type II Diabetes Mellitus [1-3]. Globally 31.6 crores of people suffer from IGT related symptoms [1]. These numbers globally shares 6.9% of the present total adult population [1]. Moreover, large proportion of these population resides in lower as well as middle income territories. Worldwide 15.3 crores of the adult population suffer from these IGT symptoms within 50 year span of their life [1]. They also possess increased possibility to extend it towards Type II Diabetes Mellitus in later part of their life [1]. The global dominance of IGT resembles diabetes pervasiveness, with enormous proportion in African and European countries as compared to South-East Asian countries [1]. As per future estimations during the year 2035 A.D, 47.1 crores of peoples from the world population will suffer from IGT related symptoms or nearly 8.0% from the then total adult world population [1].The crucial points of care for robust supervision of elevated blood glucose level include its 3 to 4 times regular monitoring on a daily basis [1-5]. Invasive glucometers rigorously perform such measurements each and every time with respective tissue puncturing procedures []. Consequently the diabetic patients generally experiences mental agony for regular tissue puncturing and patient incompliance occurs [3-5]. All this factors drives the urge for a nascent, novel noninvasive blood glucose determining technology with successful clinical applications [4, 5, 20-22]. In recent years, several optical techniques based noninvasive blood glucose level determining approaches had arrived with good potentiality and promising aspects [4-6, 20-22]. Such novel approaches mainly consists of Infrared Spectroscopy [7, 8, 20-22], Raman Spectroscopy [9, 20-22], Scattering Spectroscopy [10], Fluorescent Spectroscopy [11, 20-22], Polarimetry [12, 13, 20-22], Thermal Gradient Spectroscopy [14, 20-22], Photo-Acoustic Technology [15, 20-22], OCT (Optical Coherence Tomography) [16, 20-22], Occlusion Spectroscopy [17, 20-22], Photo-Thermal Technology [18], Ultrasound-Modulated Optical Techniques [19], etc.The blood glucose exhibit weak signals and overlapped by numerous interferences from the surrounding optically active similar molecules [20-22]. To override such signal interferences we had applied Amplitude Modulated Ultrasound and Infrared Techniques altogether here. Moreover, this research paper focuses on the performance evaluation of indigenously developed noninvasive technique for determination of blood glucose levels in Normal and Prediabetic human volunteers. Rest of Research Paper organizations are as follows: Section II describes the principle and working methodology. Section III illustrates the Instrumental block diagram, light wavelength and ultrasound band selection criteria. Section IV contains the experimental results and discussion portions. Section V provides the conclusive part of the research paper followed by acknowledgment and references.
2. Principle and Working Methodology
- Noninvasive technique includes mainly amplitude modulated ultrasonic waves, infrared light beam and its respective IR (Infra Red) detector for determining blood glucose levels in Normal and Prediabetic Human Volunteers. When standing ultrasonic waves propagates through the finger based blood tissue complex medium of human volunteers, it initiates the process of vibration throughout that respective medium. The molecules vibrate depending upon their respective mass, physical, chemical properties [23-25]. Moreover, the effect of radiation forces applied over the molecules had been derived from the gradient of molecular acoustic potential energy [23-32] and expressed as follows:
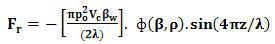 | (1) |
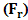 ,
, 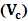 , (z), (P0), and () stands for radiation force characteristics, volume of the respective molecules, space form the node of pressure, ultrasonic wave peak amplitude and wavelength of ultrasound respectively [23-32].When compressibility aspects
, (z), (P0), and () stands for radiation force characteristics, volume of the respective molecules, space form the node of pressure, ultrasonic wave peak amplitude and wavelength of ultrasound respectively [23-32].When compressibility aspects 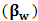 of the suspending (blood tissue complex) segment present in human volunteers finger are considered, the mathematical expression had been represented as given below:
of the suspending (blood tissue complex) segment present in human volunteers finger are considered, the mathematical expression had been represented as given below: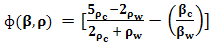 | (2) |
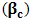 stands for compressibility of the molecules and
stands for compressibility of the molecules and 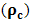 ,
, 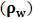 signifies molecular density of the suspending molecules and suspending segment respectively [23-32].When infrared light beam propagates through these ultrasound (amplitude modulated ultrasonic waves) excited (blood tissue complex) optical medium, the glucose molecule vibration specific signatures are captured by the respective IR sensitive detectors. This light interaction phenomenon had been represented by Beer-Lambert Law [23-32] as follows:
signifies molecular density of the suspending molecules and suspending segment respectively [23-32].When infrared light beam propagates through these ultrasound (amplitude modulated ultrasonic waves) excited (blood tissue complex) optical medium, the glucose molecule vibration specific signatures are captured by the respective IR sensitive detectors. This light interaction phenomenon had been represented by Beer-Lambert Law [23-32] as follows: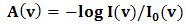 | (3) |
3. MUS-IR Experimental Setup and Its Functional Part Depictions
- Main functional parts of the MUS-IR Experimental setup had been illustrated as follows:Synchronous square wave generator: This functional part produces square wave based pulses to the IR LED (Infra Red Light Emitting Diode) light source.
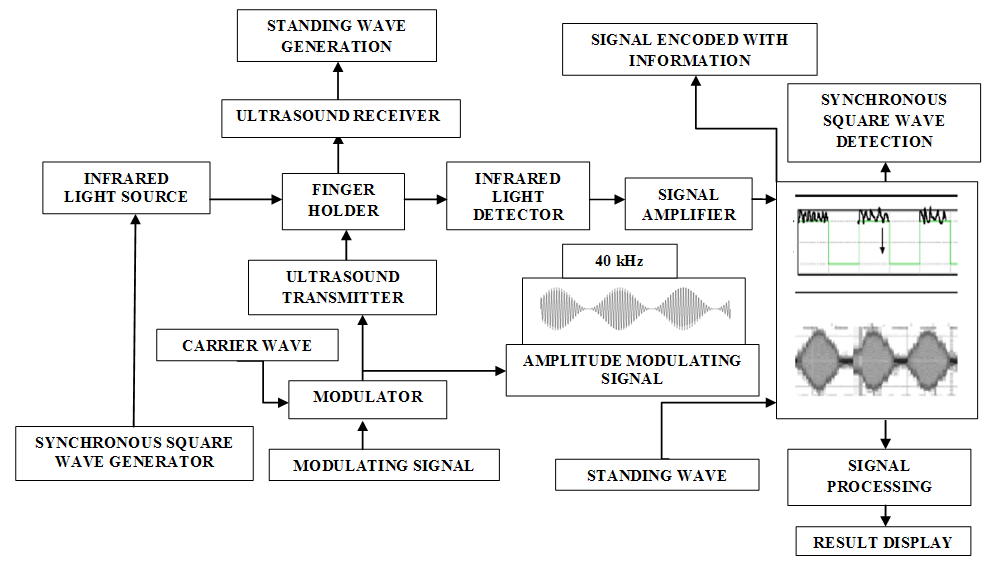 | Figure 1. Block diagram of the MUS-IR (Modulated Ultra Sound-Infrared) Experimental Setup |
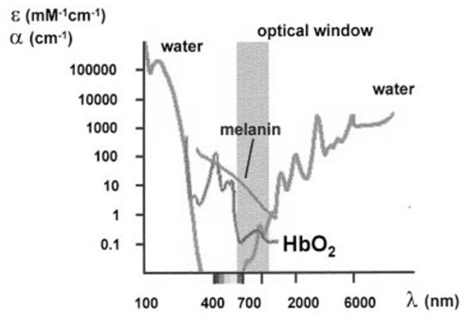 | Figure 2. Absorption characteristics of chief intracellular components within the light spectral domain extending from 100nm to 6000nm [4, 33] |
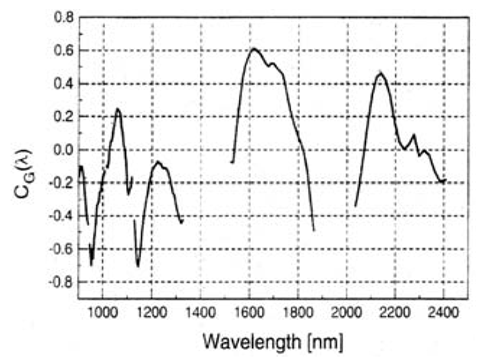 | Figure 3. Absorption coefficient characteristics of Glucose within the light spectral domain extending from 900nm to 2400nm [4, 34-36] |
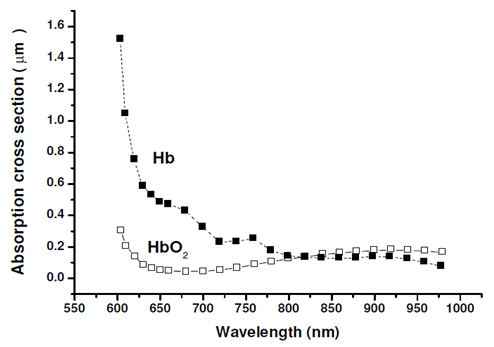 | Figure 4. Absorption characteristics of Oxygenated Hemoglobin (HbO2) and Deoxygenated Hemoglobin (Hb) within the light spectral domain extending from 550nm to 1000nm [4, 34-36] |
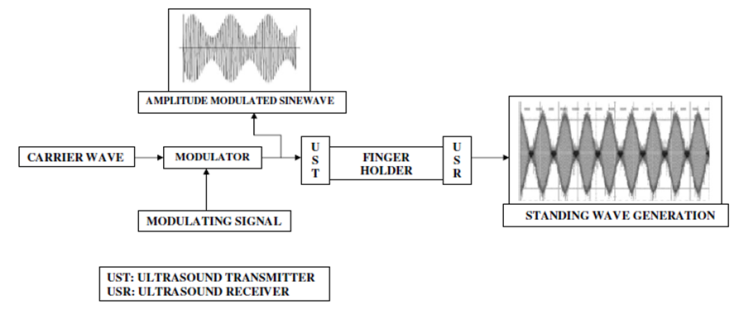 | Figure 5. Shows the generation of amplitude modulated ultrasonic waves in MUS-IR Experimental unit |
4. Experimental Results and Discussions
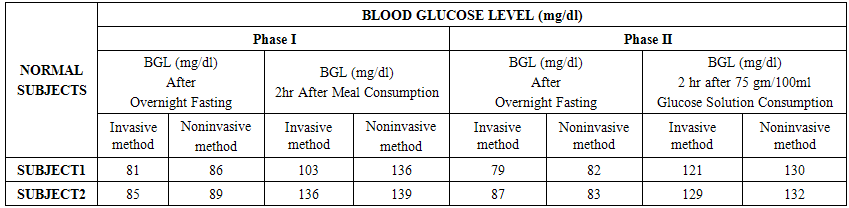
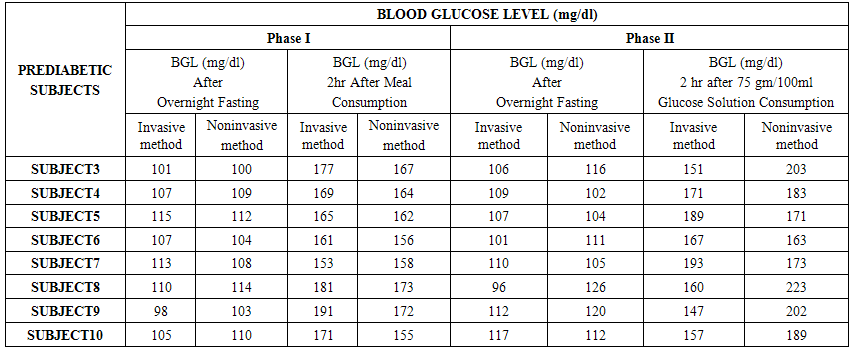
- The experiments were conducted in two phases. The Phase I includes determination of invasive (reference) and noninvasive (predicted) BGL values of both the Normal and Prediabetic volunteers after overnight fasting and after 02 hours of meal consumption.The Phase II includes determination of invasive (reference) and noninvasive (predicted) BGL values of both the Normal and Prediabetic volunteers next day after overnight fasting and 02 hours after 75gm/100ml of glucose solution consumption.For invasive blood glucose level determinations the Accu-chek Active blood glucose monitoring system of Roche Diagnostics GmbH had been utilized here. Similarly, for noninvasive blood glucose level predictions we had utilized our indigenously designed and developed MUS-IR (Modulated Ultra Sound-Infra Red) unit. Table No.1 and 2 shows the comparison of invasive (reference) and noninvasive (predicted) Blood Glucose Levels (BGL) as obtained from the Normal and Prediabetic subjects during Phase I and II experimental pilot studies respectively.
5. Conclusions
- The hybridized potential aspect of utilizing amplitude modulated ultrasound and Infra Red technique for determining noninvasive blood glucose levels had been reported in this research paper. For cross validations of acquired noninvasive BGL values, the invasive glucometer of Roche diagnostics had been applied here. Furthermore, the 940nm LED and 40 kHz-generating central frequency based Ultrasound Transmitter forms the main instrumental base for this noninvasive technology. For validating the performance of noninvasive blood glucose detecting technology the Error Grid Analytical approaches had also been applied here. Error Grid Analysis shows that all the invasive (reference) and noninvasive (predicted) BGL values occupy within the medically significant A and B zones. At present all new noninvasive BGL determining techniques requires invasive glucose sensors for calibration purposes. Hope our nascent noninvasive BGL determining technology will be successful in near future with all the aspects.
ACKNOWLEDGMENTS
- All authors of the manuscript wish to appreciate the Coordinator and other Faculty members of School of Biomedical Engineering, IIT-(BHU), Varanasi for their support during the experimental works and manuscript writing.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML