-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biomedical Engineering
p-ISSN: 2163-1050 e-ISSN: 2163-1077
2013; 3(6): 119-131
doi:10.5923/j.ajbe.20130306.01
Electrochemical Synthesis, Characterisation, and Preliminary Biological Evaluation of an Anodic Aluminium Oxide Membrane with a pore size of 100 nanometres for a Potential Cell Culture Substrate
Gérrard Eddy Jai Poinern1, Xuan Thi Le1, Marilyn Hager2, Tom Becker3, Derek Fawcett1
1Murdoch Applied Nanotechnology Research Group, Department of Physics, Energy Studies and Nanotechnology, School of Engineering and Energy, Murdoch University, Murdoch, Western Australia 6150, Australia
2Animal Health Laboratories, Animal Virology, Department of Agriculture and Food, 3 Baron Hay Court, Kensington, Western Australia 6150, Australia
3Department of Chemistry, Curtin University of Technology, Bentley, Western Australia 6102, Australia
Correspondence to: Gérrard Eddy Jai Poinern, Murdoch Applied Nanotechnology Research Group, Department of Physics, Energy Studies and Nanotechnology, School of Engineering and Energy, Murdoch University, Murdoch, Western Australia 6150, Australia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
In this study we investigate the electrochemical synthesis and characterisation of a nanometre scale porous anodic aluminium oxide (AAO) membranes with a mean pore diameter of 100 nm. The membranes exhibit interesting properties such as controllable pore diameters, periodicity and density distribution. These properties can be preselected by adjusting the controlling parameters of a temperature controlled two-step anodization process. The surface features of the nanometre scale membrane such as pore density, pore diameter and inter-pore distance were quantified using field emission scanning electron microscopy (FESEM) and atomic force microscopy (AFM). A preliminary biological evaluation of the membranes was carried out to determine cell adhesion and morphology using the Cercopithecus aethiops[African green monkey – (Vero)] kidney epithelial cell line. Optical microscopy, FESEM and AFM investigations revealed the presence of focal adhesion sites over the surface of the porous membranes. The positive outcomes of the study, indicates that AAO membranes can be used as a viable tissue scaffold for potential tissue engineering applications in the future.
Keywords: Regenerative Tissues, Nano-porous Anodic Aluminium Oxide, Cell Substrate, Tissue Scaffold
Cite this paper: Gérrard Eddy Jai Poinern, Xuan Thi Le, Marilyn Hager, Tom Becker, Derek Fawcett, Electrochemical Synthesis, Characterisation, and Preliminary Biological Evaluation of an Anodic Aluminium Oxide Membrane with a pore size of 100 nanometres for a Potential Cell Culture Substrate, American Journal of Biomedical Engineering, Vol. 3 No. 6, 2013, pp. 119-131. doi: 10.5923/j.ajbe.20130306.01.
Article Outline
1. Introduction
- The primary function of tissue engineering is to create an environment that can promote productive biological processes and cellular signals, which can create or repair damaged tissues by rational design[1]. However, this environment is influenced by four main factors: 1) the types of cells; 2) the structure and physiochemical properties of the scaffold; 3) the biological processes, and 4) cellular and scaffold signals[2]. Individual cells are generally in the micron-size range, but their component structures and surrounding environment are in the nanometre to micrometre scale range. Cells actively interact with the surrounding topography and the molecular building blocks of life such as proteins, which are crucial for controlling cell functions such as proliferation, migration, and the production of the extracellular matrix, (ECM)[3]. Furthermore, the chemical and physical structure of the topography directly influences the behaviour of the cell in contact with the surface. For example, the adhesive attachment of a cell to a substrate surface is via protein adsorption, which is highly dependent on surface properties such as surface charge, surface chemistry[4], wettability[5], surface density of cell-binding ligands[6] and nanometre scale topography[7]. Therefore, to achieve a successful clinical outcome it is crucial that a tissue scaffold be completely biocompatible, i.e. no cytotoxicity, immunological reactions and inflammatory responses from the surrounding tissues[8-10]. The surface chemistry and wettability of the tissue scaffold are also important factors, since they must be chemically compatible with both the ECM and cellular tissue. By mimicking the ECM, the tissue scaffold promotes an environment, which actively supports the creation of new ECM, cell adhesion, intercellular interaction, proliferation and migration[11]. While the wettability of materials used to construct a tissue scaffold must also be carefully considered, since hydrophobic materials will not promote successful cell adhesion and attachment. It is also extremely important at the cell level, that the tissue scaffold also contains a network of pores and interconnecting channels to facilitate the diffusion of nutrients, oxygen, metabolites and waste products. Both pore size and pore surface area to volume ratio have been found to be important parameters in encouraging the in-growth of cells into the matrix of the tissue scaffold forming cellular associations[12].The earliest clinical use of an aluminium oxide (Alumina) ceramic as a biomaterial has its origins in the 1970’s, when Hamadouche et al. successfully used it in a total hip arthroplasty procedure[13]. However, the study of porous aluminium oxide membranes derived from the anodization of aluminium metal made in a variety of polyprotic acids or electrolytes, temperatures and Anodization voltages goes back more than sixty years[14]. During anodization in a polyprotic acid (e.g., oxalic, phosphoric or sulphuric acid), the electrochemical process produces a nanometre scale porous oxide layer over the metal surface. A typical schematic of an experimental setup is presented in Figure 1. The first systematic study of this porous oxide layer was carried out by Keller and co-workers in 1953. This study revealed that the structure of the pore formed during anodization was the result of the solvent action of different electrolytes on the oxide coating and the dependence of anodic voltage on pore size[15].The anodization process begins with Al3+ ions migrating from the Al metal across the metal/oxide interface into the forming surface oxide layer[16]. Meanwhile at the oxide/electrolyte interface, O2- ions formed in the electrolyte migrate into the oxide layer. The forming oxide layer (or barrier layer) consumes 70% of the available Al3+ ions, while the remaining Al3+ ions are dissolved into the electrolyte[17]. This balanced flow of Al3+ ions is a prerequisite for porous oxide growth and also takes into account the Al-O bonds in the oxide lattice breaking to release Al3+ ions[18]. The steady state growth in the pore structure results from the balance between the field-enhanced oxide dissolution at the oxide/electrolyte interface and the oxide growth at the metal/oxide interface. The electric field is high enough in the hemispherical shaped pore bases to propel the Al3+ ions through the barrier layer (the electric field strength in the pore walls is too small to make any significant contribution to the flow of ions), while the migration of O2- and OH- ions into the pore base oxide maintains the layers constant thickness[14, 19, 20]. The oxidation takes place over the entire pore base, with pronounced perpendicular oxide growth occurring at the pore boundaries, see Figure 2. In addition, the presence of neighbouring pore growth prevents oxide growth in any other direction and also explains the dependence of pore diameter to the strength of the electric field produced by the anodizing voltage. The net result of the oxide growth is a porous structure that contains numerous columnar structures, with a high aspect ratio and each containing a central circular channel, which extends from the base of the pore to the surface of the oxide layer.
 | Figure 1. Schematic of a typical experimental setup for anodization of aluminium |
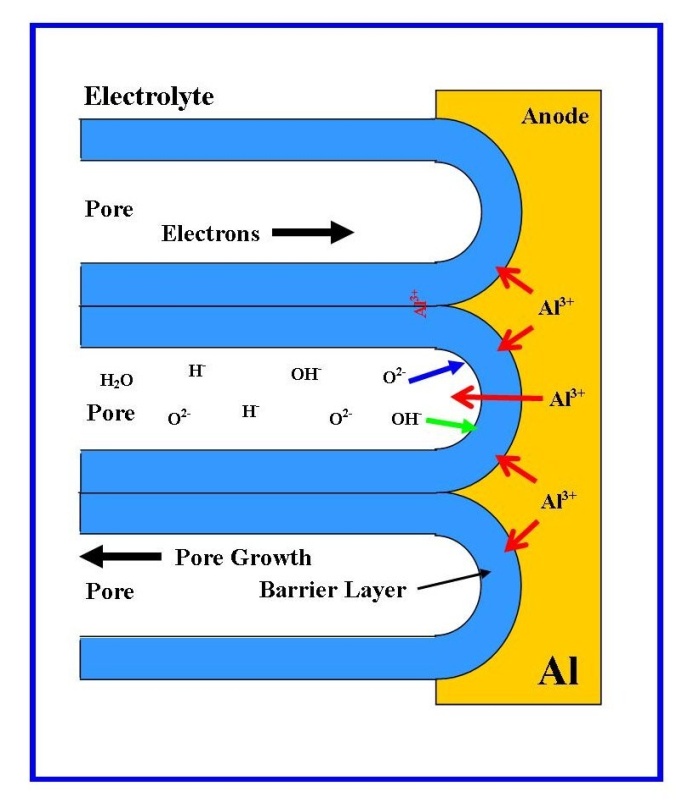 | Figure 2. Schematic of pore formation mechanism in an acidic electrolyte |
2. Materials and Methods
2.1. Materials
- All chemicals were purchased from Sigma-Aldrich (Castle Hill: NSW, Australia) and used without further purification. Milli-Q® water (18.3 MΩ cm-1) was used in all aqueous solution preparations and was produced from a Barnstead Ultrapure Water System D11931 (Thermo Scientific, Dubuque, IA). The 99.99% pure aluminium foil (0.25 mm thick) was supplied by Alfa Aesar (USA) and the Anodisc membrane (diameter 25 mm, pore size 0.1 µm) used for comparative purposes was supplied by Whatman® Anopore (UK).
2.2. Fabrication of in-house Nano-porous AAO Membranes
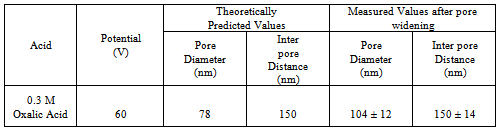
- Fabrication of the in-house membranes begins with a 100 mm square Aluminium (Al) high purity (99.99%) sheet, 0.25 mm thick supplied by Alfa Aesar, USA being cut into 50 mm x 20 mm strips. The strips are then placed into a tube furnace and annealed in a nitrogen atmosphere at 500 °C for 5 hours to initiate re-crystallisation and release mechanical stresses in the strips. After annealing, the strips were degreased in acetone and then etched in a 3.0 M sodium hydroxide solution for 5 minutes before being thoroughly washed in Milli-Q® water. The strips were then dried before a thin layer of polymer was applied to one side of the strip. Once the polymer had set, the strip was ready for the first step of the two-step anodization procedure. During the first step, each strip was anodized using a voltage of 60 V in an electrolyte solution consisting of 0.3 M oxalic acid for 5 hours. At the end of the first anodization step: the resulting thin oxide layer formed on the non polymer coated side of the strip was removed from the substrate by immersion in a stirred acidic solution composed of phosphoric and chromic acid (70 mL/L and 20 g/L, respectively) at 60 °C for 1 hour. This is an important stage of the process, since it selectively removes the first oxide layer and exposes a highly periodic and indented landscape covering the surface of the Al substrate. These indentations will form the initiation sites for pores formed in the second anodization step. The second anodization step is performed under the same conditions as the first step, except that the period of anodization is only for 3 hours. During the second step, a regular, honeycomb array of nanometre-sized pores are formed across the surface of the oxide layer. After the second anodization, the pores were then widened by chemical etching in a 5% solution of phosphoric acid at 35°C for 15 minutes. Then a thin layer of Acrifix® 192 polymer was applied to the anodized side of the Al strip. This serves as a physical support for the membrane during the removal of the Al substrate using an acidic solution mixture composed of 0.1 M copper chloride and 7% hydrochloric acid. Following the removal of the Al substrate, the barrier layer was removed from the membrane by etching in a 0.3 M solution of phosphoric acid. The final etching stage, results in the complete dissolution of the barrier layer and the acrylic support leaving an off white coloured oxide membrane. The final stage in producing the membrane is sterilization, which involved immersing the membranes in a 30% solution of hydrogen peroxide at 60ºC for 15 minutes. This was followed by quickly dipping the membrane into a solution of Milli-Q® water for 10 seconds to remove any hydrogen peroxide from the membrane surface and then placed under ultraviolet light for 2 h. The membranes are then placed in airtight containers, wrapped in Al foil and stored for future use. Figure 1 presents a field emission scanning electron microscopy micrograph of a typical AAO membrane fabricated in-house using the two-step anodization procedure.
2.3. Characterization of Substrates and Cells
- The in-house fabricated nano-porous AAO membranes and Whatmann® Anodisc membranes were examined using a field emission scanning electron microscopy (FESEM). The micrographs were taken using the Zeiss Neon 40EsB FIBSEM (Carl Zeiss, Oberkochen, Germany) located at the Centre for Materials Research (CMR) at Curtin University of Technology. The field emission electron gun provided both high brightness and high resolution (0.8 nm). Samples were mounted on individual substrate holders using carbon adhesive tape before being sputter coated with a 2 nm layer of platinum to prevent charge build up using a Cressington 208HR High Resolution Sputter coater. Micrographs were taken at various magnifications ranging from 2 to 5 kV using the SE2 and In-Lens detectors. The AFM images were taken using the Digital Instruments Dimension 3100 AFM set for Tapping Mode. The AFM tips used were supplied by Nano-world innovative technologies and the silicon SPM sensor (non contact mode) was supplied by NCH-W Point-Probe[Thickness 4 µm, Width 30 µm and Length 125 µm]. The AFM’s force constant was 42 N/m and its resonance frequency was 320 kHz. Optical microscopy (OM) was used throughout the cell studies to examine cell-membrane interactions such as attachment, migration and proliferation. An Olympus BX51 compound microscope (Olympus Optical Co. Ltd., Tokyo, Japan) was used for all optical studies and photographs were taken using the DP 70 camera attachment.Before optical microscopy observations, cells adhering to each respective membrane were fixed using a 1:1 solution of acetone and methanol. The cells were then stained using an aqueous solution containing 1% Fuchsin acid. After 1 h the excess stain was rinsed off the membranes using Milli-Q® water. After the membranes had dried, they were then mounted onto microscope slides ready for optical microscopy investigation at various magnifications (4x, 10x, 20x and 40x). Before FESEM observation could be carried out, the cell/membrane samples needed to be washed in increasing concentrations of ethanol prior to being sputter coated. Initially the cell/membranes were washed in a 30% solution of ethanol several times before being allowed to soak for 15 minutes in the ethanol solution. At the end of this period, sequential drying of the samples using progressively increasing concentrations of ethanol washes (2 washes of 50%, 70%, 80%, 90%, and 95%) was carried out until finally being washed in a solution of 100% ethanol for 30 minutes. Following the ethanol washing procedure, the samples were then treated with a 50:50 solution of ethanol:amylacetate for 30 minutes. This was then followed by 2 immersions in amyl acetate over a period of 1 h before being placed into a critical point dryer. Finally, the dried cell/membrane samples were mounted on FESEM stubs before being sputter-coated with a 2 nm layer of platinum metal for imaging purposes. The samples were then ready for FESEM investigations.
2.4. Cell Culturing and Growth on Membranes
2.4.1. Cell Seeding and Culture
- The cell line used in this in vitro study was the Cercopithecus aethiops (African green monkey) Kidney (Vero) epithelial, and were supplied by the Animal Health Laboratories, Animal Virology, Department of Agriculture and Food, 3 Baron Hay Court, Kensington, Western Australia 6151, Australia. The cell culturing protocol was carried out in accordance with the Animal Health Laboratories procedure VIW-17 using a Cell Growth Medium 199 (Sigma-Aldrich) and 10 % fetal calf serum (FCS)[35]. A standard cell culturing procedure used in previous AAO cell line studies by the authors is presented and discussed in reference[34].
2.4.2. Cell Adhesion and Morphology Studies
- Both types of nano-porous membranes (in-house and Whatmann® Anodisc) were used without surface treatments, other than sterilization along with the glass controls prior to cell seeding. The cell adhesion studies started with making up a series of sample sets. Each sample set consisted of adding 2 samples of each nano-membrane type and 2 glass controls to a 6 well culture plate (No 657-160) supplied by CellStar® Greiner Bio-One, Germany. Then a 3 mL solution of Vero cells (approximately 3x105 cells/mL) suspended in culture medium and 10% FCS were transferred to each well of the culture plate using a pipette. The well plates were then gently oscillated to disperse the cells and then incubated at 37˚C under a 5 % CO2 atmosphere. After 24 h the well culture plate was removed from the incubator and the individual membranes and glass controls were washed and then fixed using a 1:1 solution of methanol: acetone. After 10 minutes, the membranes and glass controls were removed from the fixing solution and allowed to air dry at room temperature. Then the cells on both membranes and glass controls were stained using an aqueous solution containing 1% Fuchsin acid. After soaking the samples for 1 h, the excess stain was rinsed off Milli-Q® water and allowed to air dry at room temperature. After the samples had dried, each sample was mounted onto microscope slide before a cover slip was added. The samples were then ready for optical microscopic investigation. The cell adhesion procedure was carried out in triplicate to ensure consistency in the study.
2.5. Statistical Analysis
- The frequency and size of the particular surface features such as pore diameter, pore density and inter-pore distance was determined by counting and physically measuring the size of the features found within 10 randomly selected 1 µm square grids. From this analysis the mean ± standard deviation of each surface feature was calculated.
3. Results and Discussions
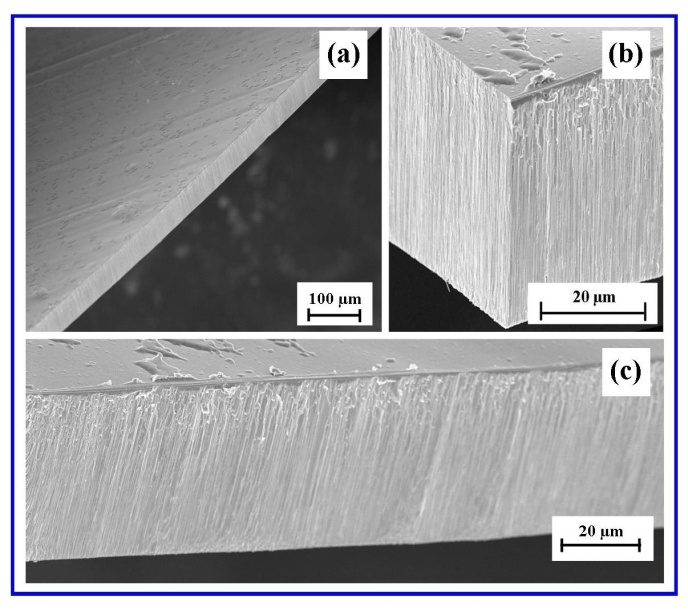
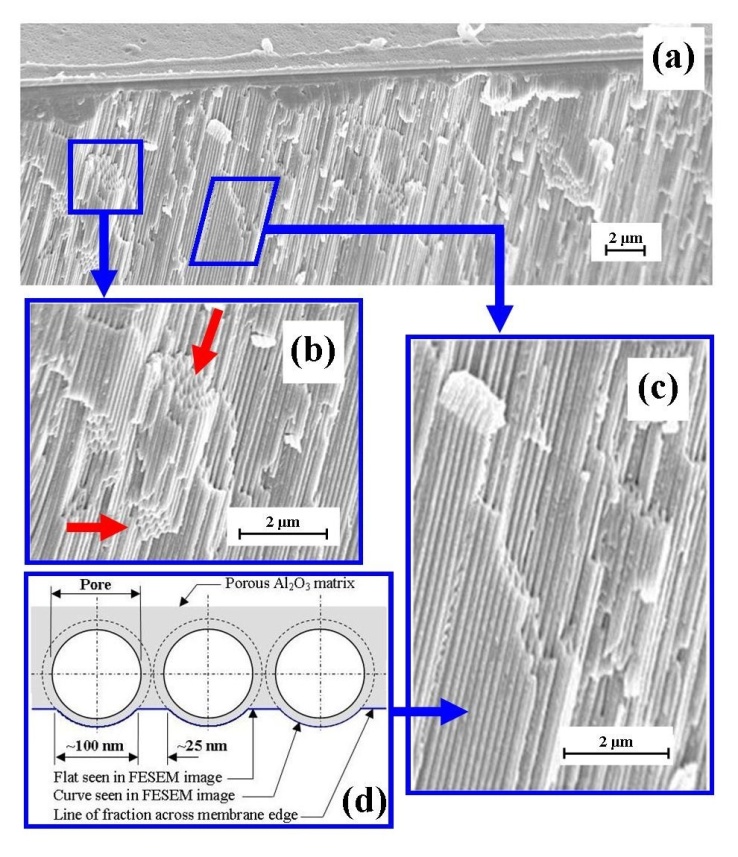
- FESEM characterisation of the surface terrain of the in-house AAO membranes produced by the two-step anodization procedure revealed an architecture that was highly ordered, with close packed hexagonal arrays of uniformly sized pores. The ordered pore domains are tessellated across the undulating landscape of the membrane surface as shown in Figure 3(a).Examination of the FESEM micrographs revealed that between the pore domains were occasional non-ordered pores resulting from point defects, dislocations and grain boundaries in the Al substrate and have also been reported by other researchers[36, 37]. In addition, the FESEM micrographs also revealed the presence of a smaller number of minor pores merging with larger, nearby neighbouring pores to form elliptical shaped pores. The bottom of the membrane, which was etched to remove the barrier layer and open up the pores, had a similar surface topography to the upper surface. The second step of the two-step anodization process, which lasted 3 hours’ produced a highly porous and permeable membrane composed of highly linear channels that traverse the membrane, see Figure 3(b).
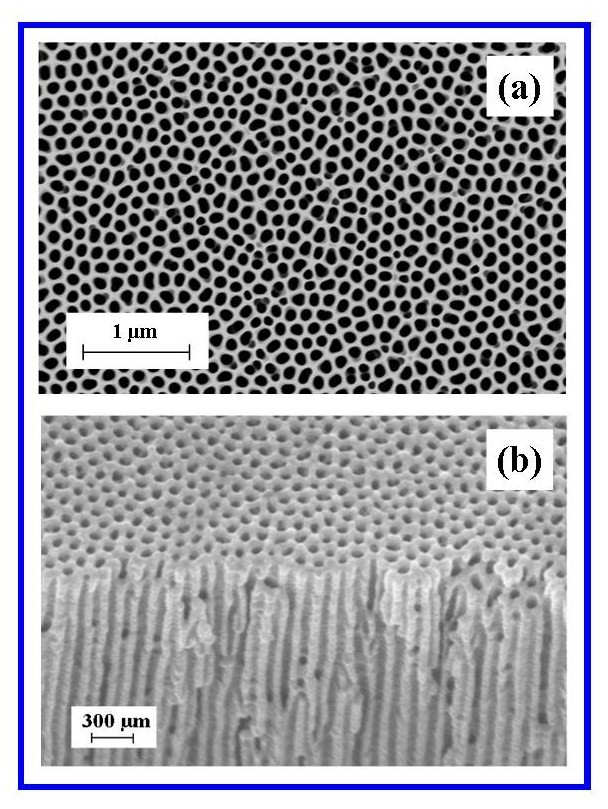 | Figure 3. (a) A typical FESEM image of an AAO top view, surface landscape showing ordered pore domains; (b) A typical image of nano-channels traversing the AAO membrane |
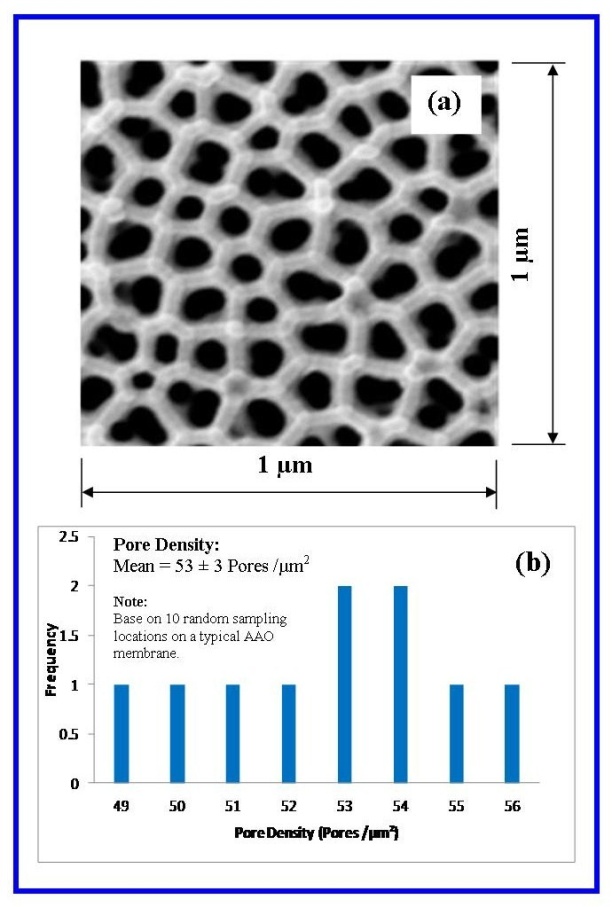 | Figure 5. (a) A representative counting square used for determining pore density; (b) Statistical analysis of pore numbers counted in 10 random locations on a typical AAO membrane[34] |
|
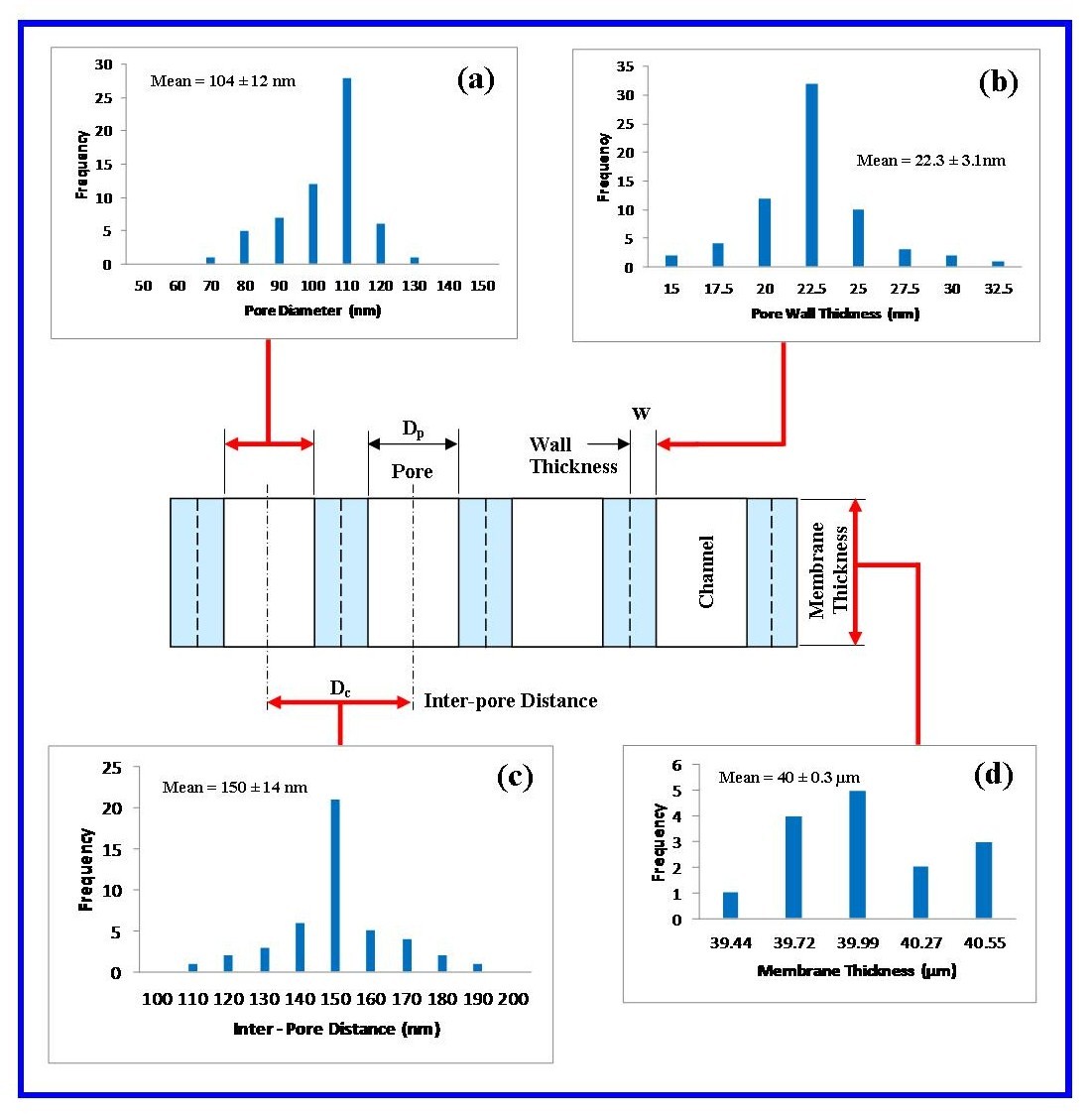 | Figure 6. Results of the investigation into nano-scale features found in AAO membranes: (a) Pore diameter analysis; (b) Pore wall thickness; (c) Inter-pore distance and (d) Membrane thickness |
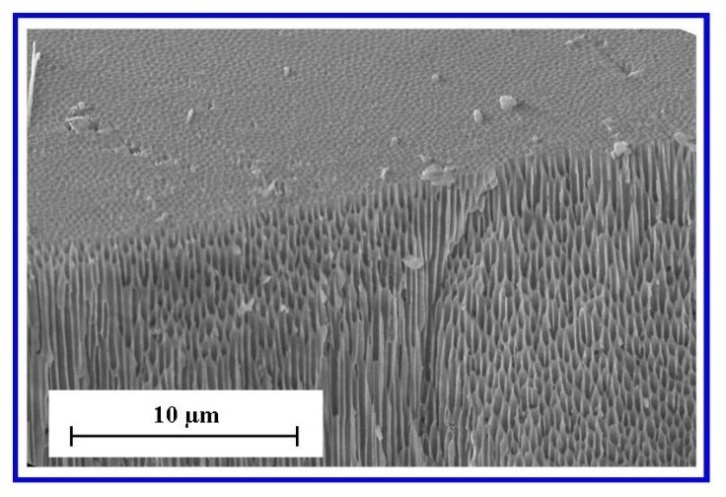 | Figure 8. Cross-sectional view of angularly cleaved Anodisc membrane showing nano-channels and channel openings |
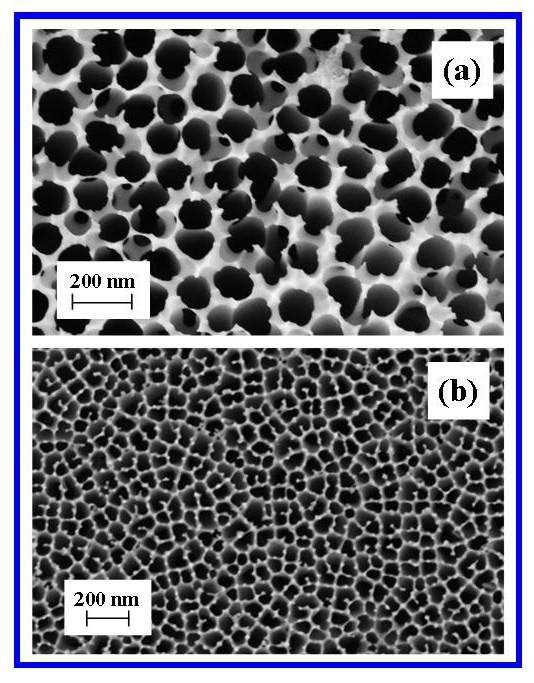 | Figure 9. FESEM images of commercially available Whatman® Anopore (Anodisc) membrane (0.1 µm pore diameter), (a) Top surface and (b) Bottom surface |
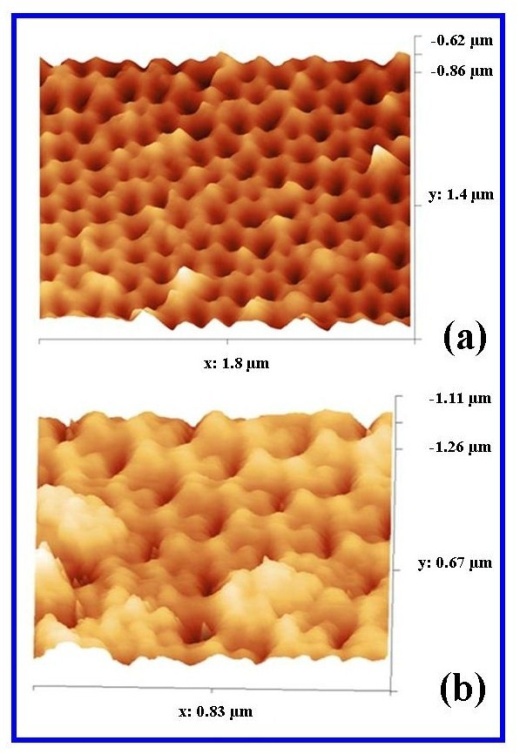 | Figure 10. AFM surface analysis of (a) in-house AAO membrane and (b) Whatman® Anopore (Anodisc) |
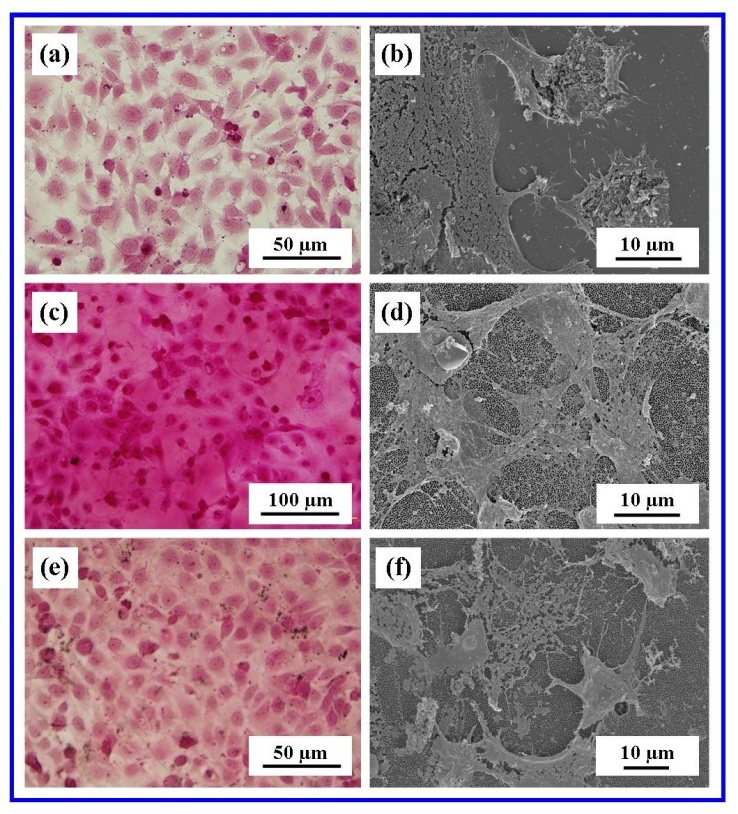
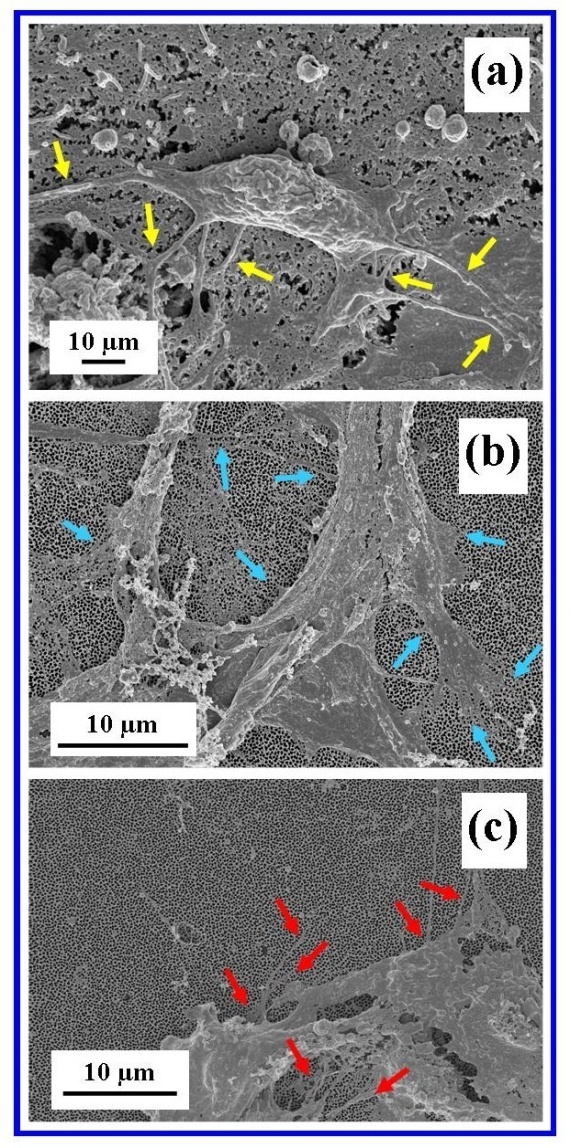 | Figure 12. Cell filopodia attaching cell to substrate surfaces (a) Glass control, (b) Whatman® Anodisc and (c) in-house AAO membrane. (Arrows indicate major filopodia) |
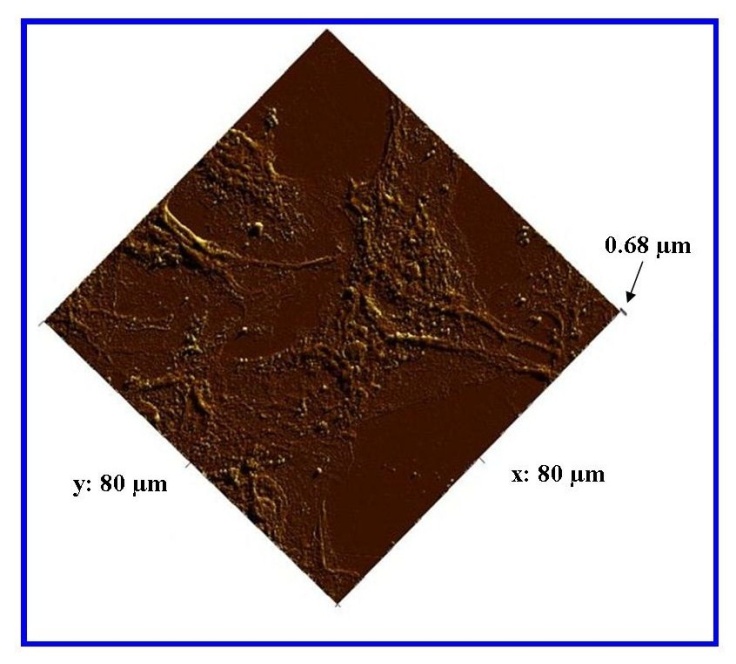 | Figure 13. AFM analysis of the in-house AAO membrane showing Vero cells and major filopodia anchoring the cells to the membrane surface |
4. Conclusions
- The present study has successfully demonstrated that an in-house nano-porous AAO membrane and a commercial AAO membrane have the biological potential for attracting, interacting and promoting cell attachment. The two-step anodization process used to synthesize the in-house AAO membranes was able to produce highly reproducible nanometre scale features by adjusting the controlling experimental parameters. The membranes were used without any further surface modification and showed no cytotoxic effects during the attachment studies. Both AFM analysis and FESEM microscopy provided images that confirmed cell attachment to both nano-porous membrane types. Images revealed the presence of thin filopodia emanating from the cells, spreading across the membrane surfaces and attaching to the nanometre scale topographical surface features of the membranes. Further studies are needed to confirm the long-term survivability of Vero cells on both membranes types. Also the influence of surface texturing on cell attachment and proliferation needs to be quantified before establishing nano-porous AAO membranes as a viable tissue scaffold.
ACKNOWLEDGEMENTS
- The authors would like to thank Dr Mark O’Dea and Mr Cameron Loomes of the Animal Health Laboratories, Animal Virology, Department of Agriculture and Food, Western Australia for their assistance with the cell culture study. The authors would also like to thank Mr Gordon Thompson from the School of Veterinary and Life Sciences, Murdoch University for his assistance with the optical microscopy images.
Disclosure
- The authors report no conflict of interest in this work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML