-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biomedical Engineering
p-ISSN: 2163-1050 e-ISSN: 2163-1077
2013; 3(1): 9-13
doi:10.5923/j.ajbe.20130301.02
Biodegradable Poly-pentadecalactone (PDL) Synthesis via Synergistic Lipase and Microwave Catalysis
Anil Mahapatro1, Taína D. Matos Negrón2
1Bioengineering Program & Department of Industrial and Manufacturing Engineering, Wichita State University, Wichita, KS-67260, USA
2Center for Materials Research,(CMR), Norfolk State University, Norfolk, VA-23508, USA
Correspondence to: Anil Mahapatro, Bioengineering Program & Department of Industrial and Manufacturing Engineering, Wichita State University, Wichita, KS-67260, USA.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
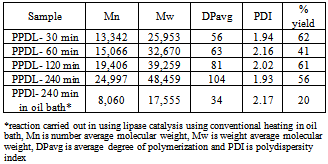
A large number of currently used synthetic biodegradable polymers in biomedical engineering applications are polyesters based materials and thus research on the synthesis, properties, manufacturing and processing of aliphatic polyesters continues to be of great importance. Poly-ω-pentadecalactone (PPDL) a lactone based ring opening polymer has good mechanical properties and the presence of hydrolysable ester linkages along the polymer chain making it desirable as a biodegradable material for diversified biomedical engineering applications. In this paper we report the formation of PPDL using the synergistic effects of lipase and microwave (MW) technology. The effect of reaction time on the PPDL polymer chain growth has been investigated. PPDL have been formed using lipase and MW irradiation at varying reaction time intervals (30-240 mins). Synergistic MW and lipase catalyzed polymerization of PPDL gave a number average molecular weight (Mn) of 24,997 g/mol and a polydispersity index (PDI) of 1.93 in 240 mins as compared to Mn of 8,060 g/mol and PDI of 2.17 using lipase and traditional heating. Thermal characterization of PPDL formed using MW and lipase catalysis showed that MW did not have a detrimental effect on the thermal properties of the polymer obtained.
Keywords: Microwave, Lipase, Biodegradable Polymer, Poly-pentadecalactone
Cite this paper: Anil Mahapatro, Taína D. Matos Negrón, Biodegradable Poly-pentadecalactone (PDL) Synthesis via Synergistic Lipase and Microwave Catalysis, American Journal of Biomedical Engineering, Vol. 3 No. 1, 2013, pp. 9-13. doi: 10.5923/j.ajbe.20130301.02.
Article Outline
1. Introduction
- Biodegradable polymers find various biomedical engineering applications that could be categorized as temporary support device (sutures, bone fixation devices), temporary barrier (artificial skin), drug delivery device (nano, micro particles), tissue engineering scaffold and multifunctional devices (biodegradable drug eluting stents)[1]. Although numerous biodegradable polymers find use in biomedical engineering applications a large number of currently used synthetic biodegradable polymers are polyesters based materials[2]. Thus research on the synthesis, properties, manufacturing and processing of aliphatic polyesters continues to be of great importance. The production of both aliphatic and semi-aliphatic polyesters follows two synthetic strategies (a) either ring-opening polymerization (ROP) of cyclic esters (lactones or cyclic oligoesters) or (b) polycondensation of diacids and diols, hydroxyacids and/or their di-methyl esters[2]. Both of the above methodologies require use of a catalyst or an initiator. In case of biodegradable polymers for pharmaceutical or medical applications, the potential toxicity of the catalyst plays a critical role in the choice of the catalyst used as a residual trace of the catalyst could lead to cytotoxicity[3].This has led to academic and industrial interest to look for a less toxic group of catalysts, initiators and processes for the synthesis and manufacturing of biodegradable polyesters.Microwave and enzymatic assisted polymer chemistry both individually have been gaining interest in green manufacturing as an alternative energy source and catalyst respectively[4, 5]. Microwave assisted synthesis provides several advantages and benefits such as localized heating, reduced chemical reactions times, increased product yield and increased selectivity[6]. Microwave assisted synthesis has expanded to a variety of biological molecules such as peptides, oligomers and carbohydrates[7]. Comprehensive reviews have covered the use of microwave in organic chemistry[4] and in recent years in polymer chemistry[7]. Enzyme catalyzed polymerizations have been explored intensively since the 1990’s. Enzymatic catalysts provide an alternate green catalytic substitute for the toxic metal complexes usually employed in polyester polymer synthesis[5, 8, 9]. The main advantages of enzyme catalyst are 1) high catalytic activity, 2) high efficiency under mild conditions (temperature, solvent), 3) biocompatibility, 4) reusability, and 5) stereo and regioselectivity[5,10]. Enzymatic catalyzed polymerizations serve as an environmental friendly synthetic process and provide an example of green polymeric chemistry[10].Relatively fewer reports exist on the applications of microwave assisted enzymatic reactions, with emphasis on organic small molecule transformations[11-16].Enhancements in the initial rate of reaction,[17, 18] product yields[15, 19] and enantioselectivity[13] have been reported when using microwave heating as compared to conventional heating. However understanding of this area is poor and often controversial. Leadbeater[12] and co-workers investigated the effect of microwave irradiation on lipase catalyzedtransesterification of methyl acetoacetate in toluene. They reported minor differences between conventional and microwave heating. Rejasse et al.[20] studied the effect of microwave heating on the stability of Candida antarctica Lipase B (CALB) and the kinetics of butyl butyrate synthesis. They reported an increase in enzymatic stability in organic medium under microwave field suggesting a possible explanation for an increase in conversion rates under microwave heating[20]. The field of microwave assisted enzymaticpolymerizations has been recently receiving attention for exploiting the synergistic benefits of lipase and microwave processed in the field of polymer chemistry[21, 22]. Kerep and Ritter investigated the influence of MW irradiation on lipase catalyzed ring opening polymerization of ε-caprolactone[22]. They reported that MW assisted enzymatic polymerizations had accelerating effects depending on the kind of boiling solvent used[22]. Recently our laboratory investigated the effects of microwave process parameters (power, intensity, MW irradiation time and temperature) on lipase catalyzed polymerization of caprolactone[23]. In this paper we report the formation of poly-ω-pentadecalactone (PPDL) a lactone based ring opening polymer using the synergistic effects of lipase and microwave technology. Good mechanical properties of PPDL and the presence of hydrolysable ester linkages along the polymer chain of PPDL have led to significant interest and consideration of PPDL as a biodegradable material for diversified biomedical engineering applications.
2. Materials and Methods
2.1. Reagents
- The monomer ω-pentadecalactone (PDL)[98%,molecular weight 240.38], Novozyme-435 (Candida antartica Lipase B), chloroform (CHCl3), methanol (CH3OH) were purchased from Sigma-Aldrich and used without further purification.
2.2. Microwave Assisted enzyme-catalyzed ring-opening Polymerization of PDL
- All reactions were carried in solvent-free environment. The PDL monomer (500 mg, 2.08 mmol) and lipase (50 mg of CALB) was used for the reaction. These reagents consisting of an enzyme to monomer ratio of 1/10 wt/wt wereplaced in a 7 mL microwave (MW) reaction vials with temperature monitored using an in situ optical probe. The reaction was set in a ‘CEM’ microwave synthesizer ‘Explorer’ at constant temperature of 70℃, constant power of 200W and at predetermined reaction time periods of 30 min, 60 min, 120 min and 240 min. The temperature and power setting were determined based on previous protocols established in our laboratory[23]. Control reaction was carried out using enzyme catalyzed polymerization in a traditional oil bath for 240 min. The reactions were terminated by dissolving the residual monomer and polymer in chloroform and separating the insoluble enzyme by filtration using 10-15 mm glass-fritted filters. The polymer was then dissolved in chloroform and precipitated using methanol. The insoluble materials was filtrated and washed three times with 5 mL portions of fresh methanol. The polymer was dried using vacuum oven and subsequently characterized.
2.3. Characterization
2.3.1. Nuclear Magnetic Resonance (NMR)
- Proton (1H) NMR spectra were obtained on a Bruker Advanced 300 MHz spectrometer at 300 and 75.13 MHz. The chemical shifts in parts per million (ppm) were referenced relative to tetramethylsilane (TMS).
2.3.2. Gel Permeation Chromatography (GPC)
- The molecular weights of polymers were measured on a Viscotek GPC max 2001 TDA 360 triple-detector system at a temperature of 35℃.Narrow molecular weight polystyrene standards obtained from Sigma-Aldrich were used to generate the conventional calibration curve. The mobile phase utilized to study these systems was tetrahydrofuran (THF) at a flow rate of 1ml/min.
2.3.3. Thermogravimetric Analysis (TGA)
- Thermal analysis was performed on a Perkin-Elmer TGA6/DSC6 system with constant N2 flow. Weight of sample was in the range 8-10 mg. The analyses were performed at a rate of 10℃/ min from room temperature to 600℃
3. Results and Discussion
- Figure 1 represents the schematic representation of MW assisted Lipase catalysis of poly-ω-pentadecalactone. The reactions were carried out at 70℃ in bulk for up to 4 hours in MW irradiation and a control reaction using traditional oil bath heating.
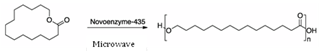 | Figure 1. Schematic representation of synergistic microwave and novozyme-435 catalyzed polymerization of poly-pentadecalactone from the cyclic monomer ω-pentadecalactone |
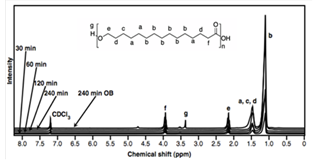 | Figure 2. 1H NMR spectra of poly-pentadecalactone in CD3Cl at reaction intervals of 30, 60, 120 and 240 minutes and with conventional heating in an oil bath (240min OB) |
|
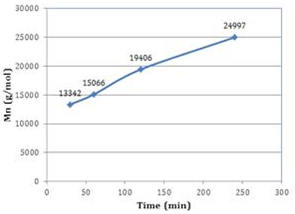 | Figure 3. Effect of number average molecular weight (Mn) of the PDL formed vs reaction time for microwave assisted Novozyme-435 catalyzed polymerizations |
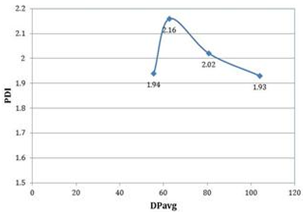 | Figure 4. Effect of polydispersity index (PDI) of PDL formed vs reaction time for microwave assisted Novozyme-435 catalyzed polymerizations |
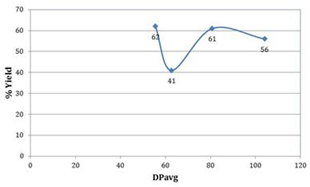 | Figure 5. Effect of % yield on DPavg for microwave assisted Novozyme-435 catalyzed polymerizations |
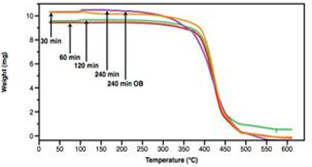 | Figure 6. TGA curves of Novozyme-435 catalyzed PPDL |
4. Summary and Conclusions
- In summary we have demonstrated the synergistic MW and lipase catalyzed polymerization of PDL. Synergistic MW and lipase catalyzed polymerization of PPDL gave an average molecular weight (Mn) of 24,997 g/mol and a polydispersity index (PDI) of 1.93 in 240 mins as compared to Mn of 8,060 g/mol and PDI of 2.17 using lipase and traditional heating. Thermal characterization of PPDL formed using MW and lipase catalysis did not have a detrimental effect on the thermal properties of the polymer obtained.
ACKNOWLEDGEMENTS
- We would like to acknowledge Wichita State University, the Center of Biotechnology and Biomedical Sciences (CBBS) at Norfolk State University for partial support of the work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML