-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biomedical Engineering
p-ISSN: 2163-1050 e-ISSN: 2163-1077
2012; 2(6): 241-250
doi:10.5923/j.ajbe.20120206.03
Depth of Cure and Microhardness of Nanofilled, Packable and Hybrid Dental Composite Resins
Mohamed El-Nawawy 1, Lubna Koraitim 1, Ossama Abouelatta 2, Hanan Hegazi 1
1Conservative Dentistry Dept., Faculty of Dentistry, Mansoura University, 35516, Mansoura, Egypt
2Production Engineering and Mechanical Design Dept., Faculty of Engineering, Mansoura University, 35516, Mansoura, Egypt
Correspondence to: Ossama Abouelatta , Production Engineering and Mechanical Design Dept., Faculty of Engineering, Mansoura University, 35516, Mansoura, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Resin-based composites are used worldwide in dentistry as they are used in a huge variety of clinical applications, as an esthetic restorative material with excellent physical and mechanical properties when adequate polymerization is obtained. In this study, depth of cure and microhardness of three composites were measured and compared. A total of sixty human mandibular first molars were used. The teeth were divided into three main groups (20 teeth each) according to the composite resins that were used. In group I, Surefil (packable composite) was used as the restorative material. In group II, Esthet-X-improved (nanofilled composite) was used, while in group III Glacier (hybrid composite) was used. Each group was subdivided into four subgroups (five teeth each) according to the storage intervals (24 hours, one week, two weeks, and three weeks). In each group, occlusomesial cavities were prepared with diamond burs and restored with the composite, according to manufacturer's instructions. In all specimens, composite was applied to the cavity using incremental technique. All the restored teeth were subjected to in vitro thermal cycling and mechanical loading simulating a total of six months in vivo function. Depth of cure was evaluated using penetrometer and microhardness was measured using Vicker’s microhardness tester. A significant difference in depth of cure and microhardness were found between the three composites used. Depth of cure and microhardness of the packable composite was better than the other two composites used. There was a fairly good correlation between the microhardness and the depth of cure for the three composite materials.
Keywords: Depth of Cure, Microhardness, Nanofilled, Packable, Hybrid, Composite Resins
Cite this paper: Mohamed El-Nawawy , Lubna Koraitim , Ossama Abouelatta , Hanan Hegazi , Depth of Cure and Microhardness of Nanofilled, Packable and Hybrid Dental Composite Resins, American Journal of Biomedical Engineering, Vol. 2 No. 6, 2012, pp. 241-250. doi: 10.5923/j.ajbe.20120206.03.
Article Outline
1. Introduction
- The evolutionary development of filling materials leads to an increasing need for better tooth colored restorative materials to replace missing tooth structure and to modify tooth color and contour, thus enhance facial esthetics. Polymeric restoratives have continued to evolve into the direct restorative materials of choice mainly because of their superior aesthetic characteristics. Currently, composites are the most widely used materials in restorative dentistry[1]. Composites consist of a mixture of two or more materials. Each of these materials contributes to the overall properties of the composite. Resin based composites are possibly the most universal material available in dentistry as they are used in a huge variety of clinical applications, ranging from filling material, luting agent, indirect restorations and metal facing for endodontic posts and cores[2].There is no one ideal dental composite materials available to the clinician, but the commercial materials that comprise the current armamentarium are of high quality and when used appropriately, have proven to deliver excellent clinical outcomes of adequate longevity[3].The depth of polymerization is of vital importance not only in order to achieve optimum mechanical properties including hardness but also to ensure that the clinical problems do not arise due to partially polymerized material in the base of the cavity[4].Hybrid or blended composites contain a graded blend of small and colloidal silica filler particles to achieve an optimal balance among the properties of strength, polymerization shrinkage, wear resistance and polish ability[5].Dental composite restorations have a major drawback: the degree to which they cure which is proportional to the amount of light to which they are exposed. So, they polymerize to a certain depth which varies with the penetration of a light beam in the bulk material. This extent of cure has been termed (depth of cure) and has significant influence on both physical and biological properties of restorations[6].The depth of cure is the depth to which the light is able to harden the material. This does not mean that the hardness in the lower areas is of the same magnitude at the top of the irradiated sample. Although there exists no general accepted definition on depth of cure and how to determine it quantitatively, there is a sort of agreement that depth of cure is limited to that distance from the top surface of a cylindrical sample where no more resinous material can be scratched off[7]. Because dental restorations need an optimal polymerization level, investigators have studied the effect of different parameters on the depth of cure. It was found that the depth of cure of light activated composite resins depends on the material filler composition, its shade, translucency, the intensity of light source and the distance from the curing tip[8-12].The depth of cure of visible light activated composites has been accomplished by many researchers[13-23]. Overall depth of cure of the 15 micron filler series was somewhat better than that for the 2 micron filler series[13].Large particle hybrids had the greatest depth of cure, followed by small particle hybrid then micro-filled composite[14]. The depth of cure was affected bycomposite composition rather than irradiance from light units[15]. The hardness of composites decreased with increasing the depth[16]. At the composite surface, filler type, exposure duration and resin shade predominated as the most influential factors, respectively[17]. The cure of top surfaces of light activated materials was not greatly affected by light intensities but curing of the inner aspects of the materials was affected by light intensities[18]. By comparing he physical properties of three packable hybrid resin-based composites with those of a conventional hybrid and a microfill composite material advocated for use as posterior restorative materials, packable composites had a significantly greater depth of cure than all other resin-based composites, followed in decreasing order by conventional hybrid composite and microfilled composite[19]. By studying depth of cure, polymerization shrinkage and microhardness of packable, ion-releasing and hybrid composites, the depth of cure showed no statistically significant differences among all materials tested[20]. The curing depth of polyacid-modified composite resins was independent of post-cure, but differed significantly among materials and shade and the curing depth greatly varied among the materials[21]. New-formulated resin-based composites showed better performance concerning depth of cure compared to conventional materials[22]. Properties currently used to evaluate depth of cure (microhardness, degree of conversion or scraping methods) fail to detect this transition, which results in overestimation of the depth of cure[23].On the other hand, hardness is defined as the ability of the material to resist permanent surface indentation, penetration and abrasion.Hardness is indicative for the ease of finishing of a structure and its resistance toscratching[24]. Besides, the indentation produced on the surface of a material from an applied force of a sharp point or an abrasive particle results from the interaction of numerous properties. Among the properties that are related to the hardness of the material, there are strength, proportional limit and ductility. Hardness of a brittle material such as composite resin can be determined by Vickers and knooptests, which are classified as microhardness tests in comparison with Rockwell and Brinellmacrohardness tests[25]. When studying the effect of volume fraction and particle size on the microhardness of eight visible light cured composite resins with a wide range of particle size, a direct proportional relation existed, as the particle size increased the hardness increased[26]. A significant correlation between the volume fraction of filler and knoop hardness number and no correlation was observed between the degree of conversion and the surface hardness in any of the composite resins tested[27]. A progressive increase in microhardness for four weeks measurements in testing microhardness of eight visible light-cured and self-cured composite resin specimens (dry or wet), followed by stabilization until the end of the test[28]. Filler type was found to be the most important variable at the top surface[29], when studying the effect of the light intensity, filler type, the duration of exposure and the thickness on the microhardness of composite resins. Microhardness values have been improved due to higher initiator concentration and exposure times themicrohardness values[30]. The microhardness values for the light cured glass ionomer were the lowest and composite resins and polyacid-modified composites had the highest values[31].In some cases, packable composites were unlikely to offer improved clinical performance over well-placed non-packable composites[32]. Besides the filler content level and filler size, some factors like matrix-filler interactions highly influenced the microhardness and wear behavior of the materials[33]. The microhardness of the nanofilled composites was equivalent to or higher than those of the other hybrids, microhybrids and microfill composites[34]. The indirect laboratory processed composite exhibited higher mean values for hardness and degree of conversion but at 4 mm depth, the packable composites exhibited significantly greater hardness than other direct composites tested[35]. In general, indirect resin composite shows poorer mechanical properties than hybrid composites[36]. By studying the quality of polymerization of hybrid composite resins, it was found that each product had different microhardness values and the repeated thermal stimulus had no specific effect on the change of microhardness[37]. The nanofilled resin composite displayed the higher microhardness values for curing regimes[38]. One-step polishing systems may be successfully used for polishing nanocomposites that contain nanoparticles and a micro-hybrid composite[39]. Clearfil Majesty™ Posterior demonstrated a higher microhardness, less surface roughness, and higher wear resistance when compared with the some tested materials for both polymerization types[40]. The mode of polymerization and the lightcuring time did not affect the hardness of the nanofilled composite resin, and increasing the lightcuring time did not improve the hardness of the bottom surface of the composite resin[41]. The effectiveness of cure at the top and bottom surface was not affected by soft-start polymerization mode[42]. For bulk fill materials, the ISO 4049 method overestimated depth of cure compared to depth of cure determined by Vickers hardness profiles[43]. This work aims to evaluate and to compare measurements of depth of cure and microhardness of three composites resinsat different storage time.The second research hypothesis was that the depth of cure determined by a penetrometer method would correlate with the measured microhardness.
2. Materials and Methods[44]
2.1. Material
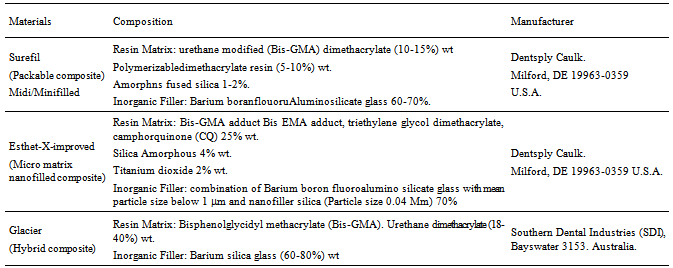
- Sixty sound human mandibular first molars were collected from a dental clinic from patients with ages ranged from 25-35 years. The collected teeth were cleaned from debris and blood by a tooth brush and soap under running water after storing them in 1% H2O2 for 24 hours. The collected teeth were examined using light microscope (PHMG, Olympus Optical Co. Ltd., Tokyo, Japan) to select molars that were free from cracks[45].The extracted teeth were stored in 0.2% Sodium Azide (has antibacterial activity) at room temperature before preparation andrestoration[46].The materials used in this study are listed in Table 1.
2.2. Specimen Preparation
- Intact teeth were mounted in auto-polymerizing acrylic resin blocks (Pekatray, Bayer Dental Leverkusen, Germany) that were confined by stainless steel holders. Each holder held three teeth positioned with crowns in proximal contacts and long axis parallel to the sides[47]. The tooth in between the axial teeth was used only to obtain firm contact areas as shown in Figure 1(a). Class II (occlusomesial) cavities were prepared with an occlusal box of dimensions 2.5 mm depth and 2 mm width. A proximal box of 3.5 mm depth and 3 mm width was made as shown in Figure1(b), using high speed handpiece (NSK, Tokyo, Japan) with continuous air-water cooling system[45].In order to create standardized occlusomesial cavities with a definite form and size, the preparation was made using a cylindrical diamond bur (Mani, Tokyo, Japan) with a rubber stop adjusted to the required depth[48-48], as shown in Figure 1(c). For adjusting the cavity width, a vernier caliper was used. A new point was used for every three preparations to standardize the sharpness and cutting efficiency of the diamond instruments for all the specimens.
2.3. Specimens Grouping
- Sixty molars were divided into three main groups according to the type of composite resins that were used. Each group consisted of 20 teeth. In group I Surefil was used as the restorative material, in group II Esthet–X–Improved was used, while in group III Glacier was used. Each group was subdivided into four subgroups (five teeth each) according to the storage intervals (24 hours, one week, two weeks and three weeks)[50].
2.4. Restorative Procedures
- After the preparation of each tooth, the cavity was dried using gentle air blast and a transparent matrix in a tofflemire matrix retainer (ProduitsDentaires SE, Switzerland) was used and held in place with light reflecting wedges (Dentalez Group, Malvern, USA) as shown in Figure 1(d). Phosphoric acid etchant gel 37% was applied to the enamel and dentin as shown in Figure 1(e), and left for 15 seconds. After that it washed with water spray for ten seconds and air dried by oil free compressed air. Excess water was removed without over drying the dentin[45]. The Prime & Bond NT bonding agent was applied to the etched enamel and dentin using a disposable applicator tip as shown in Figure 1(f), for 20 seconds.
|
2.5. Curing Procedures
- According to the manufacturer instructions, the first layer was applied with a suitable condenser (Compo-Sculp: DD 3, Sutr dental manufacturing, CHICO, CA). The composite resin was adapted to the cavity floor and walls, then light cured for 20 seconds. The second layer was applied similarly, then the anatomical occlusal surface was carved using shaping-sculpting instruments (Compo-Sculp: DD 1, 2, Sutr dental manufacturing, CHICO, CA) and light cured for 20 seconds as shown in Figure 1(i).
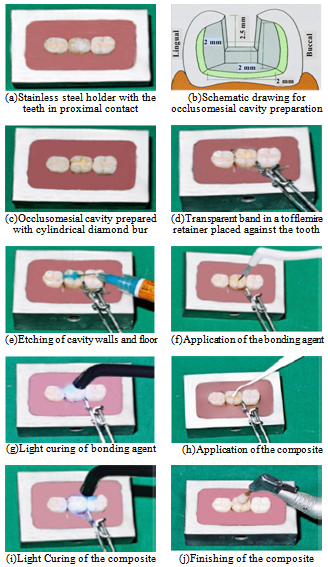 | Figure 1. Specimen preparation |
2.6. Finishing and Polishing
- The finishing was immediately done, gross excess was removed and a general outline form was established with diamond finishing instruments. Additional finishing was done by the use of "Enhance" finishing system (Dentsply Caulk, Mil ford , DE 19963-0359, USA) as shown in Figure 1(j). A high luster was established by using "Pogo" one step diamond micro-polisher system (Dentsply Caulk, Mil ford, DE 19963-0359, USA), according to the manufacturer's instructions. All the specimens were subjected to a total of six months in vitro simulated in vivo function[51], by exposing the restoration to thermal cycling and mechanical loading (Conservative Department Lab, faculty of Dentistry, Tanta University, Egypt).
2.7. Thermal Cycling
- For thermal cycling, all the specimens were thermocycled between 5±2C and 55±2C for 300 cycles, with a dwell time 2 minutes and 10 seconds transfer time[51].
2.8. Load Cycling
- All specimens were fixed in mounting rings. The mounting ring was attached to the lower member of a custom – made loading machine which acted as the mandibular element, while a uniaxial load of 49 N was applied using a rounded end metal rod which was fascinated to the upper member of the machine, Figure 2.
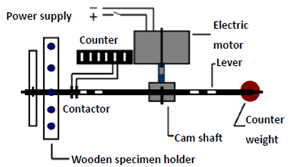 | Figure 2. Schematic drawing of load cycling machine |
2.9. Depth of Cure Test
- The depth of cure of a visible-light activated resin has been the subject of considerable laboratory research. Even after more than 25 years of clinical use, there are still controversies about the depth of cure of a visible light activated resin. A number of different techniques have been employed to measure the properties of the polymerized resin composite most distant from the light source[52].Tefflon cylindrical moulds were prepared, as shown in Figure 3. Its outside dimensions were 24 mm in diameter and 6 mm height. Cylindrical cavities of 4 mm in diameter were prepared in the center line of the moulds[4]. The cylindrical mould was placed on a cellulose acetate matrix strip resting on a flat black disk and the mould filled was with the composite. The composite was dispensed directly from its container into the cavity of the mould as shown in Figure 4.
 | Figure 3. Tefflon cylindrical mould |
 | Figure 4. Applying a glass slab to remove the excess of material |
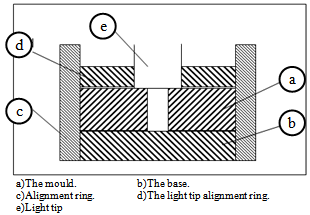 | Figure 5. Line drawing of the mould and alignment rings |
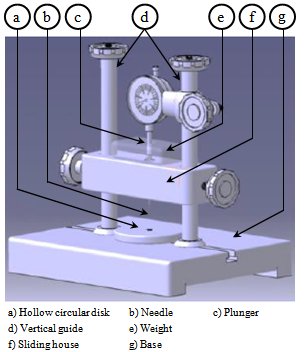 | Figure 6. Penetrometer apparatus |
2.10. Microhardness Test
- The microhardness test is a simple and reliable method to reflect the degree of conversion at different depths of resin composite. In this study microhardnessmeasurements were used as an indicator of the degree of polymerization of light-curing resin composite materials which is a relative simple and accurate technique[53]. Microhardness measurements are in particular useful since a small change in degree of polymerization may yield a large change in hardness. A total of 30 specimens, ten specimens of each composite type of the same shade (A2) were prepared in a round stainless steel mould of 6mm diameter and 3mm thickness[36].The round mould was placed on a cellulose acetate strip resting on a flat surface, then the mould was filled with the composite restorative material which was dispensed directly from its container into the cavity of the mould, a second layer of a cellulose strip was placed on the top, a glass slab was pressed on the top of this to express the excess material. The composite was cured from both sides for ten seconds each using a light curing unit. Cellulose acetate strips(Vicker’s hardness tester, SM-7, future grope, Japan.) were used in this test to produce a very smooth surface of the composite specimens, Figure 7.
 | Figure 7. Specimen preparation for microhardness test |
 | Figure 8. Vicker’s microhardness tester |
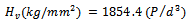 whereP=applied indentation load.d=diagonal length of the impression.All the collected results were statistically analyzed using analysis of variance (ANOVA). A Duncan’s multiple range test was used to interpret the data. The statistical analysis of data was conducted using Microsoft Excel and SPSS software 12.0 (SPSS Software, München, Germany).First part of the data is descriptive in form of mean(X), standard deviation(+SD)and probability (P). Second part isanalytic to test statistical significant difference between groups.One way ANOVA test was used for determination of significant difference between groups, paired sample student’s t-test was used to compare significance different for different storage interval periods in the same group and Post Hoc Test: LSD[least significant difference] to test significant difference intra - groups.
whereP=applied indentation load.d=diagonal length of the impression.All the collected results were statistically analyzed using analysis of variance (ANOVA). A Duncan’s multiple range test was used to interpret the data. The statistical analysis of data was conducted using Microsoft Excel and SPSS software 12.0 (SPSS Software, München, Germany).First part of the data is descriptive in form of mean(X), standard deviation(+SD)and probability (P). Second part isanalytic to test statistical significant difference between groups.One way ANOVA test was used for determination of significant difference between groups, paired sample student’s t-test was used to compare significance different for different storage interval periods in the same group and Post Hoc Test: LSD[least significant difference] to test significant difference intra - groups.3. Results
3.1. Depth of Cure
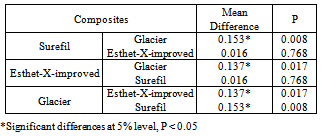
- The analysis of variance test ANOVA was used to compare the mean values of the depth of cure of the three restorative materials. The mean values, the standard deviation and results of the ANOVA test were represented in Figure 9.
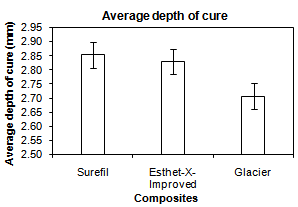 | Figure 9. Comparison between mean values of the depth of cure of the three composite resins |
|
3.2. Microhardness
- The analysis of variance test ANOVA was used to compare between the mean values of the microhardness of the three restorative materials. The mean values, the standard deviation and results of the ANOVA test were represented in Figure 10.
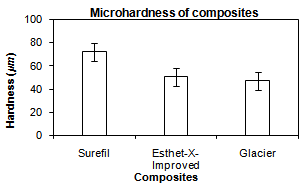 | Figure 10. Comparison between the mean values of the microhardness of the three composite resins |
3.3. Correlation between Depth of Cure and Microhardness Tests
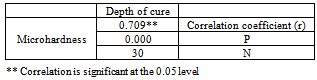
- In the standard tests for correlation, a correlation coefficient is tested against the hypothesis of no correlation, i.e., R = 0. It is possible to test whether the correlation coefficient is equal to or different from another fixed value. Correlation coefficient test for depth of cure and microhardness of the three composites showed a good correlation as (R = 0.709) and (P<0.05) as shown in Table 3.
|
4. Discussion
- Despite the improved characteristics of composite restorations, polymerization shrinkage remains a challenge and still imposes limitation in the application of direct techniques. This volumetric shrinkage ranges from 2-4%[51]. In this study,a nanofilled, packable and hybrid composites were compared at different filler particle size and different storage intervals (one day, one week, two weeks and three weeks) using artificial saliva[52]. Nanofilled composite was used because of the new technology used in its fabrication where its filler particle size ranges from (0.005-0.01 microns). These very small particles don’t react with the visible light and don’t produce scattering resulting in significant absorption of light which leads to improvement in modulus of elasticity, depth of cure and esthetics[55, 56].The packable composite was selected for its different advantages. A major advantage is using a new inter-locking particle technology. This technology uses a precisely engineered mixture of different-sized filler particles. These particles are made of a patented fluoride-infused glass. When these particles are packed together, the larger particles mechanically interlock with the smaller particles which lead to improvement of the depth of cure and microhardness[32].Depth of cure still remains a challenge in the application of direct composite techniques. The depth of polymerization is of vital importance not only in order to achieve optimum physical and mechanical properties but also to ensure that, the clinical problems do not arise due to partially polymerized material in the base of the cavity[4,6]. Depth of cure of dental composites and shrinkage stresses induced during polymerization affect the marginal integrity of composite restorations. For this reason, investigation of depth of cure is of scientific interest[57]. The marginal adaptation andreduction of polymerization shrinkage of composite resin could be improved by increasing depth of cure which could decrease width and length of gaps without interfering with the mechanical properties of the composite restorative material[58].Evaluation of depth of cure, in this study, was performed using penetrometer. Penetrometer applies a constant stress of 62MPa allowing consistency of results. Also, penetrometer needs just a single reading to give the value of depth of cure[2].The results of depth of cure showed that, a significant difference was found between the three tested composites, where the packable composite recorded the best depth of cure followed by nanofilled compositethen hybrid composite. This may be attributed to theinterlocking particle technology used in the fabrication of the packable composite, where elongated fibrous filler particles of about 100micron in length, and/or textured surfaces tend to interlock and resist flow. Rough surfaces, blends of fibrous and particulate fillers produce a packable consistency and enable physical and mechanical properties to be optimized for clinical performance[59].In addition, the results were found to be in agreement with the results ofCobb et al.[19],who compared the depth of cure of packable composites, hybrid composites and microfilled composites. They found that, depth of cure of packable composites is better than hybrid composites and microfilled composites. Also, the findings of the present study were close to those ofErsoyet al.[20],who studied the depth of cure and polymerization shrinkage of packable and hybrid composites and reported that, a significant difference was found between materials used, where the packable composites were much better than the hybrid composites used. Because the packable composites consist ofa mixture of different sized filler particles, when packed together result ina precisely engineered mixture that allows light transmission for the deep layers.The results of the present study go with those of Rueggeberget al.[17],who concluded that, the filler type is one of the most influential factors in the depth of cure.On the other hand, material is considered hard if it strongly resists indentation by a hard material such as diamond. Also, hardness is indicative for the ease of finishing of a structure and its resistance to scratching [24].The Vicker’s microhardness test was selected in this study because it is the most accurate, available and simple test for measuring the microhardness of a brittle material like composite restorations[31].The results of microhadness test showed that, there was a high significant difference between the three composites used where packable composite recorded the highest microhardness values followed by nanofilled composite, then hybrid composite. This may be attributed to the increased depth of cure of the packable composite than the other two composites.The results of this study were in agreement with the results of Knoblochet al.[35],who studied the microhardness and degree of conversion of packable and microhybrid composites. They concluded that, the packable composites exhibited significantly greater hardness than the other composites. In addition, the results were in agreement with the results ofChoi et al.[32] whostudied the depth of cure, microhardness and polymerization shrinkage of packable composite and non-packable composite. They concluded that, the microhardness of packable composite was higher than non-packable composite when the composite was used in increments not thicker thantwo millimeter.The results were also close to the results of Manhartet al.[33],who found thatthe filler size and filler content have a high influence on microhardness. Also,the results of this study were in accordance with the results of Mitraet al.[34],who found that microhardness of nanofilled composite was better than the hybrid composite.In the present study, there was a good correlation between the depth of cure and microhardness of the three composites where (R =0.709). This could possibly due to that, the increased depth of cure will improve the process of conversion of the monomer to the polymer stage and accelerates the polymerization reaction.
5. Conclusions
- This study was divided into two parts. The first part dealt with measuring and comparing depth of cure of the three composites,while the second part evaluated and compared the microhardness of these composites. Depth of cure and microhardness of the composite specimens was measured using penetrometer and Vicker’s microhardness tester, respectively. Six measurements for each specimen were recorded and average was calculated. It can be concluded that:1. A significant differences in depth of cure and microhardness were found between the three composites used.2. Depth of cure and microhardness of the packable composite were better than the other two composites used.3. There was a fairly good correlation between the microhardness and the depth of cure for the three composite materials. The results obtained in the present study lead the authors to partially accept the research hypothesis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML