-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biomedical Engineering
p-ISSN: 2163-1050 e-ISSN: 2163-1077
2012; 2(6): 212-217
doi: 10.5923/j.ajbe.20120206.01
Common Pesticide Rotenone Interference with Neuronal Transmission in Hippocampus
Fatih Akkentli, Yusuf P. Tan, Hale Saybasili
Institute of Biomedical Engineering, Bogazici University, Istanbul, 34684, Istanbul
Correspondence to: Hale Saybasili, Institute of Biomedical Engineering, Bogazici University, Istanbul, 34684, Istanbul.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Rotenone is a pesticide and piscicide, which causes behavioral and biochemical changes in rats that closely resemble PD symptoms in humans. Rotenone is a naturally occurring retinoid plant extract derived from the roots of certain tropical and subtropical legume plants and interferes with energy production in the cell. Rotenone is highly lipophilic, so it can easily cross the blood brain barrier and cellular membrane for intracellular entry.It is known that this pesticide causes superoxide release and results in decreased energy production by inhibiting electron transport chain of mitochondria from NADH ubiquinone reductaseand may lead to neuronal death. The object of the current research is to investigate the effect of rotenone on synaptic transmission in hippocampus, especially on glutamatergic transmission for a brief exposure time. For this purpose, hippocampal CA1 pyramidal neuronal response upon low frequency stimulation of Schaffer collateral (0.1 Hz) was recorded by the patch clamp tight-seal whole cell recording technique. Different rotenone concentrations were tested on total glutamate current; it was observed that the rotenone effect on the amplitude of glutamatergic currents is dependent on its concentration. To eliminate the rotenone induced cytoplasmic effects, ATP was excluded from the intracellular solution in experiments. Our experimental results show that the drug acutely and dose-dependently attenuates the currents that are mediated by glutamate, via a direct effect on cell membrane glutamate receptors.
Keywords: Rotenone, Hippocampus, CA1 Pyramidal Neuron, Glutamate Receptors, Patch Clamp
Cite this paper: Fatih Akkentli, Yusuf P. Tan, Hale Saybasili, "Common Pesticide Rotenone Interference with Neuronal Transmission in Hippocampus", American Journal of Biomedical Engineering, Vol. 2 No. 6, 2012, pp. 212-217. doi: 10.5923/j.ajbe.20120206.01.
Article Outline
1. Introduction
- Today, a considerable body of research exists which shows the toxicity of pesticides for human health[1-6]. Generally, these adverse effects of pesticides were demonstrated at doses previously declared as safe by industry and government[7]. Different pesticides have been linked with a variety of toxic effects on the nervous system and other organs causing carcinogenic effects, hormone system effects and general irritation[1-6]. Wide exposure of the population to pesticides in food products is the main source of the accumulation of pesticide residues in the human body. One of these pesticides, rotenone, has been shown to induce many of the major symptoms of Parkinson’s disease in rats after long-term exposure[8]. Rotenone is used as a natural broad-spectrum pesticide extracted from the derris plant. It is often formulated as dusts, powders and sprays for use in gardens and on food crops[7].Rotenone is a naturally occurring retinoid plant extract derived from the roots of certain tropical and subtropical legume plants and used as broad spectrum pesticide and Rotenone use in agriculture can cause serious pollution of streams and reservoirs, since it can easily wash of the soil with heavy rains[9]. The degradation can sometimes persist for up to six months depending on a variety of factors including light, temperature, depth, dose and presence of organic debris. The decomposition process occurs faster as the temperature of the water increases. Rotenone toxicity tests showed that goldfish (Carassius auratus) were the most resistant of the 21 species tested, where Atlantic salmon (Salmosalar) were the most sensitive[10].Rotenone is highly lipophilic, so it can easily cross the blood brain barrier and cellular membrane for intracellular entry[11]. Inside the cell, rotenone accumulates at mitochondrial complex-1 as a blocker, causing oxidative stress and a decrease in energy production, which leads to neuronal death. Hippocampus is a model system for neurophysiology since it has different neuronal cell types, which are well organised into layers. The storing of certain types of memory requires hippocampal function[12]. Damage in hippocampus and its associated pathways (such as fornix) results in a major deficit in learning to recognise new stimuli[13] and spatial memory acquisition[14]. Hippocampus receives highly processed data from association areas such as parietal cortex, the inferior temporal visual cortex and the superior temporal cortex[15]. The CA1 hippocampal area is a very vulnerable brain region affected by adverse conditions such as ischemia and anoxia[16]. Information flow in hippocampus is mostly unidirectional. Tightly packed cell-layers are paths for propagating signals. The main output region in hippocampus is CA1 pyramidal cell region[17].L-glutamate is a universal excitatory neurotransmitter of the vertebrate central nervous system. Ionotropic glutamate receptors are divided into 3 subgroups, NMDA(N-methyl-D-aspartate), kainite and AMPA(a-amino-3-hydroxy-5-methyl-4-isoxalone propionic acid)[18]. NMDA receptors allow membrane transport of sodium, potassium and calcium ions, and also work as coincidence detectors since their channel opening requires depolarization and the release of glutamate from presynaptic neurons simultaneously. Kainate and AMPA receptors allow membrane transport of sodium and potassium ions.In our current research, we studied the effect of rotenone on the CA1 pyramidal neuron functioning in rat hippocampus. Our main aim is to assess the acute influence of rotenone on glutamatergic transmission.
2. Materials and Methods
2.1. In-Vitro Hippocampal Slice Preparation
- Sprague Dawley rats on postnatal days 10-25 were provided from the Experimental Animal Center, Marmara University (Istanbul). The experiments were conducted in accordance with the guidelines of the Animal Research Committee of Bogazici University. Hippocampal slices were prepared as previously described[19]. Transversal slices (200µM in thickness) were prepared by using a vibroslicer and incubated in carbogen gas (95%O2-5%CO2) aerated artificial cerebrospinal fluid (aCSF) containing (mM): NaCl 125, KCL 2.5, CaCl2 2.0, MgCl2 1.0, NaH2PO4 1.25, NaHCO3 26, Glucose 10. Slices were maintained in aCSF solution for at least 45 minutes before performing experiments. During recordings, slices were kept submerged in a chamber perfused with aCSF, which was saturated with carbogen gas. All experiments were performed at room temperature.
2.2. Patch Clamp Tight-Seal Whole Cell Recording
- To take whole-cell patch-clamp records from pyramidal neurons in the CA1 layer of hippocampus, slices were transferred to a recording chamber placed on a microscope stage. Healthy hippocampal CA1 neurons were visualised by using a CCD camera (sensicamqe 672 LS, pco.imaging, Germany). Borosilicate recording-electrodes (with 4-7MΩ resistance) were made with a micropipette puller PP-81 (Narishige, Japan) from borosilicate capillaries (Hilfenberg, Germany). The composition of the standard pipette solution for recording post-synaptic currents was (mM): CsF 135.0, CsCl 5.0, EGTA 10.0, HEPES 10.0, CaCl2 1.0 (pH 7.3). Cesium (Cs+) was used as the main cation to substitute K+ ions and to suppress potassium conductance and fluoride (F-), which blocks the chloride conductance and calcium dynamics[20], was used as the main anion. At the time of recording, stimuli (0.1 Hz) were applied to the Schaffer collateral pathway of the hippocampal slice through a tungsten bipolar stimulation electrode. To obtain a whole-cell clamp configuration, the gigaohm seal formed between the cell membrane and recording pipette was disrupted by a slight negative pressure application at a holding potential of -60 mV. Patch-clamp amplifier was EPC-7 (List Medicals, Darmstadt, Germany), analog signals were filtered with a 3kHz Bessel filter and converted into digital signals by ITC-18 A/D converter (Instrutech, USA) at 25kHz. Stimulating current-pulses were generated from computer and delivered via a stimulus isolation unit (Iso-Flex, Israel). The glutamergic currents were recorded at a slightly depolarized voltage value (-50 mV) and rotenone was applied to observe its effect on glutamergic currents only after stable amplitude of control currents was reached.
2.3. Rotenone Application
- Rotenone (purchased from Sigma) was prepared as stock in dimethylsulfoxide (DMSO) and was diluted directly in the aCSF, and applied via the perfusion system at 0.1, 0.5, and 1µM of final concentrations. Control values were recorded for 3 minutes and neurons were treated with rotenone only if their synaptic current amplitude was stable. Then, rotenone was applied to the hippocampal slices for 3 minutes and following this, wash with aCSF was performed for 6 minutes. For the early (first 3minutes) and the late (second 3minutes) wash periods, rotenone effects on the neuronal responses were analysed separately. Former and latter halves of 6 minutes long aCSF wash are designated as E-wash and L-wash, respectively. While E-wash covers the period between 6th and 9th minutes, L-wash covers 9th-12th minute time interval of experiments.
2.4. Data Analysis
- WinWCP and MATLAB-R2009b were used for data acquisition and analysis. SPSS13 was used for statistics. WinWCP is a freeware program provided by Strathclyde Institute. MATLAB and SPSS programs are licensed for Bogazici University. Peak values of current response waves of neurons were analysed for control (before rotenone treatment), rotenone application and wash periods of each experiment. Current response waves were plotted by averaging 18 sweeps of the glutamatergic responses.
3. Results
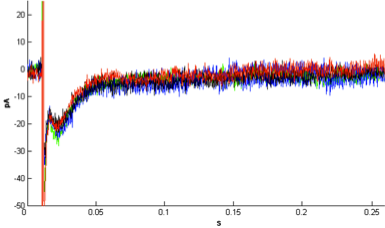
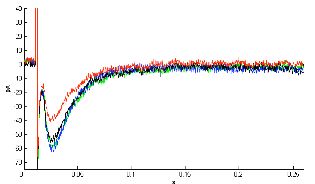
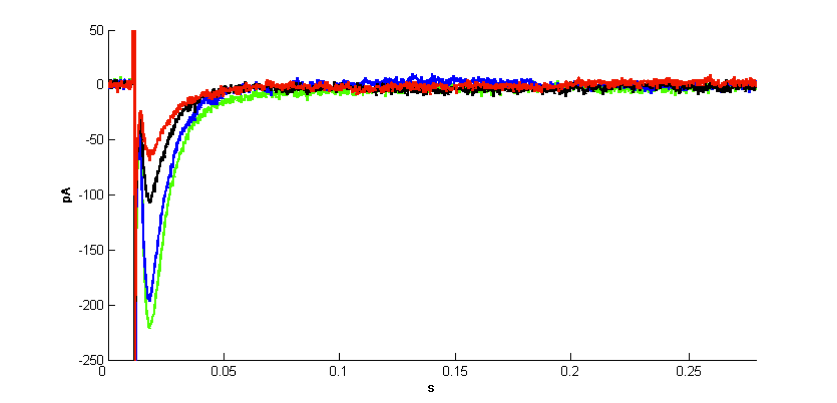
- The responses of CA1 pyramidal cells were recorded by patch clamp tight-seal whole cell recording technique at a holding potential of -50 mV. Whole cell currents were recorded from the soma of the hippocampal pyramidal neurons. Neuronal responses, inward postsynaptic currents, were evoked by a single brief current pulse (0.1 Hz frequency), which was delivered to the Schaffer collateral pathway. The intracellular pipette solution did not contain ATP to exclude the contribution of intracellular systems. Thus, only the currents originating only from membrane receptors are recorded. The effects of different concentrations of rotenone were tested on glutamate current; 0.1 mM rotenone was found to have no effect on glutamate currents“Figure.1” (F3,18=0.37, P=0.77, n=6). The excitatory postsynaptic currents did not change with rotenone application.
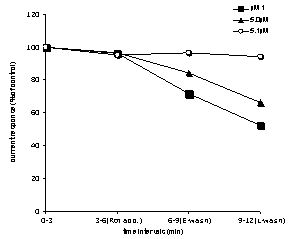 | Figure 4. Effects of different concentrations of rotenone (0.1, 0.5, 1µM) application on glutamate currents recorded from hippocampal CA1 pyramidal neuron. See text for explanation |
4. Discussion
- Rotenone is a subject of investigation in neuroscience because it causes neuropathic characteristics in rats that closely resembles to PD in humans[8]. At the neuronal level, it is used as a model to study the effect of mitochondrial inhibition[21]. As a consequence of mitochondrial inhibition, energy depletion and the generation of reactive oxygen species induce different effects on neurons from different brain regions. Understanding this diversity in rotenone effect on neurons is important for depicting the variance in the functioning of different neuron types. Rotenone is known for its toxicity in dopaminergic neurons and that is how it has a disruptive effect in brain forms a model for PD[21].In this study, we showed that the neuronal responses of pyramidal neurons in CA1 region of hippocampus are decreased with rotenone treatment. To our knowledge, this is the first report about the acute glutamatergic inhibition by using different rotenone concentrations in hippocampal slices. Single neuron responses evoked by Schaffer collateral stimulation were inhibited by 0.5 and 1µM concentrations of rotenone exposure. Unlike the effect of chronic rotenone exposure on neuronal survival in this study, a brief application of rotenone (0.5µM/1 µM for 3minutes) from perfusion solution was sufficient to inhibit the neuronal transmission.As previously indicated long exposure (for 12 hours) to 10µM rotenone did not produce toxicity on glutamatergicneurons[22]. The inhibition of glutamatergic response observed in our study is unlikely to be the result of neuron degeneration caused by exposure to rotenone (1µM for 3 minutes) because it has a short duration. In 0.1 µM rotenone application experiments no inhibition of neuronal responses was observed, while dose dependent inhibition was observed for 0.5 and 1µM concentrations. This indicates that inhibition of glutamatergic responses is caused by rotenone application rather than other nonspecific interference in the experiments.Although rotenone primarily inhibits mitochondria complex I, degeneration of dopaminergic neurons via rotenone treatment does not require the inhibition of complex-I[23]. Rotenone is known to depolymerize microtubules, the components of the cell skeleton that are involved in vesicular transportation[24]. The stabilization of microtubules with taxol prevents rotenone induced selective damage to dopaminergic and serotonergic neurons[22]. Also, the Parkin-protein prevents rotenone damage on dopaminergic neurons by stabilizing microtubules[25]. Likewise, neurotropic factors and group III metabotropic glutamate receptor activity inhibits selective rotenone toxicity on dopaminergic neurons by stabilizing microtubules[26],[27].Microtubule depolymerisation disrupts vesicular transport and causes their accumulation in the soma[28]. The accumulation of dopamine can be toxic because increased cytosolic concentration of this neuromodulator elevates oxidative stress due to dopamine oxidation[22]. Since glutamate cannot be oxidized, rotenone does not cause a similar toxicity in glutamatergic neurons but it has been claimed that it depolymerizes the microtubules[25],[28]. On the other hand, rotenone was found to generate a higher level of superoxide generation in rat hippocampal slices compared to rat striatal slices, showing that this structure is more vulnerable to oxidative stress[29].The impact of rotenone as a pesticide in controlling the habitat quality of aquatic ecosystems has been discussed before[30].In our experiments, observed irreversible rotenone interference with glutamatergic transmission might result from a deficit in glutamate transportation to the presynaptic terminals. Thus, rotenone treatment may decrease the level of presynaptic release of glutamate. Therefore, the inhibitory effect of rotenone on neuronal responses might be presynaptic rather than on the postsynaptic site. On the other hand, in the literature, rotenone’s effect on microtubules was investigated with neuron cultures exposed to rotenone treatment lasting for 12 hours[22],[23],[25],[28]). Although it is apparent that microtubule disruption with rotenone interference may require a chronic exposure, in our experiments the rotenone exposure time of slices was very brief (i.e., 3 minutes). It has been reported that rotenone decreases field potentials (fEPSP) by 17% in CA1 region of hippocampus [31]. There are other reports about rotenone enhancement of NMDA responses in rat midbrain slices, thispotentiating effect linked to NMDA receptor activation[32].
5. Conclusions
- Rotenone has been widely used to select certain fish populations over the other species and commonly used in fresh waters reservoirs. Our research showed that rotenone has an acute inhibitory effect on the hippocampal glutamatergic transmission under ATP deficient conditions. This condition excludes the contribution of mitochondrial compartment to our experimental results. To our knowledge, this is the first study that investigated the immediate effect of rotenone on membrane glutamate receptors. Rotenone effect was found to be dose dependent and irreversible. Given the impact and exposure time to rotenone, the experimental conditions of our work define a situation in which rotenone effect can be sharper compared to those in other literature that investigated some other electrophysiological and neurodegenerative features of the broad-spectrum pesticide, rotenone. Hence, our study isolated the more rotenone-sensitive conditions for neuronal transmission; in the case of ATP deficiency, rotenone can have serious dose-dependent inhibition on glutamatergic receptors. Since this pesticide is lipophilic, it can easily permeate the blood brain barrier and able to generate acute effects on neurons. As a future prospect, the rotenone effect can be studied by single cell imaging techniques and calcium dynamics in the cytoplasm can be visualized, so compartmental involvement and interactions can be revealed.
ACKNOWLEDGMENTS
- This research has been supported by Bogazici University research foundation (project no: 02S104).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML