-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biomedical Engineering
p-ISSN: 2163-1050 e-ISSN: 2163-1077
2012; 2(4): 181-184
doi: 10.5923/j.ajbe.20120204.05
Pulsed Low Intensity Electromagnetic Field (PEMF) Affects Cell Cycle of Human Osteoblast-like Cells in Vitro
Nahum Rosenberg , Orit Rosenberg , Michael Soudry
Laboratory of Musculoskeletal Research, Orthopaedic Surgery “A” Department, Rambam , Health Care Campus, Haifa 31096, Israel
Correspondence to: Nahum Rosenberg , Laboratory of Musculoskeletal Research, Orthopaedic Surgery “A” Department, Rambam , Health Care Campus, Haifa 31096, Israel.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
High frequency electromagnetic field in the range of 4 - 33 kHz given in low frequency bursts of 7-15 Hz (PEMF) is efficient in stimulating osteoblast proliferation and differentiation in vitro. We hypothesize that osteoblast cell cycle changes following this stimulatory PEMF. In order to explore this effect we used primary explant cultures of human osteoblast-like cells. These cells were subjected to low frequency of 20-30 Hz, higher frequency of 5-15 kHz electromagnetic fields and to PEMF (5-15 kHz basic frequency in pulses of 20-30 Hz) with maximal magnetic flux of 10-12 Gauss. The maturation state of the cells was estimated by the measurement of cellular alkaline phosphatase activity and the overall cell death by the LDH activity in culture media. The cell cycle was analyzed by cytometry. The PEMF stimulation significantly increased the proportion of cells in the G1 phase (p<.001) and decreased the proportion of apoptotic and necrotic cells (p<.001), with parallel decrease in cellular alkaline phosphatase and media LDH activities. Pure 20-30 Hz or 5-15 kHz electromagnetic stimulations had no effect on these parameters. Therefore we show that PEMF with a basic frequency in the kHz range provided in pulses in the infrasonic range alters human osteoblast cell cycle by reducing cell death and cellular maturation.
Keywords: PEMF, Osteoblast, Cell Cycle
1. Introduction
- Pulsed electromagnetic fields (PEMF) are in a clinical use for treatment of deficient bone lesions in order to stimulate new bone formation. The mechanism of the increased bone formation by the PEMF is attributed to the osteoblast stimulation 1 and to suppression of osteoclast bone resorption 2 by affecting, among others, osteoblast induced osteoclast stimulation. There is evidence that specific PEMFparameters are essential for stimulation of osteoblast proliferation and differentiation and they are not identical in different studies. In general basic high frequency electromagnetic field in the range of 4 - 33 kHz given in low frequency bursts of 7-15 Hz are found to be efficient in stimulating osteoblast proliferation and differentiation in vitro 1,3,4. The maximal magnetic flux in these studies was in the range of 2-30 Gauss 1,4. The mechanism of the osteoblast stimulation is based on the accumulation of cytosolic Ca+ 4, but its overall effect on cell cycle is not known. We hypothesize that osteoblast cell cycle is changed following the stimulatory PEMF. For this purpose westudied the cell cycle profile of cultured human osteoblast-like cells following exposure to a low intensity PEMF.
2. Methods
- Primary explant cultures of human osteoblast-like cells were used for the experiments. For this purpose bone chips of cancellous bone were prepared from disposable bone samples which were collected from proximal femora of six donors (three men, three women, age range 65-85 years) during fractured hip arthroplasties. No osteoarthritic changes in the femoral head were evident under direct inspection. The samples were collected from the femoral canal , at least 4 centimeters distant from subchondral area. The bone samples, 2 – 3 grams in total each, were incubated in osteogenic medium 5,6, e.g. DMEM (Dulbecco’s Modified Eagles Medium, Biological Industries Ltd) with heat-inactivated fetal calf serum (10%), 20 mM HEPES buffer, 2 mM L-glutamine, 100 μM ascorbate-2-phosphate, 10nM dexametasone, 50 U/ml penicillin, 150 μg/ml streptomycin, at 37℃ in humidified air with 5% CO2 (v:v), for 20 - 30 days. Human osteoblast-like cells grow out from the chips are adherent to the plastic tissue culture plates(non-pyrogenic polysterene). The human bone cell cultures obtained by this standard method have been shown previously to express osteoblast-like characteristics, i.e. polygonal multipolar morphology, expression of the enzyme alkaline phosphatase, synthesis of a collagen-rich extracellular matrix with predominantly type I collagen and small amounts of collagen type III and V, and non-collagenous proteins such as sialoprotein (BSP) and osteocalcin 5,6. Additionally these cells demonstrate matrix mineralization in vitro and bone formation in vivo 5,6. Furthermore, we have previously shown the osteoblast characteristics of these cells, including positive Von Kossa staining, synthesis of osteopontin, characteristic multipolar morphology and adherence to plastic surface, as well as cellular alkaline phosphatase and osteocalcin content 7. In the present study, cells were allowed to migrate from the bone chips into the medium, and proliferate in 75 cm2 culture flasks for 21 days. Then the cells were passaged into 24 well plates, where each well was seeded with 104 cells. The cells were counted by direct microscopic inspection and kept at the same conditions prior to experiment commencement. The use of these cells for the experiments was approved by the Institutional Ethical Committee. The cultures were subjected to sine shaped low frequency of 20-30 Hz and higher frequency of 5-15 kHz electromagnetic fields and to PEMF , i.e. 5-15 kHz basic frequency in pulses of 20-30 Hz (Figure 1) .
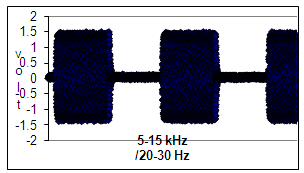 | Figure 1. Profile of a electromagnetic field with the basic frequency of 5-15 kHz in bursts of 5-15 kHz (PEMF) that was delivered to the cultured cells |
 | Figure 2. 24 well plate with cultured osteoblast-like cells is mounted on the coils that deliver controlled electromagnetic field |
3. Results
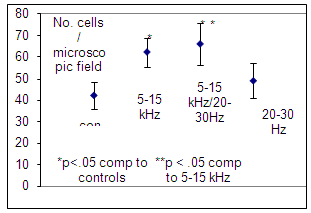
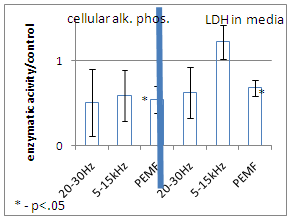
- The number of cells increased following exposure to 5-15 kHz basic electromagnetic frequency (p<0.05). Additionally when 5-15 kHz electromagnetic field was delivered in pulses of 20-30 Hz a highest increase in cell number was observed (Figure 3).Both cellular alkaline phosphatase and medium LDH activities decreased following exposure to PEMF (Figure 4).
4. Discussion
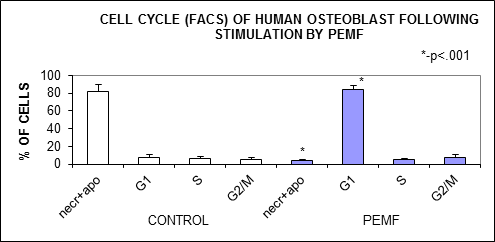
- Electromagnetic field of 5-15 kHz frequency delivered in 20-30 Hz pulses (PEMF) shifted the cultured human osteoblast-like cells toward G1 phase of the cell cycle on the expense of the proportion of necrotic and apoptotic cells. This decrease of cell death caused an increase of cell number in the culture. The increase of cell number following the PEMF shouldn't be attributed to a mitogenic activity, because the S and G2/M phases of the cell cycle were not altered by the PEMF. This observation of the decreased cell death rate following PEMF is supported by lower activity of LDH in culture media which is not observed following 20-30 Hz or 5-15 kHz electromagnetic stimulation. In parallel to the decrease of cell death PEMF caused a suppression of osteoblast maturation, which is expressed by a lower alkaline phosphatase activity. This phenomenon wasn't observed following the pure 20-30 Hz or 5-15 kHz stimulation. The presented results support the previously reported evidence that rat osteoblast-like cells respond by maturation inhibition following PEMF of 15 Gauss in pulses of 48 Hz with parallel shifting of their cell cycle from G2/M and S phases 11.A different effect was previously shown by Chang WHS et al, when mice osteoblast-like cells responded by a higher proliferation rate, without change in their maturation state, following exposure to PEMF with magnetic field of 1 Gauss12. The difference from our results can be attributed to species origin of the cells, but probably the main reason is the differences in magnetic flux in these studies, 1 vs. 10-12 Gauss, and difference in exposure times to the PEMF, i.e. 8 hours vs. 4 hours daily. This diversity in cellular response with different physical parameters of PEMF should be taken into consideration when decision on the clinically optimal PEMF is made.We found that the 5-15 kHz electromagnetic stimulation cause some increase in cell number in the culture, but this weak increase wasn’t accompanied by significant change in cellular alkaline phosphatase activity , LDH activity in the culture media and, most important, in the cell cycle profile. This indicates that the effect of 5-15 kHz electromagnetic stimulation is of marginal importance.
5. Conclusions
- We show that PEMF with a basic frequency in the kHz range delivered in pulses in the infrasonic range alters human osteoblast cell cycle by reducing cell death and cellular maturation. From these results we cautiously may suggest that by using the PEMF in vivo an increase of immature osteoblast pool in the exposed to the PEMF area might occur. The clinical significance of this phenomenon should be further investigated, especially after the cessation of the exposure to the PEMF, when subsequential unopposed by the PEMF osteoblast maturation allowing matrix elaboration, is expected.
ACKNOWLEDGEMENTS
- This study was partially supported by the Appiades Meditech Ltd .
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML