-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biomedical Engineering
p-ISSN: 2163-1050 e-ISSN: 2163-1077
2012; 2(4): 175-180
doi: 10.5923/j.ajbe.20120204.04
Co-culture Microdevice with Oxygen Gradient for Tumor Microenvironment Model and Metastasis Imaging
Takahiro Shiwa 1, Hideyuki Uchida 1, Kosuke Tsukada 1, 2
1Graduate School of Fundamental Science and Technology, Keio University, Yokohama 223-8522, Japan
2Department of Applied Physics and Physico-Informatics, Faculty of Science and Technology, Keio University Yokohama 223-8522, Japan
Correspondence to: Kosuke Tsukada , Graduate School of Fundamental Science and Technology, Keio University, Yokohama 223-8522, Japan.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Tumor hypoxia is a major therapeutic problem since it decreases radiation effects and leads to metastasis. Oxygen is delivered to tumor tissue via abnormal and dysfunctional microvessels, which forms heterogeneity of tissue oxygenation in the tumor. Mimicking the oxygen gradient for cellular experiments in vitro is important to clarify the mechanisms involved in tumor biology, but the only method to produce hypoxic conditions at a constant level is using gas-controlled incubators, because there is currently no technique for creating an oxygen gradient using culture dishes. We designed a polydimethylsiloxane (PDMS) microfluidic device integrated with microchannels for cell cultures that enables visualization of cellular distribution under a microscope and co-culture to determine interactions between cancer and other cells. Phosphorescence-based partial oxygen measurements quantified the oxygen gradient, which can be controlled by the gas pressure between the inlet and outlet of the device. A monoculture of endothelial cells with an oxygen gradient in the device showed an increase in cell death in the hypoxic area. In addition, Lewis lung carcinoma cells co-cultured with endothelial cells showed gradient-dependent migration through a membrane pore filter, indicating that the interaction between tumor and endothelial cells under hypoxia is crucial in metastasis. The results suggest that the developed microdevice can be used to study the mechanisms of tumor metastasis under hypoxic conditions.
Keywords: Oxygen Gradient, Microfluidic Device, Co-Culture, Metastasis
Article Outline
1. Introduction
- Tumor cells consume oxygen during active division, and abnormal and dysfunctional microvessels form severe hypoxic conditions in the tumors[1, 2]. Tissue hypoxia causes cells to accumulate hypoxia-inducible factor-1 alpha (HIF-1α), which is an oxygen-sensing transcriptional factor that transactivates genes encoding proteins contributing to homeostatic responses to hypoxia[3] and is implicated in tumor metastasis[4]. In addition, hypoxic conditions in tumors decrease radiation effects[5]. To clarify themechanisms of tumor growth and metastasis and the effects of radiation under hypoxia, cellular experiments in an environment with a controlled oxygen concentration are required.However, difficulty in reproducing tumor internalenvironment is a barrier to tumor research in vitro. Tumor cells are constantly incubated at 37℃ under 20% O2 as a controlor at 37℃ under 1%–5% O2 as hypoxia, which does not reproduce the conditions inside tumors. Furthermore, interactions between tumor and other types of cells, such as endothelial cells, pericytes, stromal cells, and immunocompetent cells, are important in tumor metastasis. Co-culture experiments in vitro with multiple types of cells are therefore necessary in hypoxia research. Cell culture inserts with a pore membrane are available to culture different cells in wells to examine cellular migration and cell–cell interactions[6, 7]. However, only stepwise changes between normoxia and hypoxia, which do not mimic the physiological oxygen gradient, can be applied to cells.Recent microfluidic devices for controlling themicroenvironment have been used in biomedical research. Microfluidic devices for creating a concentration gradient of a reagent and protein[8] and oxygen gradient[9, 10] have been developed, and various microdevices for cell culture to facilitate microscopic observation of cellular behavior[11, 12] and drug research[13, 14] have been reported; some devices are available for cell cultures with an oxygen gradient[15, 16]. However, the absence of a technique for co-cultures in microdevices to study interactions between cells of different types, has delayed the understanding of mechanisms that relate to tumor metastasis under hypoxic conditions.In this study, we aimed to develop a co-culture microdevice with an oxygen gradient. To validate our technique, we quantified the oxygen gradient with laser-assisted phosphorimetry and imaged endothelial cells in the device for viability assay. Finally, we examined the effect of the oxygen gradient on tumor migration by performing co-culture experiments with endothelial cells.
2. Material and Methods
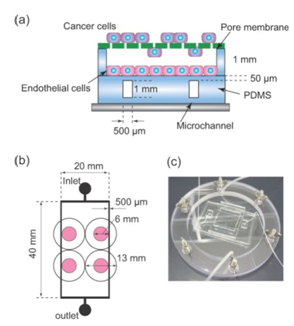
2.1. Fabricating the co-culture Microdevice
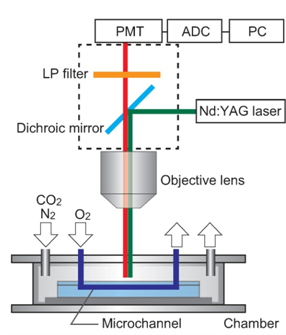
2.2. Measuring oxygen in the cell culture area
- Oxygen supplied from microchannels was diffused to the cell culture surface through PDMS. To evaluate the oxygen gradient formed on PDMS, we used laser-assisted phosphorimetry installed on a fluorescence microscope (Fig. 2). pO2 was measured by oxygen-dependent quenching of phosphorescence, as described previously[17]. In brief, Pd-meso-tetra-(4-carboxyphenyl)-porphyrin (Pd-TCPP, Frontier Scientific, Inc.) was dissolved in phosphate buffer with 5% bovine serum albumin, and the mixture was spread on PDMS. The second harmonic of a Q-switched Nd:YAG pulse laser (532-nm wavelength, 6-ns pulse width at half maximum, 1-Hz pulse recurrence frequency, 200-nJ/pulse irradiation energy) was irradiated at the measurement point through the objective lens of a microscope. Lifetime was obtained by least-squares fitting of the remaining data to a single exponential curve. pO2 was calculated using the Stern–Volmer equation,
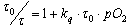 | (1) |
2.3. Cell Culture
- Mono and co-cultures can be used in our device. Lewis lung carcinomas (LLC) cells, which have a high metastatic ability, were used to visualize and quantify the distribution of tumor cells during migration, which is dependent on the oxygen gradient. LLCs were grown in RPMI media with 10% FBS, penicillin, and streptomycin. Human umbilical vein endothelial cells (HUVECs) were used for co-culture with LLCs, and were grown in EBM-2 media (Lonza Group Ltd.) with 10% FBS, penicillin, and streptomycin. To quantify the dependence of cell viability on the oxygen gradient, HUVECs were cultured in a collagen gel, rather than directly on the PDMS surface, so that the cells would adhere to the device surface while alive and could be isolated from the device surface on death. HUVECs were suspended at a cell density of 8 × 105 cells/ml in Cellmatrix (Kurabo Industries Ltd) on ice. Next, 500 µl of the suspension was spread on PDMS with a cell scraper, resulting in a final cell density of 4.4 × 104 cells/cm2. The device was incubated for 15 min for gelification, and then, 2 ml of EBM-2 was added. The device was further incubated for 12 h under the oxygen gradient.
2.4. Cancer cell migration under the oxygen gradient
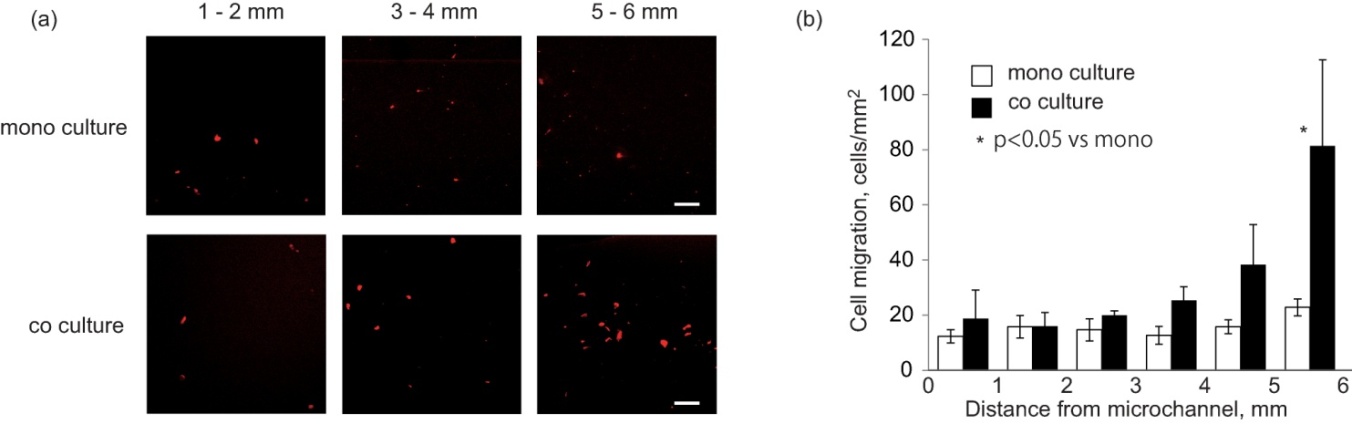
- Tumor cell migration through the pore membrane under the oxygen gradient, which implied metastasis, was quantified. A part of the pore membrane with the size of 3 µm pore was cut from a 24-well FluoroBlok (BD Falcon) and attached to a circular stainless ring (outside diameter: 16 mm, inside diameter: 6 mm). In the co-culture experiments, HUVECs were cultured on the PDMS surface coated with collagen at a density of 4.4 × 104 cells/cm2 and LLC cells were seeded at a density of 33 × 104 cells/cm2, and then incubated separately for 6 h under normoxic conditions. The ring with the membrane was installed on PDMS, and the device was incubated for 12 h under an oxygen gradient formed by the oxygen flow from microchannels. The culture medium for co-culture was a mixture of RPMI and EBM-2 in a ratio of 1:1.
2.5. Fluorescence imaging and processing
- Cells were fixed in 4% paraformaldehyde after staining with calcein-AM (Ex: 488 nm; Em: 515 nm) for living cells and propidium iodide (PI, Ex: 530 nm; Em: 615 nm) for dead cells. Fluorescent images were taken using a confocal microscope (TE 2000-U, Nikon), and the number of living and dead cells were counted offline using ImageJ software. In the experiments on migration, cells that migrated through the pore membrane were fixed and stained with PI, and the average number of cells was calculated from 10 images because the distribution of migrated cells on the membrane was non-uniform.
2.6. Statistical Analysis
- Data shown are means ± SE. The statistical significance of the results was determined using Student’s t-test. P < 0.05 was considered significant.
3. Results and Discussion
3.1. Oxygen gradient in the co-culture microdevice
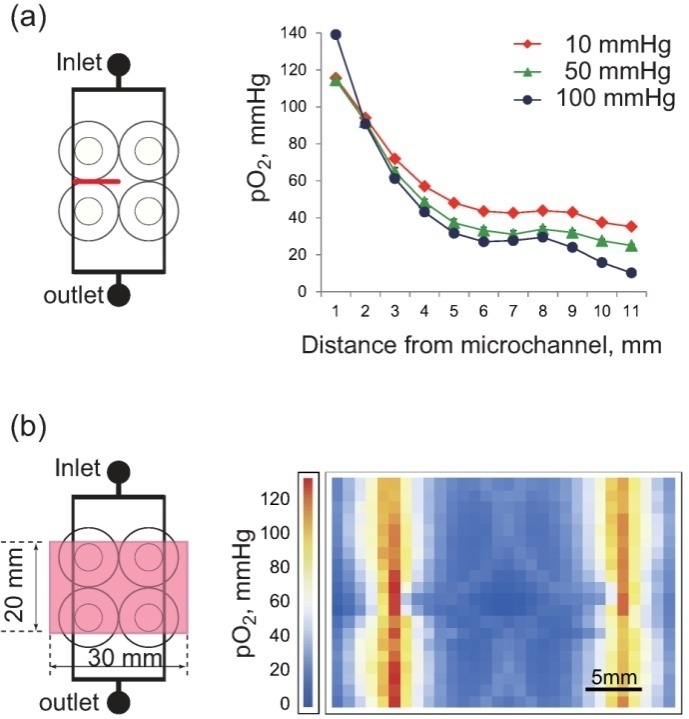
- The only oxygen supply to the co-culture microdevice was via the microchannels in PDMS, since the device was set in a chamber filled with nitrogen gas. Oxygen diffused in PDMS, forming an oxygen gradient with a maximum pO2 above the microchannels. The oxygen gradient could be controlled by changing the pressure difference of oxygen gas between the inlet and outlet. Figure 3(a) shows the measured oxygen gradient from a microchannel to the central part of the device at pressure differences of 10, 50, and 100 mmHg. pO2 peaked immediately above the microchannel and decreased exponentially 5 mm away from the channel, and then gently thereafter. The larger the difference in the pressure of the oxygen supply at the inlet and the outlet, the higher the inner pressure of the microchannel, resulting in a higher level of oxygen diffusion at the inlet than at the outlet; this in turn resulted in a steeper oxygen gradient in the device. Two-dimensional pO2 distribution on the PDMS surface at a pressure of 50 mmHg is shown in Fig. 3(b). Oxygen was diffused from the microchannels; the lowest pO2 was at the central part of the device, while the upper part of the microchannels showed no difference between the inlet and outlet.In the living body, oxygen is delivered mainly by red blood cells and diffused in microcirculation, resulting in an oxygen gradient from microvessels to tissue. In addition, the oxygen gradient depends to a large extent on vessel structure, which is specific to each organ. A major advantage of the microdevice using micro fabrication is that channels can be designed to match an organ-specific vessel structure. For instance, the lobule structure typical of the liver can be designed, producing an organ-specific oxygen gradient. Different types of theoretical models, for example, Krogh’s cylindrical model, have been used to estimate oxygen delivery to tissues. However, theoretical calculations are no match for working with actual tissue; it is extremely difficult to take into account, in vivo, the consistency of the simulation and experiments. Cellular experiments in vitro play an intermediate role between animal and theoretical experiments. However, most experiments in vitro have been performed in standard incubators, indicating that even tumor cells are cultured in about 20% O2. Most experiments in vitro may thus be unrealistic. Most recently, there has been an increase in research on regenerative medicine with ES and iPS cells, and the general consensus is that stem cells should be cultured under hypoxic conditions because a hypoxic micro-environment is critical in stem cell biology. For this reason, a microdevice with an oxygen gradient close to that of physiological tissue holds great potential as a useful tool in various biological experiments.
3.2. Cellular viability under Oxygen Gradient
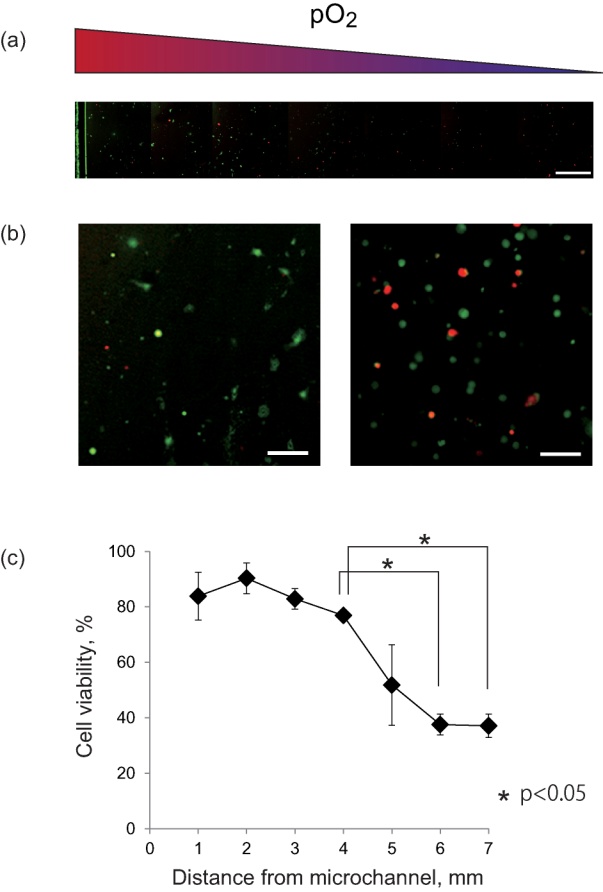
- To validate the oxygen gradient formed in the co-culture microdevice, the viability of the cultured cells was quantified. A mosaic of fluorescent images of cultured HUVECs from the microchannel to 7-mm inside at 50 mmHg of pressure is shown in Fig. 4(a). The green fluorescence of calcein-AM from living cells was dominant on the left side, while the red fluorescence of PI from the nuclei of dead cells increased with distance from the channel, indicating that viability decreased under severe hypoxia. Magnified images located within 1 and 6 mm of the channel in Fig. 4(b) showed that green fluorescence from HUVECs near the microchannels indicated normal cellular shapes, but in the hypoxic area, red fluorescence increased, and even green fluorescence-emitting cells got rounder, indicating a possible decrease in activity. In Fig. 4(c), plotting the viability of HUVECs with distance from the microchannel, a dramatic decrease in viability was indicated at a distance of 5 mm, with a further significant decrease at 6 or 7 mm. The result of Fig. 3(b) indicates that superficial pO2 on PDMS at 6 mm from the microchannel was about 30 mmHg. However, HUVECs were inside the collagen gel, and therefore, actual pO2 around the cells was assumed to be lower than 30 mmHg. These experiments demonstrated that the oxygen gradient in the microdevice affects cellular activity and that severe hypoxia cause death of endothelial cells.
3.3. Metastasis Imaging of Cancer Cells Under An Oxygen Gradient
4. Conclusions
- We developed a co-culture microdevice with an oxygen gradient to visualize cell distribution and quantify cell viability, and used the device to determine the effect of hypoxia on tumor metastasis. LLC cells co-cultured with endothelial cells showed gradient-dependent migration through a membrane pore filter, indicating that the interaction between tumor and endothelial cells under hypoxia is crucial in metastasis. The results suggest that the developed microdevice can be used to study the mechanisms of tumor metastasis under hypoxic conditions.
ACKNOWLEDGEMENTS
- Authors thank Prof. Yoshinori Matsumoto for technical assistance. This research was partially supported by the Ministry of Education, Science, Sports and Culture, Grant-in-Aid for Young Scientists (B), 2010, 22700476, and Suzuken Memorial Foundation 2010 for K.T.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML