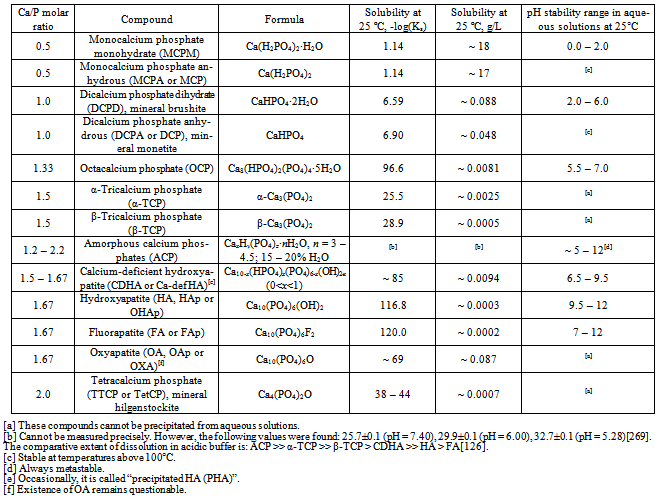
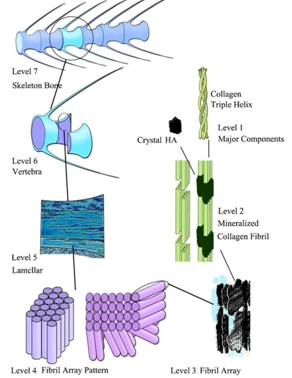
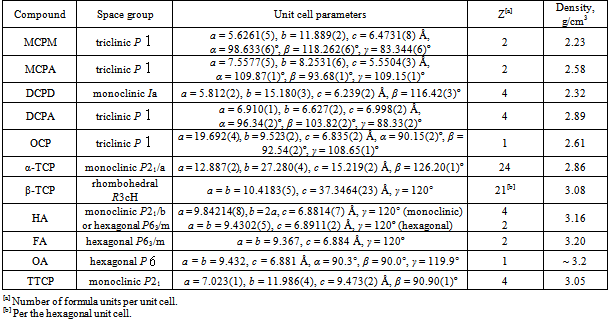
| [1] | Mann, S., Biomineralization principles and concepts in bioinorganic materials chemistry. Oxford University Press: New York, USA, 2001; pp. 216. |
| [2] | Lowenstam, H. A., and Weiner, S., On biomineralization. Oxford University Press: New York, USA, 1989; pp. 324. |
| [3] | Vallet-Regí, M., and González-Calbet, J. M., Calcium phosphates as substitution of bone tissues. Prog. Solid State Chem. 2004, 32, 1-31. |
| [4] | Weiner, S., and Addadi, L., Design strategies in mineralized biological materials. J. Mater. Chem. 1997, 7, 689-702. |
| [5] | Weiner, S., and Wagner, H. D., The material bone: structure-mechanical function relations. Ann. Rev. Mater. Sci. 1998, 28, 271-298. |
| [6] | Pasteris, J. D., Wopenka, B., and Valsami-Jones, E., Bone and tooth mineralization: why apatite? Elements 2008, 4, 97-104. |
| [7] | Giachelli, C. M. Ectopic calcification: gathering hard facts about soft tissue mineralization. Am. J. Pathol. 1999, 154, 671-675. |
| [8] | Kirsch, T. Determinants of pathological mineralization: crystal deposition diseases. Curr. Opin. Rheumatol. 2006, 18, 174-180. |
| [9] | Christian, R. C., and Fitzpatrick, L. A., Vascular calcification. Curr. Opin. Nephrol. Hypertens. 1999, 8, 443-448. |
| [10] | Boskey, A. Bone mineral crystal size. Osteoporosis Int. 2003, 14, Suppl. 5, S16-S20; discussion S20-S21. |
| [11] | Alivisatos, A. P. Enhanced naturally aligned nanocrystals. Science 2000, 289, 736-737. |
| [12] | Narayan, R. J., Kumta, P. N., Sfeir, C., Lee, D. H., Choi, D., and Olton, D., Nanostructured ceramics in medical devices: applications and prospects. JOM 2004, 56, 38-43. |
| [13] | Cai, Y., and Tang, R., Calcium phosphate nanoparticles in biomineralization and biomaterials. J. Mater. Chem. 2008, 18, 3775-3787. |
| [14] | Ginebra, M. P., Driessens, F. C. M., and Planell, J. A., Effect of the particle size on the micro and nanostructural features of a calcium phosphate cement: a kinetic analysis. Biomaterials 2004, 25, 3453-3462. |
| [15] | Nanotechnology is an application of science and engineering at the nanoscale. |
| [16] | Karch, J., Birringer, R., and Gleiter, H., Ceramics ductile at low temperature. Nature 1987, 330, 556-558. |
| [17] | Webster, T. J. Nanophase ceramics: the future of orthopedic and dental implant material. In: Nanostructured materials. Ying, J. Y. Ed., Academic Press, New York, USA, 2001; pp. 125-166. |
| [18] | Tasker, L. H., Sparey-Taylor, G. J., and Nokes, L. D., Applications of nanotechnology in orthopaedics. Clin. Orthop. Relat. Res. 2007, 456, 243-249. |
| [19] | Banfield, J. F., Welch, S. A., Zhang, H., Ebert, T. T., and Penn, R. L., Aggregation-based crystal growth and microstructure development in natural iron oxyhydroxide biomineralization products. Science 2000, 289, 751-754. |
| [20] | Cölfen, H. Bio-inspired mineralization using hydrophilic polymers. Top. Curr. Chem. 2007, 271, 1-77. |
| [21] | Oaki, Y., and Imai, H., Nanoengineering in echinoderms: the emergence of morphology from nanobricks. Small 2005, 2, 66-70. |
| [22] | Lee, S. H., and Shin, H., Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv. Drug Delivery Rev. 2007, 59, 339-359. |
| [23] | Ben-Nissan, B. Nanoceramics in biomedical applications. MRS Bulletin 2004, 29, 28-32. |
| [24] | Rehman, I. Nano bioceramics for biomedical and other applications. Mater. Technol. 2004, 19, 224-233. |
| [25] | Driessens, F. C. M., Boltong, M. G., de Maeyer, E. A. P., Wenz, R., Nies, B., and Planell, J. A., The Ca/P range of nanoapatitic calcium phosphate cements. Biomaterials 2002, 23, 4011-4017. |
| [26] | Doat, A., Fanjul, M., Pellé, F. Hollande, E., and Lebugle, A., Europium-doped bioapatite: a new photostable biological probe, internalizable by human cells. Biomaterials 2003, 24, 3365-3371. |
| [27] | Doat, A., Pellé, F., Gardant, N., and Lebugle, A., Synthesis of luminescent bioapatite nanoparticles for utilization as a biological probe. J. Solid State Chem. 2004, 177, 1179-1187. |
| [28] | Lebugle, A., Pellé, F., Charvillat, C., Rousselot, I., and Chane-Ching, J. Y., Colloidal and monocrystalline Ln3+ doped apatite calcium phosphate as biocompatible fluorescent probes. Chem. Commun. 2006, 606-608. |
| [29] | Mondejar, S. P., Kovtun, A., and Epple, M., Lanthanide-doped calcium phosphate nanoparticles with high internal crystallinity and with a shell of DNA as fluorescent probes in cell experiments. J. Mater. Chem. 2007, 17, 4153-4159. |
| [30] | Kalita, S. J., and Bhatt, H. A., Nanocrystalline hydroxyapatite doped with magnesium and zinc: synthesis and characterization. Mater. Sci. Eng. C 2007, 27, 837-848. |
| [31] | Huang, J., Jayasinghe, S. N., Best, S. M., Edirisinghe, M. J., Brooks, R. A., Rushton, N., and Bonfield, W., Novel deposition of nanosized silicon substituted hydroxyapatite by electrostatic spraying. J. Mater. Sci. Mater. Med. 2005, 16, 1137-1142. |
| [32] | Pon-On, W., Meejoo, S., and Tang, I. M., Incorporation of iron into nano hydroxyapatite particles synthesized by the microwave process. Int. J. Nanosci. 2007, 6, 9-16. |
| [33] | Predoi, D., Barsan, M., Andronescu, E., Vatasescu-Balcan, R. A., and Costache, M., Hydroxyapatite – iron oxide bioceramic prepared using nano-size powders. J. Optoelectronics Adv. Mater. 2007, 9, 3609-3613. |
| [34] | Bakunova, N. V., Fomin, A. S., Fadeeva, I. V., Barinov, S. M., and Shvorneva, L. I., Silicon-containing hydroxylapatite nanopowders. Russ. J. Inorg. Chem. 2007, 52, 1492-1497. |
| [35] | Miao, S., Weng, W., Cheng, K., Du, P., Shen, G., and Han, G., Preparation of nano-sized strontium containing tricalcium phosphate particles. Key Eng. Mater. 2007, 330-332, 263-266. |
| [36] | Liu, Y., Zhou, R., Mo, A., Chen, Z., and Wu, H., Synthesis and characterization of yttrium/hydroxyapatite nanoparticles. Key Eng. Mater. 2007, 330-332, 295-298. |
| [37] | Wu, H. C., Wang, T. W., Sun, J. S., Wang, W. H., and Lin, F. H., A novel biomagnetic nanoparticle based on hydroxyapatite. Nanotechnology 2007, 18, 165601 (9 pages). |
| [38] | Rameshbabu, N., Kumar, T. S. S., Prabhakar, T. G., Sastry, V. S., Murty, K. V. G. K., and Rao, K. P., Antibacterial nanosized silver substituted hydroxyapatite: synthesis and characterization. J. Biomed. Mater. Res. A 2007, 80A, 581-591. |
| [39] | Fujii, E., Ohkubo, M., Tsuru, K., Hayakawa, S., Osaka, A., Kawabata, K., Bonhomme, C., and Babonneau, F., Selective protein adsorption property and characterization of nano-crystalline zinc-containing hydroxyapatite. Acta Biomater. 2006, 2, 69-74. |
| [40] | Chowdhury, E. H., and Akaike, T., Fibronectin-coated nano-precipitates of calcium-magnesium phosphate for integrin-targeted gene delivery. J. Control. Release 2006, 116, e68–e69. |
| [41] | Low, H. R., Phonthammachai, N., Maignan, A., Stewart, G. A., Bastow, T. J., Ma, L. L., and White, T. J., The crystal chemistry of ferric oxyhydroxyapatite. Inorg. Chem. 2008, 47, 11774-11782. |
| [42] | Zhang, S. M., Hu, W., Zhou, W., Li, J., Liu, Y. H., and Qiu, Z. Y., Dialysis preparation of zinc-substituted nano-hydroxyapatite and its characterization. Key Eng. Mater. 2007, 330-332, 219-222. |
| [43] | Pon-On, W., Meejoo, S., and Tang, I. M., Substitution of manganese and iron into hydroxyapatite: core/shell nanoparticles. Mater. Res. Bull. 2008, 43, 2137-2144. |
| [44] | Zou, C., Weng, W., Cheng, K., Du, P., Shen, G., and Han, G., Preparation of nanosized β-tricalcium phosphate particles with Zn substitution. J. Mater. Sci. Mater. Med. 2008, 19, 1133-1136. |
| [45] | Hwang, K. S., Hwangbo, S., and Kim, J. T., Silver-doped calcium phosphate nanopowders prepared by electrostatic spraying. J. Nanoparticle Res. 2008, 10, 1337-1341. |
| [46] | Lee, D., Sfeir, C., and Kumta, P. N., Novel in-situ synthesis and characterization of nanostructured magnesium substituted β-tricalcium phosphate (β-TCMP). Mater. Sci. Eng. C 2009, 29, 69-77. |
| [47] | Petchsang, N., Pon-On, W., Hodak, J. H., and Tang, I. M., Magnetic properties of Co-ferrite-doped hydroxyapatite nanoparticles having a core/shell structure. J. Magnetism Magnetic Mater. 2009, 321, 1990-1995. |
| [48] | Hou, C. H., Hou, S. M., Hsueh, Y. S., Lin, J., Wu, H. C., and Lin, F. H., The in vivo performance of biomagnetic hydroxyapatite nanoparticles in cancer hyperthermia therapy. Biomaterials 2009, 30, 3956-3960. |
| [49] | Chen, F., Huang, P., Zhu, Y. J., Wu, J., Zhang, C. L., and Cui, D. X., The photoluminescence, drug delivery and imaging properties of multifunctional Eu3+/Gd3+ dual-doped hydroxyapatite nanorods. Biomaterials 2011, 32, 9031-9039. |
| [50] | Cacciotti, I., Bianco, A., Lombardi, M., and Montanaro, L., Mg-substituted hydroxyapatite nanopowders: synthesis, thermal stability and sintering behaviour. J. Eur. Ceram. Soc. 2009, 29, 2969-2978. |
| [51] | Bianco, A., Cacciotti, I., Lombardi, M., and Montanaro, L., Si-substituted hydroxyapatite nanopowders: synthesis, thermal stability and sinterability. Mater. Res. Bull. 2009, 44, 345-354. |
| [52] | Capuccini, C., Torricelli, P., Boanini, E., Gazzano, M., Giardino, R., and Bigi, A., Interaction of Sr-doped hydroxyapatite nanocrystals with osteoclast and osteoblast-like cells. J. Biomed. Mater. Res. A 2009, 89A, 594-600. |
| [53] | Jiang, H., Li, Y., Zuo, Y., Yang, W., Zhang, L., Li, J., Wang, L., Zou, Q., Cheng, L., and Li, J., Physical and chemical properties of superparamagnetic Fe-incorporated nano hydroxyapatite. J. Nanosci. Nanotechnol. 2009, 9, 6844-6850. |
| [54] | Al-Kattan, A., Dufour, P., Dexpert-Ghys, J., and Drouet, C., Preparation and physicochemical characteristics of luminescent apatite-based colloids. J. Phys. Chem. C 2010, 114, 2918-2924. |
| [55] | Hou, C. H., Chen, C. W., Hou, S. M., Li, Y. T., and Lin, F. H., The fabrication and characterization of dicalcium phosphate dihydrate-modified magnetic nanoparticles and their performance in hyperthermia processes in vitro. Biomaterials 2009, 30, 4700-4707. |
| [56] | Hanifi, A., Fathi, M. H., Sadeghi, H. M. M., and Varshosaz, J., Mg2+ substituted calcium phosphate nano particles synthesis for non viral gene delivery application. J. Mater. Sci. Mater. Med. 2010, 21, 2393-2401. |
| [57] | Stojanović, Z., Veselinović, L., Marković, S., Ignjatović, N., and Uskoković, D., Hydrothermal synthesis of nanosize pure and cobalt-exchanged hydroxyapatite. Mater. Manuf. Process 2009, 24, 1096-1103. |
| [58] | Veselinović, L., Karanović, L., Stojanović, Z., Bračko, I., Marković, S., Ignjatović, N., and Uskoković, D., Crystal structure of cobalt-substituted calcium hydroxyapatite nanopowders prepared by hydrothermal processing. J. Appl. Crystallogr. 2010, 43, 320-327. |
| [59] | Evis, Z., and Webster, T. J., Nanosize hydroxyapatite: doping with various ions. Adv. Appl. Ceram. 2011, 110, 311-320. |
| [60] | Al-Kattan, A., Girod-Fullana, S., Charvillat, C., Ternet-Fontebasso, H., Dufour, P., Dexpert-Ghys, J., Santran, V., Bordère, J., Pipy, B., Bernad, J., and Drouet, C., Biomimetic nanocrystalline apatites: emerging perspectives in cancer diagnosis and treatment. Int. J. Pharm. 2012, 423, 26-36. |
| [61] | Kaflak, A., and Kolodziejski, W., Complementary information on water and hydroxyl groups in nanocrystalline carbonated hydroxyapatites from TGA, NMR and IR measurements. J. Mol. Struct. 2011, 990, 263-270. |
| [62] | Kaflak, A., Ślósarczyk, A., and Kolodziejski, W., A comparative study of carbonate bands from nanocrystalline carbonated hydroxyapatites using FT-IR spectroscopy in the transmission and photoacoustic modes. J. Mol. Struct. 2011, 997, 7-14. |
| [63] | Li, Y., Widodo, J., Lim, S., and Ooi, C. P., Synthesis and cytocompatibility of manganese (II) and iron (III) substituted hydroxyapatite nanoparticles. J. Mater. Sci. 2012, 47, 754-763. |
| [64] | Li, W., and Gao, L., Fabrication of Hap-ZrO2 (3Y) nano-composite by SPS. Biomaterials 2003, 24, 937-940. |
| [65] | Wang, L., Nemoto, R., and Senna, M., Microstructure and chemical states of hydroxyapatite/silk fibroin nanocomposites synthesized via a wet-mechanochemical route. J. Nanopart. Res. 2002, 4, 535-540. |
| [66] | Nemoto, R., Wang, L., Ikoma, T., Tanaka, J., and Senna, M., Preferential alignment of hydroxyapatite crystallites in nanocomposites with chemically disintegrated silk fibroin. J. Nanopart. Res. 2004, 6, 259-265. |
| [67] | Fang, L. M., Leng, Y., and Gao, P., Processing and mechanical properties of HA/UHMWPE nanocomposites. Biomaterials 2006, 27, 3701-3707. |
| [68] | [Sugawara, A., Yamane, S., and Akiyoshi, K., Nanogel-templated mineralization: polymer-calcium phosphate hybrid nanomaterials. Macromol. Rapid Commun. 2006, 27, 441-446. |
| [69] | Pushpakanth, S., Srinivasan, B., Sreedhar, B., and Sastry, T. P., An in situ approach to prepare nanorods of titania – hydroxyapatite (TiO2 – HAp) nanocomposite by microwave hydrothermal technique. Mater. Chem. Phys. 2008, 107, 492-498. |
| [70] | Chang, M. C., Ko, C. C., and Douglas, W. H., Preparation of hydroxyapatite-gelatin nanocomposite. Biomaterials 2003, 24, 2853-2862. |
| [71] | Hao, J., Liu, Y., Zhou, S., Li, Z., and Deng, X., Investigation of nanocomposites based on semi-interpenetrating network of[L-poly(ε-caprolactone)]/[net-poly(ε-caprolactone)] and hydroxyapatite nanocrystals. Biomaterials 2003, 24, 1531-1539. |
| [72] | Deng, X. M., Hao, J. Y., and Wang, C. S., Preparation and mechanical properties of nanocomposites of poly(D,L-lactide) with Ca-deficient hydroxyapatite nanocrystals. Biomaterials 2001, 22, 2867-2873. |
| [73] | Hong, Z., Zhang, P., He, C., Qiu, X., Liu, A., Chen, L., Chena, X., and Jing, X., Nanocomposite of poly(L-lactide) and surface grafted hydroxyapatite: mechanical properties and biocompatibility. Biomaterials 2005, 26, 6296-6304. |
| [74] | Ramay, H. R. R., and Zhang, M., Biphasic calcium phosphate nanocomposite porous scaffolds for load-bearing bone tissue engineering. Biomaterials 2004, 25, 5171-5180. |
| [75] | Cross, K. J., Huq, N. L., Palamara, J. E., Perich, J. W., and Reynolds, E. C., Physicochemical characterization of casein phosphopeptide – amorphous calcium phosphate nanocomplexes. J. Biol. Chem. 2005, 280, 15362-15369. |
| [76] | Murugan, R., and Ramakrishna, S., Development of nanocomposites for bone grafting. Comp. Sci. Tech. 2005, 65, 2385-2406. |
| [77] | Liou, S. C., Chen, S. Y., and Liu, D. M., Phase development and structural characterization of calcium phosphate ceramics – polyacrylic acid nanocomposites at room temperature in water-methanol mixtures. J. Mater. Sci. Mater. Med. 2004, 15, 1261-1266. |
| [78] | Sung, Y. M., Shin, Y. K., and Ryu, J. J., Preparation of hydroxyapatite/zirconia bioceramic nanocomposites for orthopaedic and dental prosthesis applications. Nanotechnology 2007, 18, 065602 (6 pages). |
| [79] | Sreedhar, B., Aparna, Y., Sairam, M., and Hebalkar, N., Preparation and characterization of HAP / carboxymethyl chitosan nanocomposites. J. Appl. Polym. Sci. 2007, 105, 928-934. |
| [80] | Pramanik, N., Biswas, S. K., and Pramanik, P., Synthesis and characterization of hydroxyapatite/poly(vinyl alcohol phosphate) nanocomposite biomaterials. Int. J. Appl. Ceram. Technol. 2008, 5, 20-28. |
| [81] | Jevtić, M., Radulović, A., Ignjatović, N., Mitrić, M., and Uskoković, D., Controlled assembly of poly(D,L-lactide-co-glycolide)/hydroxyapatite core-shell nanospheres under ultrasonic irradiation. Acta Biomater. 2009, 5, 208-218. |
| [82] | Li, X., and Chang, J., Preparation of bone-like apatite – collagen nanocomposites by a biomimetic process with phosphorylated collagen. J. Biomed. Mater. Res. A 2008, 85A, 293-300. |
| [83] | Ohsawa, H., Ito, A., Sogo, Y., Yamazaki, A., and Ohno, T., Synthesis of albumin/DCP nano-composite particles. Key Eng. Mater. 2007, 330-332, 239-242. |
| [84] | Wilberforce, S. I., Finlayson, C. E., Best, S. M., and Cameron, R. E., The influence of the compounding process and testing conditions on the compressive mechanical properties of poly(D,L-lactide-co-glycolide)/α-tricalcium phosphate nanocomposites. J. Mech. Behav. Biomed. Mater. 2011, 4, 1081-1089. |
| [85] | Wilberforce, S. I., Finlayson, C. E., Best, S. M., and Cameron, R. E., A comparative study of the thermal and dynamic mechanical behaviour of quenched and annealed bioresorbable poly-L-lactide/α-tricalcium phosphate nanocomposites. Acta Biomater. 2011, 7, 2176-2184. |
| [86] | Degirmenbasi, N., Kalyon, D. M., and Birinci, E., Biocomposites of nanohydroxyapatite with collagen and poly(vinyl alcohol). Colloids Surf. B 2006, 48, 42-49. |
| [87] | Zhang, X., Li, Y. B., Zuo, Y., Lv, G. Y; Mu, Y. H., and Li, H., Morphology, hydrogen-bonding and crystallinity of nano-hydroxyapatite/polyamide 66 biocomposites. Composites A 2007, 38, 843-848. |
| [88] | Wei, J., Li, Y. B., and Lau, K. T., Preparation and characterization of a nano apatite/polyamide6 bioactive composite. Composites B 2007, 38, 301-305. |
| [89] | Wei, J., and Li, Y. B., Tissue engineering scaffold material of nano-apatite crystals and polyamide composite. Eur. Polym. J. 2004, 40, 509-515. |
| [90] | Rauchmann, M. A., Wichelhaus, T. A., Stirnal, V., Dingeldein, E., Zichner, L., Schnettler, R., and Alt, V., Nanocrystalline hydroxyapatite and calcium sulphate as biodegradable composite carrier material for local delivery of antibiotics in bone infections. Biomaterials 2005, 26, 2677-2684. |
| [91] | Szaraniec, B., Rosół, P., and Chłopek, J., Carbon composite material and polysulfone modified by nano-hydroxyapatite. e-Polymers 2005, no. 030. |
| [92] | Pramanik, N., Mohapatra, S., and Pramanik, P., Processing and properties of nano-hydroxyapatite (n-HAp) / poly(ethylene-co-acrylic acid) (EAA) composite using a phosphonic acid coupling agent for orthopedic applications. J. Am. Ceram. Soc. 2007, 90, 369-375. |
| [93] | Ren, Y. J., Sun, X. D., Cui, F. Z., Wei, Y. T., Cheng, Z. J., and Kong, X. D., Preparation and characterization of Antheraea pernyi silk fibroin based nanohydroxyapatite composites. J. Bioact. Compat. Polym. 2007, 22, 465-474. |
| [94] | Xu, H. H. K., Sun, L., Weir, M. D., Takagi, S., Chow, L. C., and Hockey, B., Effects of incorporating nanosized calcium phosphate particles on properties of whisker-reinforced dental composites. J. Biomed. Mater. Res. B (Appl. Biomater.) 2007, 81B, 116-125. |
| [95] | Zhou, G., Li, Y., Zhang, L., Zuo, Y., and Jansen, J. A., Preparation and characterization of nano-hydroxyapatite/chitosan/konjac glucomannan composite. J. Biomed. Mater. Res. A 2007, 83A, 931-939. |
| [96] | Liu, L., Liu, J., Wang, M., Min, S., Cai, Y., Zhu, L., and Yao, J., Preparation and characterization of nano-hydroxyapatite/silk fibroin porous scaffolds. J. Biomater. Sci. Polymer Edn. 2008, 19, 325-338. |
| [97] | Xu, F., Li, Y. B., Deng, Y., and Xiong, J., Porous nano-hydroxyapatite/poly(vinyl alcohol) composite hydrogel as artificial cornea fringe: characterization and evaluation in vitro. J. Biomater. Sci. Polymer Edn. 2008, 19, 431-439. |
| [98] | Huang, J., Lin, Y. W., Fu, X. W., Best, S. M., Brooks, R. A., Rushton, N., and Bonfield, W., Development of nanosized hydroxyapatite reinforced composites for tissue engineering scaffolds. J. Mater. Sci. Mater. Med. 2007, 18, 2151-2157. |
| [99] | Yusong, P., Dangsheng, X., and Xiaolin, C., Mechanical properties of nanohydroxyapatite reinforced poly(vinyl alcohol) gel composites as biomaterial. J. Mater. Sci. 2007, 42, 5129-5134. |
| [100] | Deng, C., Weng, J., Lu, X., Zhou, S. B., Wan, J. X., Qu, S. X., Feng, B., Li, X. H., and Cheng, Q. Y., Mechanism of ultrahigh elongation rate of poly(D,L-lactide)-matrix composite biomaterial containing nano-apatite fillers. Mater. Lett. 2008, 62, 607-610. |
| [101] | Sundaraseelan, J., and Sastry, T. P., Fabrication of a biomimetic compound containing nano hydroxyapatite – demineralised bone matrix. J. Biomed. Nanotechnol. 2007, 3, 401-405. |
| [102] | Teng, S., Chen, L., Guo, Y., and Shi, J., Formation of nano-hydroxyapatite in gelatin droplets and the resulting porous composite microspheres. J. Inorg. Biochem. 2007, 101, 686-691. |
| [103] | Meng, Y. H., Tang, C. Y., Tsui, C. P., and Chen, D. Z., Fabrication and characterization of needle-like nano-HA and HA/MWNT composites. J. Mater. Sci. Mater. Med. 2008, 19, 75-81. |
| [104] | Lin, J., Zhu, J., Gu, X., Wen, W., Li, Q., Fischer-Brandies, H., Wang, H., and Mehl, C., Effects of incorporation of nano-fluorapatite or nano-fluorohydroxyapatite on a resin-modified glass ionomer cement. Acta Biomater. 2011, 7, 1346-1353. |
| [105] | Dorozhkin, S. V., Calcium orthophosphate-based biocomposites and hybrid biomaterials. J. Mater. Sci. 2009, 44, 2343-2387. |
| [106] | Dorozhkin, S. V., Biocomposites and hybrid biomaterials based on calcium orthophosphates. Biomatter 2011, 1, 3-56. |
| [107] | Williams, D. F., The relationship between biomaterials and nanotechnology. Biomaterials 2008, 29, 1737-1738. |
| [108] | Feynman, R. P., There’s plenty of room at the bottom. J. Microelectromechanical Systems 1992, 1, 60-66. |
| [109] | European Commission, Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR). Opinion on “the scientific aspects of the existing and proposed definitions relating to products of nanoscience and nanotechnologies”. Adopted Brussels: European Commission; 29 November 2007. |
| [110] | Moriarty, P., Nanostructured materials. Rep. Prog. Phys. 2001, 64, 297-381. |
| [111] | Webster, T. J., and Ahn, E. S., Nanostructured biomaterials for tissue engineering bone. Adv. Biochem. Eng. Biotechnol. 2006, 103, 275-308. |
| [112] | Streicher, R. M., Schmidt, M., and Fiorito, S., Nanosurfaces and nanostructures for artificial orthopedic implants. Nanomedicine 2007, 2, 861-874. |
| [113] | Havancsak, K., Nanotechnology at present and its promises in the future. Mater. Sci. Forum 2003, 414-415, 85-94. |
| [114] | Duncan, R., Nanomedicines in action. Pharm. J. 2004, 273, 485-488. |
| [115] | Williams, D. F., On the nature of biomaterials. Biomaterials 2009, 30, 5897-5909. |
| [116] | Liu, H., and Webster, T. J., Nanomedicine for implants: a review of studies and necessary experimental tools. Biomaterials 2007, 28, 354-369. |
| [117] | Murugan, R., and Ramakrishna, S., Bioresorbable composite bone paste using polysaccharide based nano hydroxyapatite. Biomaterials 2004, 25, 3829-3835. |
| [118] | Murugan, R., and Ramakrishna, S., Aqueous mediated synthesis of bioresorbable nanocrystalline hydroxyapatite. J. Cryst. Growth 2005, 274, 209-213. |
| [119] | Li, G., Huang, J., Li, Y., Zhang, R., Deng, B., Zhang, J., and Aoki, H., In vitro study on influence of a discrete nano-hydroxyapatite on leukemia P388 cell behavior. Biomed. Mater. Eng. 2007, 17, 321-327. |
| [120] | Ganesan, K., Kovtun, A., Neumann, S., Heumann, R., and Epple, M., Calcium phosphate nanoparticles: colloidally stabilized and made fluorescent by a phosphate-functionalized porphyrin. J. Mater. Chem. 2008, 18, 3655-3661. |
| [121] | Kim, H. W., and Kim, H. E., Nanofiber generation of hydroxyapatite and fluor-hydroxyapatite bioceramics. J. Biomed. Mater. Res. B (Appl. Biomater.) 2005, 77B, 323-328. |
| [122] | Cihlar, J., and Castkova, K., Direct synthesis of nanocrystalline hydroxyapatite by hydrothermal hydrolysis of alkylphosphates. Monatshefte für Chemie 2002, 133, 761-771. |
| [123] | Lak, A., Mazloumi, M., Mohajerani, M., Kajbafvala, A., Zanganeh, S., Arami, H., and Sadrnezhaad, S. K., Self-assembly of dandelion-like hydroxyapatite nanostructures via hydrothermal method. J. Am. Ceram. Soc. 2008, 91, 3292-3297. |
| [124] | Mukesh, U., Kulkarni, V., Tushar, R., and Murthy, R. S. R., Methotrexate loaded self stabilized calcium phosphate nanoparticles: a novel inorganic carrier for intracellular drug delivery. J. Biomed. Nanotechnol. 2009, 5, 99-105. |
| [125] | Sun, L., Chow, L. C., Frukhtbeyn, S. A., and Bonevich, J. E., Preparation and properties of nanoparticles of calcium phosphates with various Ca/P ratios. J. Res. Natl. Inst. Stand. Technol. 2010, 115, 243-255. |
| [126] | Sylvie, J., Sylvie, T. D., Pascal, P. M., Fabienne, P., Hassane, O., and Guy, C., Effect of hydroxyapatite and β-tricalcium phosphate nanoparticles on promonocytic U937 cells. J. Biomed. Nanotechnol. 2010, 6, 158-165. |
| [127] | Sokolova, V., Knuschke, T., Kovtun, A., Buer, J., Epple, M., and Westendorf, A. M., The use of calcium phosphate nanoparticles encapsulating Toll-like receptor ligands and the antigen hemagglutinin to induce dendritic cell maturation and T cell activation. Biomaterials. 2010, 31, 5627-5633. |
| [128] | Wu, H. C., Wang, T. W., Bohn, M. C., Lin. F. H., and Spector, M., Novel magnetic hydroxyapatite nanoparticles as non-viral vectors for the glial cell line-derived neurotrophic factor gene. Adv. Funct. Mater. 2010, 20, 67-77. |
| [129] | Gergely, G., Wéber, F., Lukács, I., Illés, L., Tóth, A. L., Horváth, Z. E., Mihály, J., and Balázsi, C., Nano-hydroxyapatite preparation from biogenic raw materials. Cent. Eur. J. Chem. 2010, 8, 375-381. |
| [130] | Ergun, C., Evis, Z., Webster, T. J., and Sahin, F. C., Synthesis and microstructural characterization of nano-size calcium phosphates with different stoichiometry. Ceram. Int. 2011, 37, 971-977. |
| [131] | Ge, X., Leng, Y., Ren, F., and Lu, X., Integrity and zeta potential of fluoridated hydroxyapatite nanothick coatings for biomedical applications. J. Mech. Behav. Biomed. Mater. 2011, 4, 1046-1056. |
| [132] | Wang, J., Chen, X., Yang, X., Xu, S., Zhang, X., and Gou, Z., A facile pollutant-free approach toward a series of nutritionally effective calcium phosphate nanomaterials for food and drink additives. J. Nanopart. Res. 2011, 13, 1039-1048. |
| [133] | Sokolova, V., Knuschke, T., Buer, J., Westendorf, A. M., and Epple, M., Quantitative determination of the composition of multi-shell calcium phosphate-oligonucleotide nanoparticles and their application for the activation of dendritic cells. Acta Biomater. 2011, 7, 4029-4036. |
| [134] | Traykova, T., Aparicio, C., Ginebra, M. P., and Planell, J. A., Bioceramics as nanomaterials. Nanomedicine 2006, 1, 91-106. |
| [135] | Grainger, D. W., and Castner, D. G., Nanobiomaterials and nanoanalysis: opportunities for improving the science to benefit biomedical technologies. Adv. Mater. 2008, 20, 867-877. |
| [136] | Nelson, K. G., The Kelvin equation and solubility of small particles. J. Pharmac. Sci. 1972, 61, 479-480. |
| [137] | Fan, C., Chen, J., Chen, Y., Ji, J., and Teng, H. H., Relationship between solubility and solubility product: the roles of crystal sizes and crystallographic directions. Geochim. Cosmochim. Acta 2006, 70, 3820-3829. |
| [138] | Sato, M., and Webster, T. J., Nanobiotechnology: implications for the future of nanotechnology in orthopedic applications. Expert Rev. Med. Dev. 2004, 1, 105-114. |
| [139] | Hahn, H., Unique features and properties of nanostructured materials. Adv. Eng. Mater. 2003, 5, 277-284. |
| [140] | Aronov, D., Karlov, A., and Rosenman, G., Hydroxyapatite nanoceramics: basic physical properties and biointerface modification. J. Eur. Ceram. Soc. 2007, 27, 4181-4186. |
| [141] | Ramsden, J. J., and Freeman, J., The nanoscale. Nanotechnol. Percept. 2009, 5, 3-25. |
| [142] | Rempel, A. A., Nanotechnologies. Properties and applications of nanostructured materials. Russ. Chem. Rev. 2007, 76, 435-461. |
| [143] | Catledge, S. A., Fries, M. D., Vohra, Y. K., Lacefield, W. R., Lemons, J. E., Woodard, S., and Venugopalan, R., Nanostructured ceramics for biomedical implants. J. Nanosci. Nanotechnol. 2002, 2, 1-20. |
| [144] | Balasundarama, G., and Webster, T. J., A perspective on nanophase materials for orthopedic implant applications. J. Mater. Chem. 2006, 16, 3737-3745. |
| [145] | Balasundarama, G., and Webster, T. J., Nanotechnology and biomaterials for orthopedic medical applications. Nanomedicine 2006, 1, 169-176. |
| [146] | Padilla, S., Izquierdo-Barba, I., and Vallet-Regí, M., High specific surface area in nanometric carbonated hydroxyapatite. Chem. Mater. 2008, 20, 5942-5944. |
| [147] | Kalita, S. J., Bhardwaj, A., and Bhatt, H. A., Nanocrystalline calcium phosphate ceramics in biomedical engineering. Mater. Sci. Eng. C 2007, 27, 441-449. |
| [148] | LeGeros, R. Z., Calcium phosphates in oral biology and medicine. Karger: Basel, Switzerland, 1991; pp. 210. |
| [149] | Mann, S., The study of biominerals by high resolution transmission electron microscopy. Scan. Electron. Microsc. 1986, Pt. 2, 393-413. |
| [150] | Katsura, N., Nanospace theory for biomineralization. Dent. Jpn. (Tokyo) 1990, 27, 57-63. |
| [151] | Cuisinier, F. J. G., Voegel, J. C., Yacaman, J., and Frank, R. M., Structure of initial crystals formed during human amelogenesis. J. Cryst. Growth 1992, 116, 314-318. |
| [152] | Cuisinier, F. J. G., Steuer, P., Senger, B., Voegel, J. C., and Frank, R. M., Human amelogenesis: high resolution electron microscopy of nanometer-sized particles. Cell Tissue Res. 1993, 273, 175-182. |
| [153] | Brès, E. F., Moebus, G., Kleebe, H. J., Pourroy, G., Werkmann, J., and Ehret, G., High resolution electron microscopy study of amorphous calcium phosphate. J. Cryst. Growth 1993, 129, 149-162. |
| [154] | Layrolle, P., and Lebugle, A., Characterization and reactivity of nanosized calcium phosphate prepared in anhydrous ethanol. Chem. Mater. 1994, 6, 1996-2004. |
| [155] | Cui, F. Z., Wen, H. B., Zhang, H. B., Ma, C. L., and Li, H. D., Nanophase hydroxyapatite-like crystallites in natural ivory. J. Mater. Sci. Lett. 1994, 13, 1042-1044. |
| [156] | Li, Y. B., de Wijn, J., Klein, C. P. A. T., de Meer, S. V., and de Groot, K., Preparation and characterization of nanograde osteoapatite-like rod crystals. J. Mater. Sci. Mater. Med. 1994, 5, 252-255. |
| [157] | Li, Y. B., de Groot, K., de Wijn, J., Klein, C. P. A. T., and de Meer, S. V., Morphology and composition of nanograde calcium phosphate needle-like crystals formed by simple hydrothermal treatment. J. Mater. Sci. Mater. Med. 1994, 5, 326-331. |
| [158] | Shirkhanzadeh, M., X-ray diffraction and Fourier transform infrared analysis of nanophase apatite coatings prepared by electrocrystallization. Nanostruct. Mater. 1994, 4, 677-684. |
| [159] | Webster, T. J., Ergun, C., Doremus, R. H., Siegel, R. W., and Bizios, R., Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. J. Biomed. Mater. Res. 2000, 51, 475-483. |
| [160] | Chan, C. K., Kumar, T. S. S., Liao, S., Murugan, R., Ngiam, M., and Ramakrishnan, S., Biomimetic nanocomposites for bone graft applications. Nanomedicine 2006, 1, 177-188. |
| [161] | Okada, M., Furukawa, K., Serizawa, T., Yanagisawa, Y., Tanaka, H., Kawai, T., and Furuzono, T., Interfacial interactions between calcined hydroxyapatite nanocrystals and substrates. Langmuir 2009, 25, 6300-6306. |
| [162] | Mikołajczyk, T., Rabiej, S., and Bogun, M., Analysis of the structural parameters of polyacrylonitrile fibers containing nanohydroxyapatite. J. Appl. Polym. Sci. 2006, 101, 760-765. |
| [163] | Wilberforce, S. I. J., Finlayson, C. E., Best, S. M., and Cameron, R. E., The influence of hydroxyapatite (HA) microparticles (m) and nanoparticles (n) on the thermal and dynamic mechanical properties of poly-L-lactide. Polymer 2011, 52, 2883-2890. |
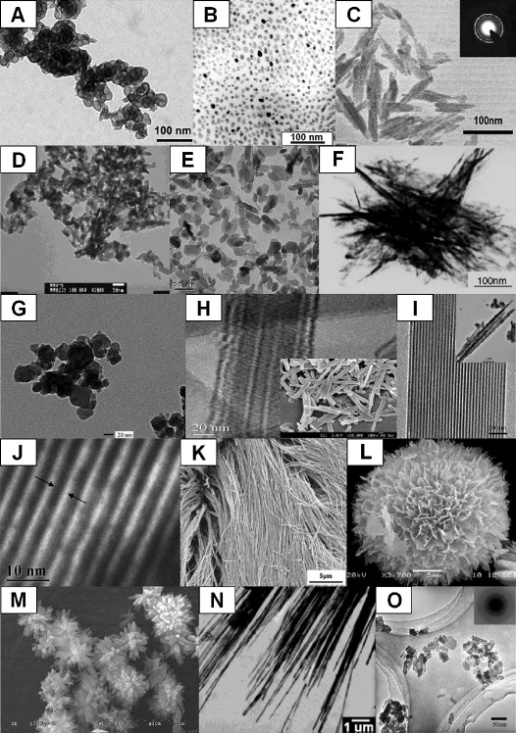
| [164] | There are both nano-sized biomaterials and nanostructured biomaterials, which should be differentiated from each other. Nano-sized biomaterials refer to individual molecular level biomaterials such as single proteins (are not considered in this review), while nanostructured biomaterials refer to any biomaterials whose structure or morphology can be engineered to get features with nanometer-scale dimensions[165]. This review is limited to calcium orthophosphate-based nanostructured biomaterials only. |
| [165] | Thomas, V., Dean, D. R., and Vohra, Y. K., Nanostructured biomaterials for regenerative medicine. Curr. Nanosci. 2006, 2, 155-177. |
| [166] | LeGeros, R. Z., Biodegradation and bioresorption of calcium phosphate ceramics. Clin. Mater. 1993, 14, 65-88. |
| [167] | Wang, J., and Shaw, L. L., Morphology-enhanced low-temperature sintering of nanocrystalline hydroxyapatite. Adv. Mater. 2007, 19, 2364-2369. |
| [168] | Fomin, A. S., Barinov, S. M., Ievlev, V. M., Smirnov, V. V., Mikhailov, B. P., Belonogov, E. K., and Drozdova, N. A., Nanocrystalline hydroxyapatite ceramics produced by low-temperature sintering after high-pressure treatment. Dokl. Chem. 2008, 418, 22-25. |
| [169] | Drouet, C., Bosc, F., Banu, M., Largeot, C., Combes, C., Dechambre, G., Estournes, C., Raimbeaux, G., and Rey, C., Nanocrystalline apatites: from powders to biomaterials. Powder Technol. 2009, 190, 118-122. |
| [170] | Ramesh, S., Tan, C. Y., Bhaduri, S. B., Teng, W. D., and Sopyan, I., Densification behaviour of nanocrystalline hydroxyapatite bioceramics. J. Mater. Process. Technol. 2008, 206, 221-230. |
| [171] | Skorokhod, V. V., Solonin, S. M., Dubok, V. A., Kolomiets, L. L., Katashinskii, V. P., and Shinkaruk, A. V., Pressing and sintering of nanosized hydroxyapatite powders. Powder Metall. Metal Ceram. 2008, 47, 518-524. |
| [172] | Sung, Y. M., Lee, J. C., and Yang, J. W., Crystallization and sintering characteristics of chemically precipitated hydroxyapatite nanopowder. J. Cryst. Growth 2004, 262, 467-472. |
| [173] | Lin, K., Chang, J., Lu, J., Wu, W., and Zeng, Y., Properties of β-Ca3(PO4)2 bioceramics prepared using nanosized powders. Ceram. Int. 2007, 33, 979-985. |
| [174] | Tanaka, Y., Hirata, Y., and Yoshinaka, R., Synthesis and characteristics of ultra-fine hydroxyapatite particles. J. Ceram. Proc. Res. 2003, 4, 197-201. |
| [175] | Wang, J., and Shaw, L. L., Nanocrystalline hydroxyapatite with simultaneous enhancements in hardness and toughness. Biomaterials 2009, 30, 6565-6572. |
| [176] | Stupp, S. I., and Ciegler, G. W., Organoapatites: materials for artificial bone. I. Synthesis and microstructure. J. Biomed. Mater. Res. 1992, 26, 169-183. |
| [177] | Webster, T. J., Ergun, C., Doremus, R. H., Siegel, R. W., and Bizios, R., Enhanced osteoclast-like cell functions on nanophase ceramics. Biomaterials 2001, 22, 1327-1333. |
| [178] | Huang, J., Best, S. M., Bonfield, W., Brooks, R. A., Rushton, N., Jayasinghe, S. N., and Edirisinghe, M. J., In vitro assessment of the biological response to nanosized hydroxyapatite. J. Mater. Sci. Mater. Med. 2004, 15, 441-445. |
| [179] | Kim, H. W., Kim, H. E., and Salih, V., Stimulation of osteoblast responses to biomimetic nanocomposites of gelatin-hydroxyapatite for tissue engineering scaffolds. Biomaterials 2005, 26, 5221-5230. |
| [180] | Webster, T. J., Siegel, R. W., and Bizios, R., Osteoblast adhesion on nanophase ceramics. Biomaterials 1999, 20, 1221-1227. |
| [181] | Webster, T. J., Ergun, C., Doremus, R. H., Siegel, R. W., and Bizios, R., Enhanced functions of osteoblast on nanophase ceramics. Biomaterials 2000, 21, 1803-1810. |
| [182] | Smith, I. O., McCabe, L. R., and Baumann, M. J., MC3T3-E1 osteoblast attachment and proliferation on porous hydroxyapatite scaffolds fabricated with nanophase powder. Int. J. Nanomed. 2006, 1, 189-194. |
| [183] | Nelson, M., Balasundaram, G., and Webster, T. J., Increased osteoblast adhesion on nanoparticulate crystalline hydroxyapatite functionalized with KRSR. Int. J. Nanomed. 2006, 1, 339-349. |
| [184] | Liu, H., Yazici, H., Ergun, C., Webster, T. J., and Bermek, H., An in vitro evaluation of the Ca/P ratio for the cytocompatibility of nano-to-micron particulate calcium phosphates for bone regeneration. Acta Biomater. 2008, 4, 1472-1479. |
| [185] | Sato, M., Sambito, M. A., Aslani, A., Kalkhoran, N. M., Slamovich, E. B., and Webster, T. J., Increased osteoblast functions on undoped and yttrium-doped nanocrystalline hydroxyapatite coatings on titanium. Biomaterials 2006, 27, 2358-2369. |
| [186] | Thian, E. S., Huang, J., Best, S. M. Barber, Z. H., Brooks, R. A., Rushton, N., and Bonfield, W., The response of osteoblasts to nanocrystalline silicon-substituted hydroxyapatite thin films. Biomaterials 2006, 27, 2692-2698. |
| [187] | Palin, E., Liu, H., and Webster, T. J., Mimicking the nanofeatures of bone increases bone-forming cell adhesion and proliferation. Nanotechnology 2005, 16, 1828-1835. |
| [188] | Sun, W., Chu, C., Wang, J., and Zhao, H., Comparison of periodontal ligament cells responses to dense and nanophase hydroxyapatite. J. Mater. Sci. Mater. Med. 2007, 18, 677-683. |
| [189] | Ergun, C., Liu, H., Webster, T. J., Olcay, E., Yılmaz, Ş., and Sahin, F. C., Increased osteoblast adhesion on nanoparticulate calcium phosphates with higher Ca/P ratios. J. Biomed. Mater. Res. A 2008, 85A, 236-241. |
| [190] | Lewandrowski, K. U., Bondre, S. P., Wise, D. L., and Trantolo, D. J., Enhanced bioactivity of a poly(propylene fumarate) bone graft substitute by augmentation with nano-hydroxyapatite. Biomed. Mater. Eng. 2003, 13, 115-124. |
| [191] | Zhou, D. S., Zhao, K. B., Li, Y., Cui, F. Z., and Lee, I. S., Repair of segmental defects with nano-hydroxyapatite / collagen / PLA composite combined with mesenchymal stem cells. J. Bioactive Compat. Polym. 2006, 21, 373-384. |
| [192] | Khanna, R., Katti, K. S., and Katti, D. R., Bone nodules on chitosan-polygalacturonic acid-hydroxyapatite nanocomposite films mimic hierarchy of natural bone. Acta Biomater. 2011, 7, 1173-1183. |
| [193] | Xu, Z., Sun, J., Changsheng, L., and Jie, W., Effect of hydroxyapatite nanoparticles of different concentrations on rat osteoblast. Mater. Sci. Forum 2009, 610-613, 1364-1369. |
| [194] | Okada, S., Nagai, A., Oaki, Y., Komotori, J., and Imai, H., Control of cellular activity of fibroblasts on size-tuned fibrous hydroxyapatite nanocrystals. Acta Biomater. 2011, 7, 1290-1297. |
| [195] | Krut’ko, V. K., Kulak, A. I., Lesnikovich, L. A., Trofimova, I. V., Musskaya, O. N., Zhavnerko, G. K., and Paribok, I. V., Influence of the dehydration procedure on the physicochemical properties of nanocrystalline hydroxylapatite xerogel. Russ. J. General Chem. 2007, 77, 336-342. |
| [196] | Severin, A. V., Komarov, V. F., Bozhevol’nov, V. E., and Melikhov, I. V., Morphological selection in suspensions of nanocrystalline hydroxylapatite leading to spheroidal aggregates. Russ. J. Inorg. Chem. 2005, 50, 72-77. |
| [197] | Biggemann, D., da Silva, M. H. P., Rossi, A. M., and Ramirez, A. J., High-resolution transmission electron microscopy study of nanostructured hydroxyapatite. Microsc. Microanal. 2008, 14, 433-438. |
| [198] | Hagmeyer, D., Ganesan, K., Ruesing, J., Schunk, D., Mayer, C., Dey, A., Sommerdijk, N. A. J. M., and Epple, M., Self-assembly of calcium phosphate nanoparticles into hollow spheres induced by dissolved amino acids. J. Mater. Chem. 2011, 21, 9219-9223. |
| [199] | Kester, M., Heakal, Y., Fox, T., Sharma, A., Robertson, G. P., Morgan, T. T., Altinoğlu, E. I., Tabaković, A., Parette, M. R., Rouse, S. M., Ruiz-Velasco, V., and Adair, J. H., Calcium phosphate nanocomposite particles for in vitro imaging and encapsulated chemotherapeutic drug delivery to cancer cells. Nano Lett. 2008, 8, 4116-4121. |
| [200] | Welzel, T., Meyer-Zaika, W., and Epple, M., Continuous preparation of functionalised calcium phosphate nanoparticles with adjustable crystallinity. Chem. Commun. 2004, 1204-1205. |
| [201] | Nichols, H. L., Zhang, N., Zhang, J., Shi, D., Bhaduri, S., and Wen, X., Coating nanothickness degradable films on nanocrystalline hydroxyapatite particles to improve the bonding strength between nanohydroxyapatite and degradable polymer matrix. J. Biomed. Mater. Res. A 2007, 82A, 373-382. |
| [202] | Bouladjine, A., Al-Kattan, A., Dufour, P., and Drouet, C., New advances in nanocrystalline apatite colloids intended for cellular drug delivery. Langmuir 2009, 25, 12256-12265. |
| [203] | Rey, C., Hina, A., Tofighi, A., and Glimcher, M. J., Maturation of poorly crystalline apatites: chemical and structural aspects in vivo and in vitro. Cell Mater. 1995, 5, 345-356. |
| [204] | Dorozhkin, S. V., Calcium orthophosphates. J. Mater. Sci. 2007, 42, 1061-1095. |
| [205] | Dorozhkin, S. V., Calcium orthophosphates in nature, biology and medicine. Materials 2009, 2, 399-498. |
| [206] | Elliott, J. C., Structure and chemistry of the apatites and other calcium orthophosphates; Elsevier: Amsterdam, Holland, 1994; pp. 404. |
| [207] | Olszta, M. J., Cheng, X., Jee, S. S., Kumar, R., Kim, Y. Y., Kaufmane, M. J., Douglas, E. P., and Gower, L. B., Bone structure and formation: a new perspective. Mater. Sci. Eng. R 2007, 58, 77-116. |
| [208] | Cui, F. Z., Li, Y., and Ge, J., Self-assembly of mineralized collagen composites. Mater. Sci. Eng. R 2007, 57, 1-27. |
| [209] | Meyers, M. A., Chen, P. Y., Lin, A. Y. M., and Seki, Y., Biological materials: structure and mechanical properties. Prog. Mater. Sci. 2008, 53, 1-206. |
| [210] | Currey, J. D., Hierarchies in biomineral structures. Science 2005, 309, 253-254. |
| [211] | Rubin, M. A., Jasiuk, I., Taylor, J., Rubin, J., Ganey, T., and Apkarian, R. P., TEM analysis of the nanostructure of normal and osteoporotic human trabecular bone. Bone 2003, 33, 270-282. |
| [212] | Hartgerink, J. D., Beniash, E., and Stupp, S. I., Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 2001, 294, 1684-1688. |
| [213] | Ji, B., and Gao, H., Elastic properties of nanocomposite structure of bone. Compos. Sci. Technol. 2006, 66, 1212-1218. |
| [214] | Wang, L., Nancollas, G. H., Henneman, Z. J., Klein, E., and Weiner, S., Nanosized particles in bone and dissolution insensitivity of bone mineral. Biointerphases 2006, 1, 106-111. |
| [215] | Xie, B., and Nancollas, G. H., How to control the size and morphology of apatite nanocrystals in bone. Proc. Natl. Acad. Sci. USA 2011, 107, 22369-22370. |
| [216] | Hu, Y. Y., Rawal, A., and Schmidt-Rohr, K., Strongly bound citrate stabilizes the apatite nanocrystals in bone. Proc. Natl. Acad. Sci. USA 2011, 107, 22425-22429. |
| [217] | Gao, H., Ji, B., Jager, I. L., Arz, E., and Fratzl, P., Materials become insensitive to flaws at nanoscale: lessons from nature. Proc. Natl. Acad. Sci. USA 2003, 100, 5597-5660. |
| [218] | Gupta, H. S., Seto, J., Wagermaier, W., Zaslansky, P., Boesecke, P., and Fratzl, P., Cooperative deformation of mineral and collagen in bone at the nanoscale. Proc. Natl. Acad. Sci. USA. 2006, 103, 17741-17746. |
| [219] | Currey, J. D., Bones: structure and mechanics. Princeton University Press: Princeton, USA, 2006; pp. 456. |
| [220] | Porter, A. E., Nalla, R. K., Minor, A., Jinschek, J. R., Kisielowski, C., Radmilovic, V., Kinney, J. H., Tomsia, A. P., and Ritchie, R. O., A transmission electron microscopy study of mineralization in age-induced transparent dentin. Biomaterials 2005, 26, 7650-7660. |
| [221] | Kirkham, J., Brookes, S. J., Shore, R. C., Wood, S. R., Smith, D. A., Zhang, J., Chen, H., and Robinson, C., Physico-chemical properties of crystal surfaces in matrix-mineral interactions during mammalian biomineralisation. Curr. Opin. Colloid Interf. Sci. 2002, 7, 124-132. |
| [222] | Daculsi, G., Mentanteau, J., Kerebel, L. M., and Mitre, D., Length and shape of enamel crystals. Calcif. Tissue Int. 1984, 36, 550-555. |
| [223] | Robinson, C., Connell, S., Kirkham, J., Shorea, R., and Smith, A., Dental enamel – a biological ceramic: regular substructures in enamel hydroxyapatite crystals revealed by atomic force microscopy. J. Mater. Chem. 2004, 14, 2242-2248. |
| [224] | Chen, H., Tang, Z., Liu, J., Sun, K., Chang, S. R., Peters, M. C., Mansfield, J. F., Czajka-Jakubowska, A., and Clarkson, B. H., Acellular synthesis of a human enamel-like microstructure. Adv. Mater. 2006, 18, 1846-1851. |
| [225] | Chen, H., Clarkson, B. H., Sun, K., and Mansfield, J. F., Self-assembly of synthetic hydroxyapatite nanorods into an enamel prism-like structure. J. Colloid Interf. Sci. 2005, 288, 97-103. |
| [226] | Robinson, C., Self-oriented assembly of nano-apatite particles: a subunit mechanism for building biological mineral crystals. J. Dental Res. 2007, 86, 677-679. |
| [227] | Cui, F. Z., and Ge, J., New observations of the hierarchical structure of human enamel, from nanoscale to microscale. J. Tissue Eng. Regen. Med. 2007, 1, 185-191. |
| [228] | He, L. H., and Swain, M. V., Enamel – a “metallic-like” deformable biocomposite. J. Dent. 2007, 35, 431-437. |
| [229] | Nelson, S. J., Wheeler’s dental anatomy, physiology and occlusion. 9th Ed., W. B. Saunders: Philadelphia, USA. 2009; pp. 368. |
| [230] | Suvorova E. I., and Buffat P. A., Electron diffraction from micro- and nanoparticles of hydroxyapatite. J. Microscopy 1999, 196, 46-58. |
| [231] | Panda, R. N., Hsieh, M. F., Chung, R. J., and Chin, T. S., X-ray diffractometry and X-ray photoelectron spectroscopy investigations of nanocrytalline hydroxyapatite synthesized by a hydroxide gel technique. Jpn. J. Appl. Phys. 2001, 40, 5030-5035. |
| [232] | Panda, R. N., Hsieh, M. F., Chung, R. J., and Chin, T. S., FTIR, XRD, SEM and solid state NMR investigations of carbonate-containing hydroxyapatite nano-particles synthesized by hydroxide-gel technique. J. Phys. Chem. Solids 2003, 64, 193-199. |
| [233] | Eichert, D. Sfihi, H., Combes, C., and Rey, C., Specific characteristics of wet nanocrystalline apatites. Consequences on biomaterials and bone tissue. Key Eng. Mater. 2004, 254-256, 927-930. |
| [234] | Rey, C., Combes, C., Drouet, C., Sfihi, H., and Barroug, A., Physico-chemical properties of nanocrystalline apatites: implications for biominerals and biomaterials. Mater. Sci. Eng. C 2007, 27, 198-205. |
| [235] | Eichert, D., Drouet, C., Sfihi, H., Rey, C., and Combes, C., Nanocrystalline apatite-based biomaterials: synthesis, processing and characterization. In: Biomaterials research advances. Kendall J. B. Ed., Nova Science Publishers, Inc., USA, 2007; Chapter 5, pp. 93-143. |
| [236] | Aronov, D., and Rosenman, G., Trap state spectroscopy studies and wettability modification of hydroxyapatite nanobioceramics. J. Appl. Phys. 2007, 101, 034701 (5 pages). |
| [237] | Jäger, C., Welzel, T., Meyer-Zaika, W., and Epple, M., A solid-state NMR investigation of the structure of nanocrystalline hydroxyapatite. Magn. Reson. Chem. 2006, 44, 573-580. |
| [238] | Isobe, T., Nakamura, S., Nemoto, R., Senna, M., and Sfihi, H., Solid-state double nuclear magnetic resonance of calcium phosphate nanoparticules synthesized by wet-mechanochemical reaction. J. Phys. Chem. B 2002, 106, 5169-5176. |
| [239] | Bertinetti, L., Tampieri, A., Landi, E., Ducati, C., Midgley, P. A., Coluccia, S., and Martra, G., Surface structure, hydration, and cationic sites of nanohydroxyapatite: UHR-TEM, IR, and microgravimetric studies. J. Phys. Chem. C 2007, 111, 4027-4035. |
| [240] | Bertinetti, L., Tampieri, A., Landi, E., Bolis, V., Busco, C., and Martra, G., Surface structure, hydration and cationic sites of nanohydroxyapatite. Key Eng. Mater. 2008, 361-363, 87-90. |
| [241] | Bertinetti, L., Drouet, C., Combes, C., Rey, C., Tampieri, A., Coluccia, S., and Martra, G., Surface characteristics of nanocrystalline apatites: effect of Mg surface enrichment on morphology, surface hydration species, and cationic environments. Langmuir 2009, 25, 5647-5654. |
| [242] | Gopi, D., Indira, J., Prakash, V. C. A., and Kavitha, L., Spectroscopic characterization of porous nanohydroxyapatite synthesized by a novel amino acid soft solution freezing method. Spectrochim. Acta A 2009, 74A, 282-284. |
| [243] | Rossi, A. M., da Silva, M. H. P., Ramirez, A. J., Biggemann, D., Caraballo, M. M., Mascarenhas, Y. P., Eon, J. G., and Moure, G. T., Structural properties of hydroxyapatite with particle size less than 10 nanometers. Key Eng. Mater. 2007, 330-332, 255-258. |
| [244] | Ramirez, C. A. O., Costa, A. M., Bettini, J., Ramirez, A. J., da Silva, M. H. P., and Rossi, A. M., Structural properties of nanostructured carbonate apatites. Key Eng. Mater. 2009, 396-398, 611-614. |
| [245] | Pasteris, J. D., Wopenka, B., Freeman, J. J., Rogers, K., Valsami-Jones, E., van der Houten, J. A. M., and Silva, M. J., Lack of OH in nanocrystalline apatite as a function of degree of atomic order: implications for bone and biomaterials. Biomaterials 2004, 25, 229-238. |
| [246] | Sakhno, Y., Bertinetti, L., Iafisco, M., Tampieri, A., Roveri, N., and Martra, G., Surface hydration and cationic sites of nanohydroxyapatites with amorphous or crystalline surfaces: a comparative study. J. Phys. Chem. C 2010, 114, 16640-16648. |
| [247] | Zyman, Z. Z., Epple, M., Rokhmistrov, D., and Glushko, V., On impurities and the internal structure in precipitates occurring during the precipitation of nanocrystalline calcium phosphate. Mat. -Wiss. u. Werkstofftech. 2009, 40, 297-301. |
| [248] | Cazalbou, S., Combes, C., Eichert, D., and Rey, C., Adaptative physico-chemistry of bio-related calcium phosphates. J. Mater. Chem. 2004, 14, 2148-2153. |
| [249] | Eichert, D., Salomé, M., Banu, M., Susini, J., and Rey, C., Preliminary characterization of calcium chemical environment in apatitic and non-apatitic calcium phosphates of biological interest by X-ray absorption spectroscopy. Spectrochim. Acta B 2005, 60B, 850-858. |
| [250] | Rosenman, G., Aronov, D., Oster, L., Haddad, J., Mezinskis, G., Pavlovska, I., Chaikina, M., and Karlov, A., Photoluminescence and surface photovoltage spectroscopy studies of hydroxyapatite nano-bio-ceramics. J. Luminescence 2007, 122-123, 936-938. |
| [251] | Melikhov, I. V., Teterin, Y. A., Rudin, V. N., Teterin, A. Y., Maslakov, K. I., and Severin, A. V., An X-ray electron study of nanodisperse hydroxyapatite. Russ. J. Phys. Chem. A 2009, 83, 91-97. |
| [252] | Aronov, D., Rosenman, G., Karlov, A., and Shashkin, A., Wettability patterning of hydroxyapatite nanobioceramics induced by surface potential modification. Appl. Phys. Lett. 2006, 88, 163902 (3 pages). |
| [253] | Rau, J. V., Generosi, A., Ferro, D., Minozzi, F., Paci, B., Albertini, V. R., Dolci, G., and Barinov, S. M., In situ time-resolved X-ray diffraction study of evolution of nanohydroxyapatite particles in physiological solution. Mater. Sci. Eng. C 2009, 29, 1140-1143. |
| [254] | Arora, A., Ceramics in nanotech revolution. Adv. Eng. Mater. 2004, 6, 244-247. |
| [255] | Mao, Y., Park, T. J., Zhang, F., Zhou, H., and Wong, S. S., Environmentally friendly methodologies of nanostructure synthesis. Small 2007, 3, 1122-1139. |
| [256] | Ioku, K., and Yoshimura, M., Stochiometric apatite fine single crystals by hydrothermal synthesis. Phosphorus Res. Bull. 1991, 1, 15-20. |
| [257] | Chen, J. D., Wang, Y. J., Wei, K., Zhang, S. H., and Shi, X. T., Self-organization of hydroxyapatite nanorods through oriented attachment. Biomaterials 2007, 28, 2275-2280. |
| [258] | Guo, X., Xiao, P., Liu, J., and Shen, Z., Fabrication of nanostructured hydroxyapatite via hydrothermal synthesis and spark plasma sintering. J. Am. Ceram. Soc. 2004, 88, 1026-1029. |
| [259] | Brown, P. W., and Constantz B., Eds., Hydroxyapatite and related materials. CRC Press: Boca Raton, FL, USA, 1994; pp. 343. |
| [260] | Amjad, Z., Ed., Calcium phosphates in biological and industrial systems. Kluwer Academic Publishers: Boston, MA, USA, 1997; pp. 529. |
| [261] | Hughes, J. M., Kohn, M., and Rakovan, J., Eds., Phosphates: geochemical, geobiological and materials importance, Series: Reviews in Mineralogy and Geochemistry. Vol. 48; Mineralogical Society of America: Washington, D. C., USA, 2002; pp. 742. |
| [262] | Chow, L. C., and Eanes, E. D., Eds., Octacalcium phosphate. Karger: Basel, Switzerland, 2001; pp. 168. |
| [263] | Brès, E., and Hardouin, P., Eds., Les matériaux en phosphate de calcium. Aspects fondamentaux. / Calcium phosphate materials. Fundamentals. Sauramps Medical: Montpellier, France, 1998; pp. 176. |
| [264] | Komarov, V. F., and Kibalchitz, V., Precipitation of apatite through highly saturated solutions. Moscow Univ. Bull. Chem. Dic. 1979, 2680-2685. |
| [265] | Prakash, K. H., Kumar, R., Ooi, C. P., Cheang, P., and Khor, K. A., Conductometric study of precursor compound formation during wet-chemical synthesis of nanocrystalline hydroxyapatite. J. Phys. Chem. B 2006, 110, 24457-24462. |
| [266] | Tao, J., Pan, H., Wang, J., Wu, J., Wang, B., Xu, X., and Tang, R., Evolution of amorphous calcium phosphate to hydroxyapatite probed by gold nanoparticles. J. Phys. Chem. C 2008, 112, 14929-14933. |
| [267] | Chane-Ching, J. Y., Lebugle, A., Rousselot, I., Pourpoint, A., and Pelle, F., Colloidal synthesis and characterization of monocrystalline apatite nanophosphors. J. Mater. Chem. 2007, 17, 2904-2913. |
| [268] | Zyman, Z. Z., Rokhmistrov, D. V., and Glushko, V. I., Structural and compositional features of amorphous calcium phosphate at the early stage of precipitation. J. Mater. Sci. Mater. Med. 2010, 21, 123-130. |
| [269] | Wei, M., Ruys, A. J., Milthorpe, B. K., and Sorrell, C. C., Solution ripening of hydroxyapatite nanoparticles: effects on electrophoretic deposition. J. Biomed. Mater. Res. 1999, 45, 11-19. |
| [270] | Zhu, X., Eibl, O., Berthold, C., Scheideler, L., and Geis-Gerstorfer, J., Structural characterization of nanocrystalline hydroxyapatite and adhesion of pre-osteoblast cells. Nanotechnology 2006, 17, 2711-2721. |
| [271] | Rusu, V. M., Ng, C. H., Wilke, M., Tiersch, B., Fratzl, P., and Peter, M. G., Size-controlled hydroxyapatite nanoparticles as self-organized organic – inorganic composite materials. Biomaterials 2005, 26, 5414-5426. |
| [272] | Wang, Y. J., Lai, C., Wei, K., and Tang, S. Q., Influence of temperature, ripening time and cosurfactant on solvothermal synthesis of calcium phosphate nanobelts. Mater. Lett. 2005, 59, 1098-1104. |
| [273] | Li, Y. B., Li, D., and Weng, W., Preparation of nano carbonate-substituted hydroxyapatite from an amorphous precursor. Int. J. Appl. Ceram. Technol. 2008, 5, 442-448. |
| [274] | Zhang, S., and Gonsalves, K. E., Preparation and characterization of thermally stable nanohydroxyapatite. J. Mater. Sci. Mater. Med. 1997, 8, 25-28. |
| [275] | Ferraz, M. P., Monteiro, F. J., and Manuel, C. M., Hydroxyapatite nanoparticles: a review of preparation methodologies. J. Appl. Biomater. Biomech. 2004, 2, 74-80. |
| [276] | Ahn, E. S., Gleason, N. J., Nakahira, A., and Ying, J. Y., Nanostructure processing of hydroxyapatite-based bioceramics. Nano Lett. 2001, 1, 149-153. |
| [277] | Mazelsky, R., Hopkins, R. H., and Kramer, W. E., Czochralski-growth of calcium fluorophosphates. J. Cryst. Growth 1968, 3-4, 260-264. |
| [278] | Loutts, G. B., and Chai, B. H. T., Growth of high-quality single crystals of FAP (Ca5(PO4)3F) and its isomorphs. Proc. SPIE – Int. Soc. Optical Eng. 1993, 1863, 31-34. |
| [279] | Siegel, R. W., Creating nanophase materials. Sci. Am. 1996, 275, 42-47. |
| [280] | Hu, J., Odom, T. W., and Lieber, C. M., Chemistry and physics in one dimension: synthesis and properties of nanowires and nanotubes. Acc. Chem. Res. 1999, 32, 435-445. |
| [281] | Schmidt, H. K., Nanoparticles for ceramic and nanocomposite processing. Mol. Cryst. Liq. Cryst. 2000, 353, 165-179. |
| [282] | Cushing, B. L., Kolesnichenko, V. L., and O’Connor, C. J., Recent advances in the liquid-phase syntheses of inorganic nanoparticles. Chem. Rev. 2004, 104, 3893-3946. |
| [283] | Wang, X., Zhuang, J., Peng, Q., and Li, Y., A general strategy for nanocrystal synthesis. Nature 2005, 437, 121-124. |
| [284] | Yin, Y., and Alivisatos, A. P., Colloidal nanocrystal synthesis and the organic-inorganic interface. Nature 2005, 437, 664-670. |
| [285] | Mao, Y., Park, T. J., Zhang, F., Zhou, H., and Wong, S. S., Environmentally friendly methodologies of nanostructure synthesis. Small 2007, 3, 1122-1139. |
| [286] | Ma, M. G., and Zhu, J. F., Recent progress on fabrication of calcium-based inorganic biodegradable nanomaterials. Recent Patents on Nanotechnology 2010, 4, 164-170. |
| [287] | Takagi, S., Chow, L. C., and Ishikawa, K., Formation of hydroxyapatite in new calcium phosphate cements. Biomaterials 1998, 19, 1593-1599. |
| [288] | Meejoo, S., Maneeprakorn, W., and Winotai, P., Phase and thermal stability of nanocrystalline hydroxyapatite prepared via microwave heating. Thermochim. Acta 2006, 447, 115-120. |
| [289] | Kumta, P., Sfeir, C., Lee, D. H., Olton, D., and Choi, D., Nanostructured calcium phosphates for biomedical applications: novel synthesis and characterization. Acta Biomater. 2005, 1, 65-83. |
| [290] | Liou, S. C., Chen, S. Y., Lee, H. Y., and Bow, J. S., Structural characterization of nanosized calcium deficient apatite powders. Biomaterials 2004, 25, 189-196. |
| [291] | Mollazadeh, S., Javadpour, J., and Khavandi, A., In situ synthesis and characterization of nanosized hydroxyapatite in poly(vinyl alcohol) matrix. Ceram. Int. 2007, 33, 1579-1583. |
| [292] | Bigi, A., Boanini, E., Gazzano, M., Rubini, K., and Torricelli, P., Nanocrystalline hydroxyapatite – polyaspartate composites. Biomed. Mater. Eng. 2004, 14, 573-579. |
| [293] | Chen, H., Sun, K., Tang, Z., Law, R. V., Mansfield, J. F., Czajka-Jakubowska, A., and Clarkson, B. H., Synthesis of fluorapatite nanorods and nanowires by direct precipitation from solution. Cryst. Growth Des. 2006, 6, 1504-1508. |
| [294] | Kong, L., Gao, Y., Cao, W., Gong, Y., Zhao, N., and Zhang, X., Preparation and characterization of nano-hydroxyapatite/chitosan composite scaffolds. J. Biomed. Mater. Res. A 2005, 75A, 275-282. |
| [295] | Kong, L., Gao, Y., G. Lu, Gong, Y., Zhao, N., and Zhang, X., A study on the bioactivity of chitosan/nano-hydroxyapatite composite scaffolds for bone tissue engineering. Eur. Polym. J. 2006, 42, 3171-3179. |
| [296] | Melikhov, I. V., Komarov, V. F., Severin, A. V., Bozhevol’nov, V. E., and Rudin, V. N., Two-dimensional crystalline hydroxyapatite. Dokl. Phys. Chem. 2000, 373, 355-358. |
| [297] | Zhao, Y., Zhang, Y., Ning, F., Guo, D., and Xu, Z., Synthesis and cellular biocompatibility of two kinds of HAP with different nanocrystal morphology. J. Biomed. Mater. Res. B (Appl. Biomater.) 2007, 83B, 121-126. |
| [298] | Ganesan, K., and Epple, M., Calcium phosphate nanoparticles as nuclei for the preparation of colloidal calcium phytate. New J. Chem. 2008, 32, 1326-1330. |
| [299] | Zhang, Y., and Lu, J., A simple method to tailor spherical nanocrystal hydroxyapatite at low temperature. J. Nanopart. Res. 2007, 9, 589-594. |
| [300] | Bouyer, E., Gitzhofer, F., and Boulos, M. I., Morphological study of hydroxyapatite nanocrystal suspension. J. Mater. Sci. Mater. Med. 2000, 11, 523-531. |
| [301] | Pang, Y. X., and Bao, X., Influence of temperature, ripening time and calcination on the morphology and crystallinity of hydroxyapatite nanoparticles. J. Eur. Ceram. Soc. 2003, 23, 1697-1704. |
| [302] | Kumar, R., Prakash, K. H., Cheang, P. and Khor, K. A., Temperature driven morphological changes of chemically precipitated hydroxyapatite nanoparticles. Langmuir 2004, 20, 5196-5200. |
| [303] | Li-yun, C., Chuan-bo, Z., and Jian-feng, H., Influence of temperature,[Ca2+], Ca/P ratio and ultrasonic power on the crystallinity and morphology of hydroxyapatite nanoparticles prepared with a novel ultrasonic precipitation method. Mater. Lett. 2005, 59, 1902-1906. |
| [304] | Afshar, A., Ghorbani, M., Ehsani, N., Saeri, M. R., and Sorrell, C. C., Some important factors in the wet precipitation process of hydroxyapatite. Mater. Des. 2003, 24, 197-202. |
| [305] | Wei, M., Ruys, A. J., Milthorpe, B. K., and Sorrell, C. C., Precipitation of hydroxyapatite nanoparticles: effects of precipitation method on electrophoretic deposition. J. Mater. Sci. Mater. Med. 2005, 16, 319-324. |
| [306] | Liu, Y., Hou, D., and Wang, G., A simple wet chemical synthesis and characterization of hydroxyapatite nanorods. Mater. Chem. Phys. 2004, 86, 69-73. |
| [307] | Saha, S. K., Banerjee, A., Banerjee, S., and Bose, S., Synthesis of nanocrystalline hydroxyapatite using surfactant template systems: role of templates in controlling morphology. Mater. Sci. Eng. C 2009, 29, 2294-2301. |
| [308] | Shanthi, P. M. S. L., Ashok, M., Balasubramanian, T., Riyasdeen, A., and Akbarsha, M. A. Synthesis and characterization of nano-hydroxyapatite at ambient temperature using cationic surfactant. Mater. Lett. 2009, 63, 2123-2125. |
| [309] | Mobasherpour, I., Heshajin, M. S., Kazemzadeh, A., and Zakeri, M., Synthesis of nanocrystalline hydroxyapatite by using precipitation method. J. Alloys Compd. 2007, 430, 330-333. |
| [310] | Phillips, M. J., Darr, J. A., Luklinska, Z. B., and Rehman, I., Synthesis and characterization of nanobiomaterials with potential osteological applications. J. Mater. Sci. Mater. Med. 2003, 14, 875-882. |
| [311] | Lee, S. J., Yoon, Y. S., Lee, M. H., and Oh, N. S., Nanosized hydroxyapatite powder synthesized from eggshell and phosphoric acid. J. Nanosci. Nanotechnol. 2007, 7, 4061-4064. |
| [312] | Monmaturapoj, N., Nanosize hydroxyapatite powders preparation by wet-chemical precipitation route. J. Metals Mater. Miner. 2008, 18, 15-20. |
| [313] | Ramesh, S., Tan, C. Y., Sopyan, I., Hamdi, M., and Teng, W. D., Consolidation of nanocrystalline hydroxyapatite powder. Sci. Technol. Adv. Mater. 2007, 8, 124-130. |
| [314] | Zhou, W., Zhang, S. M., Hu, W., Qiu, Z. Y., and Liu, Y. H., Dialysis efficiency in rapid synthesis of nano-hydroxyapatite. Key Eng. Mater. 2007, 330-332, 211-214. |
| [315] | Shi, H. B., Zhong, H., Liu, Y., Gu, J. Y., and Yang, C. S., Effect of precipitation method on stoichiometry and morphology of hydroxyapatite nanoparticles. Key Eng. Mater. 2007, 330-332, 271-274. |
| [316] | Monkawa, A., Ikoma, T., Yunoki, S., Ohta, K., and Tanaka, J., Electrophoretic deposition of hydroxyapatite nanocrystal. Key Eng. Mater. 2006, 309-311, 643-646. |
| [317] | Fujii, S., Okada, M., and Furuzono, T., Hydroxyapatite nanoparticles as stimulus-responsive particulate emulsifiers and building block for porous materials. J. Coll. Interf. Sci. 2007, 315, 287-296. |
| [318] | Ong, H. T., Loo, J. S. C., Boey, F. Y. C., Russell, S. J., Ma, J., and Peng, K. W., Exploiting the high-affinity phosphonate – hydroxyapatite nanoparticle interaction for delivery of radiation and drugs. J. Nanopart. Res. 2008, 10, 141-150. |
| [319] | Silva, G. W. C., Ma, L., Hemmers, O., and Lindle, D., Micro-structural characterization of precipitation-synthesized fluorapatite nano-material by transmission electron microscopy using different sample preparation techniques. Micron 2008, 39, 269-274. |
| [320] | Poinern, G. E., Brundavanam, R. K., Mondinos, N., and Jiang, Z. T., Synthesis and characterisation of nanohydroxyapatite using an ultrasound assisted method. Ultrason. Sonochem. 2009, 16, 469-474. |
| [321] | Doğan, Ö., and Öner, M., The influence of polymer architecture on nanosized hydroxyapatite precipitation. J. Nanosci. Nanotechnol. 2008, 8, 667-674. |
| [322] | Loo, S. C. J., Siew, Y. E., Ho, S., Boey, F. Y. C., and Ma, J., Synthesis and hydrothermal treatment of nanostructured hydroxyapatite of controllable sizes. J. Mater. Sci. Mater. Med. 2008, 19, 1389-1397. |
| [323] | Guo, X., Gough, J. E., Xiao, P., Liu, J., and Shen, Z., Fabrication of nanostructured hydroxyapatite and analysis of human osteoblastic cellular response. J. Biomed. Mater. Res. A 2007, 82A, 1022-1032. |
| [324] | Safronova, T. V., Putlyaev, V. I., Sergeeva, A. I., Kunenkov, E. V., and Tret’yakov, Y. D., Synthesis of nanocrystalline calcium hydroxyapatite from calcium saccharates and ammonium hydrogen phosphate. Dokl. Chem. 2009, 426, 118-123. |
| [325] | Iafisco, M., Palazzo, B., Marchetti, M., Margiotta, N., Ostuni, R., Natile, G., Morpurgo, M., Gandin, V., Marzano, C., and Roveri, N., Smart delivery of antitumoral platinum complexes from biomimetic hydroxyapatite nanocrystals. J. Mater. Chem. 2009, 19, 8385-8392. |
| [326] | Wang, P., Li, C., Gong, H., Jiang, X., Wang, H., and Li, K., Effects of synthesis conditions on the morphology of hydroxyapatite nanoparticles produced by wet chemical process. Powder Technol. 2010, 203, 315-321. |
| [327] | Leskiv, M., Lagoa, A. L. C., Urch, H., Schwiertz, J., da Piedade, M. E. M., and Epple, M., Energetics of calcium phosphate nanoparticle formation by the reaction of Ca(NO3)2 with (NH4)2HPO4. J. Phys. Chem. C 2009, 113, 5478-5484. |
| [328] | Rodrigues, L. R., Motisuke, M., and Zavaglia, C. A. C., Synthesis of nanostructured hydroxyapatite: a comparative study between sol-gel and aqueous solution precipitation. Key Eng. Mater. 2009, 396-398, 623-626. |
| [329] | Medvecky, L., Sopcak, T., Durisin, J., and Briancin, J., Nanohydroxyapatite prepared from non-toxic organic Ca2+ compounds by precipitation in aqueous solution. Mater. Lett. 2011, 65, 3566-3569. |
| [330] | Okada, M., and Furuzono, T., Low-temperature synthesis of nanoparticle-assembled, transparent, and low-crystallized hydroxyapatite blocks. J. Coll. Interf. Sci. 2011, 360, 457-462. |
| [331] | Sheykhan, M., Heydari, A., Ma’mani, L., and Badiei, A., The synthesis and spectroscopic characterization of nano calcium fluorapatite using tetra-butylammonium fluoride. Spectrochim. Acta A 2011, 83, 379-783. |
| [332] | Kazemzadeh, R., Behnamghader, A., and Hesaraki, S., Effect of synthesis temperature on phase and morphological characteristics of hydroxyapatite nanoparticles. Adv. Mater. Res. 2011, 264-265, 1329-1333. |
| [333] | López-Macipe, A., Gómez-Morales, J., and Rodríguez-Clemente, R., Nanosized hydroxyapatite precipitation from homogeneous calcium/citrate/phosphate solutions using microwave and conventional heating. Adv. Mater. 1998, 10, 49-53. |
| [334] | Siddharthan, A., Seshadri, S. K., and Kumar, T. S. S., Rapid synthesis of calcium deficient hydroxyapatite nanoparticles by microwave irradiation. Trends Biomater. Artif. Organs 2005, 18, 110-113. |
| [335] | Ioku, K., Yamauchi, S., Fujimori, H., Goto, S., and Yoshimura, M., Hydrothermal preparation of fibrous apatite and apatite sheet. Solid State Ionics 2002, 151, 147-150. |
| [336] | Kasahara, H., Ogata, N., and Ogihara, T., Effect of starting solution on the formation of calcium phosphate nano particles by hydrothermal process. J. Ceram. Soc. Jpn. 2004, 112, 650-654. |
| [337] | Lemos, A. F., Rocha, J. H. G., Quaresma, S. S. F., Kannana, S., Oktar, F. N., Agathopoulos, S., and Ferreira, J. M. F., Hydroxyapatite nano-powders produced hydrothermally from nacreous material. J. Eur. Ceram. Soc. 2006, 26, 3639-3646. |
| [338] | Chaudhry, A. A., Haque, S., Kellici, S., Boldrin, P., Rehman, I., Khalid, F. A., and Darr, J. A., Instant nano-hydroxyapatite: a continuous and rapid hydrothermal synthesis. Chem. Commun. 2006, 2286-2288. |
| [339] | Cao, M., Wang, Y., Guo, C., Qi, Y., and Hu, C., Preparation of ultrahigh-aspect-ratio hydroxyapatite nanofibers in reverse micelles under hydrothermal conditions. Langmuir 2004, 20, 4784-4786. |
| [340] | Jinlong, N., Hydrothermal synthesis of nano-crystalline hydroxyapatite. Key Eng. Mater. 2007, 330-332, 247-250. |
| [341] | Ryu, I. Y., Kim, D. J., Han, J. S., and Lee, M. H., Influence of two-step sintering variables on phase stability of hydrothermally prepared HAp nano powders. Key Eng. Mater. 2008, 361-363, 91-94. |
| [342] | Han, J. K., Song, H. Y., Saito, F., and Lee, B. T., Synthesis of high purity nanosized hydroxyapatite powder by microwave-hydrothermal method. Mater. Chem. Phys. 2006, 99, 235-239. |
| [343] | Suchanek, W. L., Shuk, P., Byrappa, K., Riman, R. E., TenHuisen, K. S., and Janas, V. F., Mechanochemical-hydrothermal synthesis of carbonated apatite powders at room temperature. Biomaterials 2002, 23, 699-710. |
| [344] | Guo, X., and Xiao, P., Effects of solvents on properties of nanocrystalline hydroxyapatite produced from hydrothermal process. J. Eur. Ceram. Soc. 2006, 26, 3383-3391. |
| [345] | Xin, R., and Yu, K., Ultrastructure characterization of hydroxyapatite nanoparticles synthesized by EDTA-assisted hydrothermal method. J. Mater. Sci. 2009, 44, 4205-4209. |
| [346] | Zhang, C., Yang, J., Quan, Z., Yang, P., Li, C., Hou, Z., Lin, J. Hydroxyapatite nano- and microcrystals with multiform morphologies: controllable synthesis and luminescence properties. Cryst. Growth Des. 2009, 9, 2725-2733. |
| [347] | Zhang, H. B., Zhou, K. C., Li, Z. Y., and Huang, S. P., Plate-like hydroxyapatite nanoparticles synthesized by the hydrothermal method. J. Phys. Chem. Solids 2009, 70, 243-248. |
| [348] | Abdel-Aal, E. A., El-Midany, A. A., and El-Shall, H., Mechanochemical-hydrothermal preparation of nano-crystallite hydroxyapatite using statistical design. Mater. Chem. Phys. 2008, 112, 202-207. |
| [349] | Sun, Y., Guo, G., Tao, D., and Wang, Z., Reverse microemulsion-directed synthesis of hydroxyapatite nanoparticles under hydrothermal conditions. J. Phys. Chem. Solids 2007, 68, 373-377. |
| [350] | Du, X., Chu, Y., Xing, S., and Dong, L., Hydrothermal synthesis of calcium hydroxyapatite nanorods in the presence of PVP. J. Mater. Sci. 2009, 44, 6273-6279. |
| [351] | Xin, R., Ren, F., and Leng, Y., Synthesis and characterization of nano-crystalline calcium phosphates with EDTA-assisted hydrothermal method. Mater. Des. 2010, 31, 1691-1694. |
| [352] | Yan, L., Li, Y., Deng, Z., Zhuang, J., Sun, X. Surfactant-assisted hydrothermal synthesis of hydroxyapatite nanorods. Int. J. Inorg. Mater. 2001, 3, 633-637. |
| [353] | Zhang, F., Zhou, Z., Yang, S., Mao, L., Chen, H., and Yu, X., Hydrothermal synthesis of hydroxyapatite nanorods in the presence of anionic starburst dendrimer. Mater. Lett. 2005, 59, 1422-1425. |
| [354] | Pathi, S. P., Lin, D. D., Dorvee, J. R., Estroff, L. A., and Fischbach, C., Hydroxyapatite nanoparticle-containing scaffolds for the study of breast cancer bone metastasis. Biomaterials 2011, 32, 5112-5122. |
| [355] | Zhu, A., Lu, Y., Si, Y., and Dai, S., Frabicating hydroxyapatite nanorods using a biomacromolecule template. Appl. Surf. Sci. 2011, 257, 3174-3179. |
| [356] | Wang, Y. Z., and Fu, Y., Microwave-hydrothermal synthesis and characterization of hydroxyapatite nanocrystallites. Mater. Lett. 2011, 65, 3388-3390. |
| [357] | Manafi, S., and Rahimipour, M. R., Synthesis of nanocrystalline hydroxyapatite nanorods via hydrothermal conditions. Chem. Engin. Technol. 2011, 34, 972-976. |
| [358] | Byrappa, K., and Haber, M., Handbook of hydrothermal technology: a technology for crystal growth and materials processing. Noyes Publications: New Jersey, USA, 2002, 893 pp. |
| [359] | Yu-Song, P., Surface modification of nanocrystalline hydroxyapatite. Micro and Nano Lett. 2011, 6, 129-132. |
| [360] | Chai, C. S., and Ben-Nissan, B., Bioactive nanocrystalline sol-gel hydroxyapatite coatings. J. Mater. Sci. Mater. Med. 1999, 10, 465-469. |
| [361] | Ben-Nissan, B., Green, D. D., Kannangara, G. S. K., Chai, C. S., and Milev, A., 31P NMR studies of diethyl phosphite derived nanocrystalline hydroxyapatite. J. Sol-Gel Sci. Technol. 2001, 21, 27-37. |
| [362] | Gopi, D., Govindaraju, K. M., Victor, C. A. P., Kavitha, L., and Rajendiran, N., Spectroscopic investigations of nanohydroxyapatite powders synthesized by conventional and ultrasonic coupled sol–gel routes. Spectrochim. Acta A: Mol. Biomol. Spectrosc. 2008, 70, 1243-1245. |
| [363] | Natarajan, V. U., and Rajeswari, S., Influence of calcium precursors on the morphology and crystallinity of sol–gel-derived hydroxyapatite nanoparticles. J. Cryst. Growth 2008, 310, 4601-4611. |
| [364] | Ben-Nissan, B., and Choi, A. H., Sol-gel production of bioactive nanocoatings for medical applications. Part 1: an introduction. Nanomedicine 2006, 1, 311-319. |
| [365] | Choi, A. H., and Ben-Nissan, B., Sol-gel production of bioactive nanocoatings for medical applications. Part 2: current research and development. Nanomedicine 2007, 2, 51-61. |
| [366] | Kim, T. S., and Kumta, P. N., Sol-gel synthesis and characterization of nanostructured hydroxyapatite powder. Mater. Sci. Eng. B 2004, 111, 232-236. |
| [367] | Rajabi-Zamani, A. H., Behnamghader, A., and Kazemzadeh, A., Synthesis of nanocrystalline carbonated hydroxyapatite powder via nonalkoxide sol-gel method. Mater. Sci. Eng. C 2008, 28, 1326-1329. |
| [368] | Sopyan, I., Toibah, A. R., and Natasha, A. N., Nanosized bioceramic hydroxyapatite powders via sol-gel method. Int. J. Mech. Mater. Eng. 2008, 3, 133-138. |
| [369] | Padmanabhan, S. K., Balakrishnan, A., Chu, M. C., Lee, Y. J., Kim, T. N., and Cho, S. J., Sol–gel synthesis and characterization of hydroxyapatite nanorods. Particuology 2009, 7, 466-470. |
| [370] | Yuan, Y., Liu, C., Zhang, Y., and Shan, X., Sol-gel auto-combustion synthesis of hydroxyapatite nanotubes array in porous alumina template. Mater. Chem. Phys. 2008, 112, 275-280. |
| [371] | Kuriakose, T. A., Kalkura, S. N., Palanichamy, M., Arivuoli, D., Dierks, K., Bocelli, G., and Betzel, C., Synthesis of stoichiometric nano crystalline hydroxyapatite by ethanol-based sol-gel technique at low temperature. J. Cryst. Growth 2004, 263, 517-523. |
| [372] | Jahandideh, R., Behnamghader, A., Rangie, M., Youzbashi, A., Joughehdoust, S., and Tolouei, R., Sol-gel synthesis of FHA nanoparticles and CDHA agglomerates from a mixture with a nonstochiometric Ca/P ratio. Key Eng. Mater. 2009, 396-398, 607-610. |
| [373] | Pang, X., Zeng, H., Liu, J., Wei, S., and Zheng, Y., The properties of nanohydroxyapatite materials and its biological effects. Mater. Sci. Applications 2010, 1, 81-90. |
| [374] | Sanosh, K. P., Chu, M. C., Balakrishnan, A., Lee, Y. J., Kim, T. N., and Cho, S. J., Synthesis of nano hydroxyapatite powder that simulate teeth particle morphology and composition. Curr. Appl. Phys. 2009, 9, 1459-1462. |
| [375] | Darroudi, M., Eshtiagh-Hosseini, H., Housaindokht, M. R., and Youssefi, A., Preparation and characterization of fluorohydroxyapatite nanopowders by nonalkoxide sol-gel method. Digest J. Nanomater. Biostruct. 2010, 5, 29-33. |
| [376] | Montazeri, N., Jahandideh, R., and Biazar, E., Synthesis of fluorapatite-hydroxyapatite nanoparticles and toxicity investigations. Int. J. Nanomedicine 2011, 6, 197-201. |
| [377] | Li, B., Wang, X. L., Guo, B., Xiao, Y. M., Fan, H. S., and Zhang, X. D., Preparation and characterization of nano hydroxyapatite. Key Eng. Mater. 2007, 330-332, 235-238. |
| [378] | Tas, A. C., Synthesis of biomimetic Ca-hydroxyapatite powders at 37°C in synthetic body fluids. Biomaterials 2000, 21, 1429-1438. |
| [379] | Wu, Y. S., Lee, Y. H., and Chang, H. C., Preparation and characteristics of nanosized carbonated apatite by urea addition with coprecipitation method. Mater. Sci. Eng. C 2009, 29, 237-241. |
| [380] | Swain, S. K., and Sarkar, D., A comparative study: hydroxyapatite spherical nanopowders and elongated nanorods. Ceram. Int. 2011, 37, 2927-2930. |
| [381] | Rameshbabu, N., Kumar, T. S. S., Murugan, R., and Rao, K. P., Mechanochemical synthesis of nanocrystalline fluorinated hydroxyapatite. Int. J. Nanosci. 2005, 4, 643-649. |
| [382] | Yeong, K. C. B., Wang, J., and Ng, S. C., Mechanochemical synthesis of nanocrystalline hydroxyapatite from CaO and CaHPO4. Biomaterials 2001, 22, 2705-2712. |
| [383] | Coreno, J. A., Coreno, O. A., Cruz, R. J. J., and Rodriguez, C. C., Mechanochemical synthesis of nanocrystalline carbonate-substituted hydroxyapatite. Optical Mater. 2005, 27, 1281-1285. |
| [384] | el Briak-Ben Abdeslam, H., Mochales, C., Ginebra, M. P., Nurit, J., Planell J. A., and Boudeville, P., Dry mechanochemical synthesis of hydroxyapatites from dicalcium phosphate dihydrate and calcium oxide: a kinetic study. J. Biomed. Mater. Res, A 2003, 67A, 927-937. |
| [385] | Nakamura, S., Isobe, T., and Senna, M., Hydroxyapatite nano sol prepared via a mechanochemical route. J. Nanopart. Res. 2001, 3, 57-61. |
| [386] | Nasiri-Tabrizi, B., Honarmandi, P., Ebrahimi-Kahrizsangi, R., and Honarmandi, P., Synthesis of nanosize single-crystal hydroxyapatite via mechanochemical method. Mater. Lett. 2009, 63, 543-546. |
| [387] | Sharifah, A., Iis, S., Mohd, H., and Singh, R., Mechanochemical synthesis of nanosized hydroxyapatite powder and its conversion to dense bodies. Mater. Sci. Forum 2011, 694, 118-122. |
| [388] | Fathi, M. H., and Zahrani, E. M., Fabrication and characterization of fluoridated hydroxyapatite nanopowders via mechanical alloying. J. Alloys Compd. 2009, 475, 408-414. |
| [389] | Fathi, M. H., and Zahrani, E. M., Mechanical alloying synthesis and bioactivity evaluation of nanocrystalline fluoridated hydroxyapatite. J. Cryst. Growth 2009, 311, 1392-1403. |
| [390] | Silva, C. C., Graça, M. P. F., Valente, M. A., and Sombra, A. S. B., Crystallite size study of nanocrystalline hydroxyapatite and ceramic system with titanium oxide obtained by dry ball milling. J. Mater. Sci. 2007, 42, 3851-3855. |
| [391] | Zahrani, E. M., and Fathi, M. H., The effect of high-energy ball milling parameters on the preparation and characterization of fluorapatite nanocrystalline powder. Ceram. Int. 2009, 35, 2311-2323. |
| [392] | Mochales, C., Wilson, R. M., Dowker, S. E. P., and Ginebra, M. P., Dry mechanosynthesis of nanocrystalline calcium deficient hydroxyapatite: structural characterization. J. Alloys Compounds 2011, 509, 7389-7394. |
| [393] | Xu, J. L., Khor, K. A., Dong, Z. L., Gu, Y. W., Kumar, R., and Cheang, P., Preparation and characterization of nanosized hydroxyapatite powders produced in a radio frequency (rf) thermal plasma. Mater. Sci. Eng. A 2004, 374, 101-108. |
| [394] | Xu, J. L., Khor, K. A., Kumar, R., and Cheang, P., RF induction plasma synthesized calcium phosphate nanoparticles. Key Eng. Mater. 2006, 309-311, 511-514. |
| [395] | Ruksudjarit, A., Pengpat, K., Rujijanagul, G., and Tunkasiri, T., Synthesis and characterization of nanocrystalline hydroxyapatite from natural bovine bone. Curr. Appl. Phys. 2008, 8, 270-272. |
| [396] | Cho, J. S., and Kang, Y. C., Nano-sized hydroxyapatite powders prepared by flame spray pyrolysis. J. Alloys Compd. 2008, 464, 282-287. |
| [397] | Wang, X., Zhuang, J., Peng, Q., and Li, Y., Liquid-solid-solution synthesis of biomedical hydroxyapatite nanorods. Adv. Mater. 2006, 18, 2031-2034. |
| [398] | Shirkhanzadeh, M., Direct formation of nanophase hydroxyapatite on cathodically polarized electrodes. J. Mater. Sci. Mater. Med. 1998, 9, 67-72. |
| [399] | Montalbert-Smith, R., Palma, C. A., Arias, J. D., and Montero, M. L., Formation of hydroxyapatite nanosized and other apatites by electrolysis process. Key Eng. Mater. 2009, 396-398, 579-582. |
| [400] | Gao, J. H., Guan, S. K., Chen, J., Wang, L. G., Zhu, S. J., Hu, J. H., and Ren, Z. W., Fabrication and characterization of rod-like nano-hydroxyapatite on MAO coating supported on Mg–Zn–Ca alloy. Appl. Surf. Sci. 2011, 257, 2231-2237. |
| [401] | Liu, J., Li, K., Wang, H., Zhu, M., Xu, H., and Yan, H., Self-assembly of hydroxyapatite nanostructures by microwave irradiation. Nanotechnology 2005, 16, 82-87. |
| [402] | Rameshbabu, N., Rao, K. P., and Kumar, T. S. S., Accelerated microwave processing of nanocrystalline hydroxyapatite. J. Mater. Sci. 2005, 40, 6319-6323. |
| [403] | Ran, X., Chen, J., Ran, J., Gou, L., and Zhang, X., Synthesis of nanosized carbonated hydroxyapatite under microwave irradiation. Key Eng. Mater. 2007, 330-332, 303-306. |
| [404] | Siddharthan, A., Seshadri, S. K., and Kumar, T. S. S., Microwave accelerated synthesis of nanosized calcium deficient hydroxyapatite. J. Mater. Sci. Mater. Med. 2004, 15, 1279-1284. |
| [405] | Liu, J., Li, K., Wang, H., Zhu, M., and Yan, H., Rapid formation of hydroxyapatite nanostructures by microwave irradiation. Chem. Phys. Lett. 2004, 396, 429-432. |
| [406] | Krishna, D. S. R., Siddharthan, A., Seshadri, S. K., and Kumar, T. S. S., A novel route for synthesis of nanocrystalline hydroxyapatite from eggshell waste. J. Mater. Sci. Mater. Med. 2007, 18, 1735-1743. |
| [407] | Seo, D. S., Hwang, K. H., and Lee, J. K., Nanostructured hydroxyapatite by microwave sintering. J. Nanosci. Nanotechnol. 2008, 8, 944-948. |
| [408] | Arami, H., Mohajerani, M., Mazloumi, M., Khalifehzadeh, R., Lak, A., and Sadrnezhaad, S. K., Rapid formation of hydroxyapatite nanostrips via microwave irradiation. J. Alloys Compd. 2009, 469, 391-394. |
| [409] | Lak, A., Mazloumi, M., Mohajerani, M. S., Zanganeh, S., Shayegh, M. R., Kajbafvala, A., Arami, H., and Sadrnezhaad, S. K., Rapid formation of mono-dispersed hydroxyapatite nanorods with narrow-size distribution via microwave irradiation. J. Am. Ceram. Soc. 2008, 91, 3580-3584. |
| [410] | Rameshbabu, N., Kumar, T. S. S., and Rao, K. P., Synthesis of nanocrystalline fluorinated hydroxyapatite by microwave processing and its in vitro dissolution study. Bull. Mater. Sci. 2006, 29, 611-615. |
| [411] | Kumar, A. R., Kalainathan, S., and Saral, A. M., Microwave assisted synthesis of hydroxyapatite nano strips. Cryst. Res. Technol. 2010, 45, 776-778. |
| [412] | Kalita, S. J., and Verma, S., Nanocrystalline hydroxyapatite bioceramic using microwave radiation: synthesis and characterization. Mater. Sci. Eng. C 2010, 30, 295-303. |
| [413] | Vani, R., Raja, S. B., Sridevi, T. S., Savithri, K., Devaraj, S. N., Girija, E. K., Thamizhavel, A., and Kalkura, S. N., Surfactant free rapid synthesis of hydroxyapatite nanorods by a microwave irradiation method for the treatment of bone infection. Nanotechnology 2011, 22, 285701. |
| [414] | Cabrera, J. L., Velázquez-Castillo, R., and Rivera-Muñoz, E. M., Synthesis of hydroxyapatite nanostructures using microwave heating. J. Nanosci. Nanotechnol. 2011, 11, 5555-5561. |
| [415] | Zyman, Z., Goncharenko, A., Rokhmistrov, D., and Epple, M., Nanocrystalline calcium-deficient hydroxyapatite prepared by a microwave-assisted solvent-free reaction. Mat. -Wiss. u. Werkstofftech. 2011, 42, 154-157. |
| [416] | Shih, W. J., Chen, Y. F., Wang, M. C., and Hon, M. H., Crystal growth and morphology of the nanosized hydroxyapatite powders synthesized from CaHPO4·2H2O and CaCO3 by hydrolysis method. J. Cryst. Growth 2004, 270, 211-218. |
| [417] | Zhang, Y., and Lu, J., The transformation of single-crystal calcium phosphate ribbon-like fibres to hydroxyapatite spheres assembled from nanorods. Nanotechnology 2008, 19, 155608 (10 pages). |
| [418] | Furuichi, K., Oaki, Y., and Imai, H., Preparation of nanotextured and nanofibrous hydroxyapatite through dicalcium phosphate with gelatin. Chem. Mater. 2006, 18, 229-234. |
| [419] | Yoruç, A. B. H., and Koca, Y., Double step stirring: a novel method for precipitation of nano-sized hydroxyapatite powder. Digest J. Nanomater. Biostructures 2009, 4, 73-81. |
| [420] | Jarudilokkul, S., Tanthapanichakoon, W., and Boonamnuayvittaya, V., Synthesis of hydroxyapatite nanoparticles using an emulsion liquid membrane system. Colloids Surf. A 2007, 296, 149-153. |
| [421] | Lim, G. K., Wang, J., Ng, S. C., and Gan, L. M., Nanosized hydroxyapatite powders from microemulsions and emulsions stabilized by a biodegradable surfactant. J. Mater. Chem. 1999, 9, 1635-1639. |
| [422] | Guo, G., Sun, Y., Wang, Z., and Guo, H., Preparation of hydroxyapatite nanoparticles by reverse microemulsion. Ceram. Int. 2005, 31, 869-872. |
| [423] | Lim, G. K., Wang, J., Ng, S. C., and Gan, L. M., Formation of nanocrystalline hydroxyapatite in nonionic surfactant emulsions. Langmuir 1999, 15, 7472-7477. |
| [424] | Sun, Y., Guo, G., Wang, Z., and Guo, H., Synthesis of single-crystal HAP nanorods. Ceram. Int. 2006, 32, 951-954. |
| [425] | Bose, S., and Saha, S. K., Synthesis and characterization of hydroxyapatite nanopowders by emulsion technique. Chem. Mater. 2003, 15, 4464-4469. |
| [426] | Lai, C., Tang, S. Q., Wang, Y. J., and Wei, K., Formation of calcium phosphate nanoparticles in reverse microemulsions. Mater. Lett. 2005, 59, 210-214. |
| [427] | Jiang, F. X., Lu, X. Y., Zhang, M. L., and Weng, J., Regulating size, morphology and dispersion of nano-crystallites of hydroxyapatite by pH value and temperature in microemulsion system. Key Eng. Mater. 2008, 361-363, 195-198. |
| [428] | Sato, K., Hotta, Y., Nagaoka, T., Yasuoka, M., and Watari, K., Agglomeration control of hydroxyapatite nano-crystals grown in phase-separated microenvironments. J. Mater. Sci. 2006, 41, 5424-5428. |
| [429] | Li, H., Zhu, M. Y., Li, L. H., and Zhou, C. R., Processing of nanocrystalline hydroxyapatite particles via reverse microemulsions. J. Mater. Sci. 2008, 43, 384-389. |
| [430] | Koetz, J., Baier, J., and Kosmella, S., Formation of zinc sulfide and hydroxylapatite nanoparticles in polyelectrolyte-modified microemulsions. Colloid Polym. Sci. 2007, 285, 1719-1726. |
| [431] | Lim, H. N., Kassim, A., and Huang, N. M., Preparation and characterization of calcium phosphate nanorods using reverse microemulsion and hydrothermal processing routes. Sains Malaysiana 2010, 39, 267-273. |
| [432] | Furuzono, T., Walsh, D., Sato, K., Sonoda, K., and Tanaka, J., Effect of reaction temperature on the morphology and size of hydroxyapatite nanoparticles in an emulsion system. J. Mater. Sci. Lett. 2001, 2, 111-114. |
| [433] | Sadjadi, M. A. S., Akhavan, K., and Zare, K., Preparation of hydroxyapatite nanoparticles by reverse microemulsions and polyelectrolyte-modified microemulsions. Res. J. Chem. Environment 2011, 15, 959-962. |
| [434] | Shen, S. C., Chia, L., Ng, W. K., Dong, Y. C., and Tan, R. B. H., Solid-phase steam-assisted synthesis of hydroxyapatite nanorods and nanoparticles. J. Mater. Sci. 2010, 45, 6059-6067. |
| [435] | Jevtić, M., Mitrić, M., Škapin, S., Jančar, B., Ignjatović, N., and Uskoković, D., Crystal structure of hydroxyapatite nano-rods synthesized by sonochemical homogenous precipitation. Cryst. Growth Des. 2008, 8, 2217-2222. |
| [436] | Wang, Y. J., Lai, C., Wei, K., Chen, X., Ding, Y., and Wang, Z. L., Investigations on the formation mechanism of hydroxyapatite synthesized by the solvothermal method. Nanotechnology 2006, 17, 4405-4412. |
| [437] | Cao, L. Y., Zhang, C. B., and Huang, J. F., Synthesis of hydroxyapatite nanoparticles in ultrasonic precipitation. Ceram. Int. 2005, 31, 1041-1044. |
| [438] | Liu, J., Wu, Q., and Ding, Y., Self-assembly and fluorescent modification of hydroxyapatite nanoribbon spherulites. Eur. J. Inorg. Chem. 2005, 20, 4145-4149. |
| [439] | Huang, J., Jayasinghe, S. N., Su, X., Ahmad, Z., Best, S. M., Edirisinghe, M. J., Brooks, R. A., Rushton, N., and Bonfield, W., Electrostatic atomisation spraying: a novel deposition method for nano-sized hydroxyapatite. Key Eng. Mater. 2006, 309-311, 635-638. |
| [440] | Hwang, K. S., and Kim, B. H., Preparation of calcium phosphate nano-powders prepared by sol-gel assisted-electrostatic spraying method. J. Mater. Sci. 2005, 40, 4665-4666. |
| [441] | Uota, M., Arakawa, H., Kitamura, N., Yoshimura, T., Tanaka, J., and Kijima, T., Synthesis of high surface area hydroxyapatite nanoparticles by mixed surfactant-mediated approach. Langmuir 2005, 21, 4724-4728. |
| [442] | Chu, M., and Liu, G., Preparation and characterization of hydroxyapatite/liposome core – shell nanocomposites. Nanotechnology 2005, 16, 1208-1212. |
| [443] | Huang, F., Shen, Y., Xie, A., Zhu, J., Zhang, C., Li, S., and Zhu, J., Study on synthesis and properties of hydroxyapatite nanorods and its complex containing biopolymer. J. Mater. Sci. 2007, 42, 8599-8605. |
| [444] | Wang, A., Liu, D., Yin, H., Wu, H., Wada, Y., Ren, M., Jiang, T., Cheng, X., and Xu, Y., Size-controlled synthesis of hydroxyapatite nanorods by chemical precipitation in the presence of organic modifiers. Mater. Sci. Eng. C 2007, 27, 865-869. |
| [445] | Ye, F., Guo, H., and Zhang, H., Biomimetic synthesis of oriented hydroxyapatite mediated by nonionic surfactants. Nanotechnology 2008, 19, 245605 (7 pages). |
| [446] | Han, Y., Wang, X., and Li, S., A simple route to prepare stable hydroxyapatite nanoparticles suspension. J. Nanoparticle Res. 2009, 11, 1235-1240. |
| [447] | Tseng, Y. H., Kuo, C. S., Li, Y. Y., and Huang, C. P., Polymer-assisted synthesis of hydroxyapatite nanoparticle. Mater. Sci. Eng. C 2009, 29, 819-822. |
| [448] | Klinkaewnarong, J., Swatsitang, E., and Maensiri, S., Nanocrystalline hydroxyapatite powders by a chitosan-polymer complex solution route: synthesis and characterization. Solid State Sci. 2009, 11, 1023-1027. |
| [449] | Li, Y., Li, D., and Xu, Z., Synthesis of hydroxyapatite nanorods assisted by Pluronics. J. Mater. Sci. 2009, 44, 1258-1263. |
| [450] | Nayar, S., Sinha, M. K., Basu, D., and Sinha, A., Synthesis and sintering of biomimetic hydroxyapatite nanoparticles for biomedical applications. J. Mater. Sci. Mater. Med. 2006, 17, 1063-1068. |
| [451] | Yao, X., Yao, H., Li, G., and Li, Y., Biomimetic synthesis of needle-like nano-hydroxyapatite templated by double-hydrophilic block copolymer. J. Mater. Sci. 2010, 45, 1930-1936. |
| [452] | Hong, Y., Fan, H., Li, B., Guo, B., Liu, M., and Zhang, X., Fabrication, biological effects, and medical applications of calcium phosphate nanoceramics. Mater. Sci. Eng. R 2010, 70, 225-242. |
| [453] | Mostaghaci, B., Fathi, M. H., Sheikh-Zeinoddin, M., and Soleimanian-Zad, S., Bacterial synthesis of nanostructured hydroxyapatite using Serratia marcescens PTCC 1187. Int. J. Nanotechnol. 2009, 6, 1015-1030. |
| [454] | Nathanael, A. J., Hong, S. I., Mangalaraj, D., and Chen, P. C., Large scale synthesis of hydroxyapatite nanospheres by high gravity method. Chem. Eng. J. 2011, 173, 846-854. |
| [455] | Parisi, M., Stoller, M., and Chianese, A., Production of nanoparticles of hydroxy apatite by using a rotating disk reactor. Chem. Eng. Trans. 2011, 24, 211-216. |
| [456] | Yuan, J., Wu, Y., Zheng, Q., and Xie, X., Synthesis and characterization of nano hydroxylapatite by reaction precipitation in impinging streams. Adv. Mater. Res. 2011, 160-162, 1301-1308. |
| [457] | Mohn, D., Doebelin, N., Tadier, S., Bernabei, R. E., Luechinger, N. A., Stark, W. J., and Bohner, M., Reactivity of calcium phosphate nanoparticles prepared by flame spray synthesis as precursors for calcium phosphate cements. J. Mater. Chem. 2011, 21, 13963-13972. |
| [458] | Tadic, D., Veresov, A., Putlayev, V. I., and Epple, M., In-vitro preparation of nanocrystalline calcium phosphates as bone substitution materials in surgery. Mat. -Wiss. u. Werkstofftech. 2003, 34, 1048-1051. |
| [459] | Qiu, Y., Xia, H., and Jiang, H., Fabrication of nano-hydroxyapatite using a novel ultrasonic atomization precipitation method. J. Nanosci. Nanotechnol. 2010, 10, 2213-2218. |
| [460] | Rouhani, P., Taghavinia, N., and Rouhani, S., Rapid growth of hydroxyapatite nanoparticles using ultrasonic irradiation. Ultrasonics Sonochemistry 2010, 17, 853-856. |
| [461] | Giardina, M. A., and Fanovich, M. A., Synthesis of nanocrystalline hydroxyapatite from Ca(OH)2 and H3PO4 assisted by ultrasonic irradiation. Ceram. Int. 2010, 36, 1961-1969. |
| [462] | Sadjadi, M. S., Meskinfam, M., Sadeghi, B., Jazdarreh, H., and Zare, K., In situ biomimetic synthesis, characterization and in vitro investigation of bone-like nanohydroxyapatite in starch matrix. Mater. Chem. Phys. 2010, 124, 217-222. |
| [463] | Mhin, S. W., Ryu, J. H., Kim, K. M., Park, G. S., Ryu, H. W., Shim, K. B., Sasaki, T., and Koshizaki, N., Simple synthetic route for hydroxyapatite colloidal nanoparticles via a Nd:YAG laser ablation in liquid medium. Appl. Phys. A 2009, 96A, 435-440. |
| [464] | Musaev, O. R., Dusevich, V., Wieliczka, D. M., Wrobel, J. M., and Kruger, M. B., Nanoparticle fabrication of hydroxyapatite by laser ablation in water. J. Appl. Phys. 2008, 104, 084316 (5 pages). |
| [465] | Boutinguiza, M., Lusquiños, F., Riveiro, A., Comesaña, R., and Pou, J., Hydroxylapatite nanoparticles obtained by fiber laser-induced fracture. Appl. Surf. Sci. 2009, 255, 5382-5385. |
| [466] | Boutinguiza, M., Pou, J., Lusquiños, F., Comesaña, R., and Riveiro, A., Production of calcium phosphate nanoparticles by laser ablation in liquid. Physics Procedia 2011, 12, 54-59. |
| [467] | Boutinguiza, M., Comesaña, R., Lusquiños, F., Riveiro, A., and Pou, J., Production of nanoparticles from natural hydroxylapatite by laser ablation. Nanoscale Res. Lett. 2011, 6, 1-5. |
| [468] | Zuo, Y., Li, Y. B., Wei, J., and Yan, Y., Influence of ethylene glycol on the formation of calcium phosphate nanocrystals. J. Mater. Sci. Technol. 2003, 19, 628-630. |
| [469] | Barinov, S. M., Belonogov, E. K., Ievlev, V. M., Kostyuchenko, A. V., Putlyaev, V. I., Tret’yakov, Y. D., Smirnov, V. V., and Fadeeva, I. V., Synthesis of dense nanocrystalline hydroxyapatite films. Dokl. Phys. Chem. 2007, 412, 15-18. |
| [470] | Mello, A., Mavropoulos, E., Hong, Z., Ketterson, J. B., and Rossi, A. M., Nanometer coatings of hydroxyapatite characterized by glancing-incidence X-ray diffraction. Key Eng. Mater. 2009, 396-398, 369-372. |
| [471] | Iafisco, M., Morales, J. G., Hernández-Hernández, M. A., García-Ruiz, J. M., and Roveri, N., Biomimetic carbonate-hydroxyapatite nanocrystals prepared by vapor diffusion. Adv. Eng. Mater. 2010, 12, B218-B223. |
| [472] | Luo, P., and Nieh, T. G., Synthesis of ultrafine hydroxyapatite particles by a spray dry method. Mater. Sci. Eng. C 1995, 3, 75-78. |
| [473] | Chen, F., Wang, Z. C., and Chang, J. L., Preparation and characterization of nanosized hydroxyapatite particles and hydroxyapatite / chitosan nano-composite for use in biomedical materials. Mater. Lett. 2002, 57, 858-861. |
| [474] | Sarig, S., and Kahana, F., Rapid formation of nanocrystalline apatite. J. Cryst. Growth 2002, 237-239, 55-59. |
| [475] | Bose, S., and Saha, S. K., Synthesis of hydroxyapatite nanopowders via sucrose-templated sol-gel method. J. Am. Ceram. Soc. 2003, 86, 1055-1057. |
| [476] | Han, Y., Li, S., Wang, X., and Chen, X., Synthesis and sintering of nanocrystalline hydroxyapatite powders by citric acid sol-gel combustion method. Mater. Res. Bull. 2004, 39, 25-32. |
| [477] | Liu, D. M., Yang, Q., Troczynski, T., and Tseng, W. J., Structural evolution of sol-gel-derived hydroxyapatite. Biomaterials 2002, 23, 1679-1687. |
| [478] | Liu, D. M., Troczynski, T., and Tseng, W. J., Water-based sol-gel synthesis of hydroxyapatite: process development. Biomaterials 2001, 22, 1721-1730. |
| [479] | Wang, F., Li, M. S., Lu, Y. P., and Ge, S. S., Synthesis of nanocrystalline hydroxyapatite powders in stimulated body fluid. J. Mater. Sci. 2005, 40, 2073-2076. |
| [480] | Wang, J., and Shaw, L. L., Synthesis of high purity hydroxyapatite nanopowder via sol-gel combustion process. J. Mater. Sci. Mater. Med. 2009, 20, 1223-1227. |
| [481] | Varma, H. K., Kalkura, S. N., and Sivakumar, R., Polymeric precursor route for the preparation of calcium phosphate compounds. Ceram. Int. 1998, 24, 467-470. |
| [482] | Ghosh, S. K., Roy, S. K., Kundu, B., Datta, S., and Basu, D., Synthesis of nano-sized hydroxyapatite powders through solution combustion route under different reaction conditions. Mater. Sci. Eng. B 2011, 176, 14-21. |
| [483] | Loher, S., Stark, W. J., Maciejewski, M., Baiker, A., Pratsinis, S. E., Reichardt, D., Maspero, F., Krumeich, F., and Günther, D., Fluoro-apatite and calcium phosphate nanoparticles by flame synthesis. Chem. Mater. 2005, 17, 36-42. |
| [484] | Trommer, R. M., Santos, L. A., and Bergmann, C. P., Nanostructured hydroxyapatite powders produced by a flame-based technique. Mater. Sci. Eng. C 2009, 29, 1770-1775. |
| [485] | Chow, L. C., Sun, L., and Hockey, B., Properties of nanostructured hydroxyapatite prepared by a spray drying technique. J. Res. Natl. Inst. Stand. Technol. 2004, 109, 543-551. |
| [486] | Li, J., Chen, Y. P., Yin, Y., Yao, F., and Yao, K., Modulation of nano-hydroxyapatite size via formation on chitosan-gelatin network film in situ. Biomaterials 2007, 28, 781-790. |
| [487] | Zhai, Y., Cui, F. Z., and Wang, Y., Formation of nano hydroxyapatite on recombinant human like collagen fibrils. Curr. Appl. Phys. 2005, 5, 429-432. |
| [488] | Liou, S. C., Chen, S. Y., and Liu, D. M., Synthesis and characterization of needlelike apatitic nanocomposite with controlled aspect ratios. Biomaterials 2003, 24, 3981-3988. |
| [489] | Liou, S. C., Chen, S. Y., and Liu, D. M., Manipulation of nanoneedle and nanosphere apatite/poly(acrylic acid) nanocomposites. J. Biomed. Mater. Res. B (Appl. Biomater.) 2005, 73B, 117-122. |
| [490] | Amjad, Z., Performance of polymeric additives as HA crystal growth inhibitors. Phosphorus Res. Bull. 1995, 5, 1-12. |
| [491] | Kamitahara, M., Kawashita, M., Kokubo, T., and Nakamura, T., Effect of polyacrylic acid on the apatite formation of a bioactive ceramic in a simulated body fluid: fundamental examination of the possibility of obtaining bioactive glass-ionomer cements for orthopedic use. Biomaterials 2001, 22, 3191-3196. |
| [492] | Wang, X., Li, Y., Wei, J., and de Groot, K., Development of biomimetic nano-hydroxyapatite/poly(hexamethylene adipamide) composites. Biomaterials 2002, 23, 4787-4791. |
| [493] | Sinha, A., Nayar, S., and Agrawak, A. C., Synthesis of nanosized and microporous precipitated hydroxyapatite in synthetic polymers and biopolymers. J. Am. Ceram. Soc. 2003, 86, 357-359. |
| [494] | Liao, S., Watari, F., Zhu, Y., Uo, M., Akasaka, T., Wang, W., Xu, G., and Cui, F., The degradation of the three layered nano-carbonated hydroxyapatite/collagen/PLGA composite membrane in vitro. Dental Mater. 2007, 23, 1120-1128. |
| [495] | Gonzalez-McQuire, R., Chane-Ching, J. Y., Vignaud, E., Lebugle, A., and Mann, S., Synthesis and characterization of amino acid-functionalized hydroxyapatite nanorods. J. Mater. Chem. 2004, 14, 2277-2281. |
| [496] | Rosseeva, E. V., Golovanova, O. A., and Frank-Kamenetskaya, O. V., The influence of amino acids on the formation of nanocrystalline hydroxyapatite. Glass Phys. Chem. 2007, 33, 283-286. |
| [497] | Zhan, J., Tseng, Y. H., Chan, J. C. C., and Mou, C. Y., Biomimetic formation of hydroxyapatite nanorods by a single-crystal-to-single-crystal transformation. Adv. Funct. Mater. 2005, 15, 2005-2010. |
| [498] | Xu, A. W., Ma, Y., and Cölfen, H., Biomimetic mineralization. J. Mater. Chem. 2007, 17, 415-449. |
| [499] | Pileni, M., The role of soft colloidal templates in controlling the size and shape of inorganic nanocrystals. Nature Mater. 2003, 12, 145-150. |
| [500] | Wu, Y., and Bose, S., Nanocrystalline hydroxyapatite: micelle templated synthesis and characterization. Langmuir 2005, 21, 3232-3234. |
| [501] | Wei, K., Lai, C., and Wang, Y., Solvothermal synthesis of calcium phosphate nanowires under different pH conditions. J. Macromolec. Sci. A 2006, 43A, 1531-1540. |
| [502] | Lai, C., Tang, S. Q., Wang, Y. J., Wei, K., and Zhang, S. Y., Insight into shape control mechanism of calcium phosphate nanopartiles in reverse micelles solution. Synth. React. Inorg. Met. Met. Org. Nano-Metal Chem. 2005, 35, 717-725. |
| [503] | Banerjee, A., Bandyopadhyay, A., and Bose, S., Hydroxyapatite nanopowders: synthesis, densification and cell-materials interaction. Mater. Sci. Eng. C 2007, 27, 729-735. |
| [504] | Han, J. Y., Tan, T. T. Y., and Loo, J. S. C., Utilizing inverse micelles to synthesize calcium phosphate nanoparticles as nano-carriers. J. Nanoparticle Res. 2011, 13, 3441-3454. |
| [505] | Shchukin, D. G., Sukhorukov, G. B., and Möhwald, H., Biomimetic fabrication of nanoengineered hydroxyapatite/polyelectrolyte composite shell. Chem. Mater. 2003, 15, 3947-3950. |
| [506] | Mateus, A. Y. P., Ferraz, M. P., and Monteiro, F. J., Microspheres based on hydroxyapatite nanoparticles aggregates for bone regeneration. Key Eng. Mater. 2007, 330-332, 243-246. |
| [507] | Cai, Y., Liu, Y., Yan, W., Hu, Q., Tao, J., Zhang, M., Shi, Z., and Tang, R., Role of hydroxyapatite nanoparticle size in bone cell proliferation. J. Mater. Chem. 2007, 17, 3780-3787. |
| [508] | Mossaad, C., Tan, M. C., Starr, M., Payzant, E. A., Howe, J. Y., and Riman, R. E., Size-dependent crystalline to amorphous uphill phase transformation of hydroxyapatite nanoparticles. Cryst. Growth Des. 2011, 11, 45-52. |
| [509] | Quantum dots, first developed in the early 1980’s, are crystalline semi-conducting nanodimensional particles comprised of a metalloid crystalline core and a “cap” or “shell” that shields the core and renders the dots biologically compatible. They are used or being developed for use in electronics, biomedical imaging and surveillance. |
| [510] | Guo, Y., Shi, D., Lian, J., Dong, Z., Wang, W., Cho, H., Liu, G., Wang, L., and Ewing, R. C., Quantum dot conjugated hydroxylapatite nanoparticles for in vivo imaging. Nanotechnology 2008, 19, 175102 (6 pages). |
| [511] | Liu, Q., de Wijn, J. R., de Groot, K., and van Blitterswijk, C. A., Surface modification of nano-apatite by grafting organic polymer. Biomaterials 1998, 19, 1067-1072. |
| [512] | Palazzo, B., Iafisco, M., Laforgia, M., Margiotta, N., Natile, G., Bianchi, C. L., Walsh, D., Mann, S., and Roveri, N., Biomimetic hydroxyapatite-drug nanocrystals as potential bone substitutes with antitumor drug delivery properties. Adv. Funct. Mater. 2007, 17, 2180-2188. |
| [513] | Lee, H. J., Choi, H. W., Kim, K. J., and Lee, S. C., Modification of hydroxyapatite nanosurfaces for enhanced colloidal stability and improved interfacial adhesion in nanocomposites. Chem. Mater. 2006, 18, 5111-5118. |
| [514] | Lee, S. C., Choi, H. W., Lee, H. J., Kim, K. J., Chang, J. H., Kim, S. Y., Choi, J., Oh, K. S., and Jeong, Y. K., In-situ synthesis of reactive hydroxyapatite nanocrystals for a novel approach of surface grafting polymerization. J. Mater. Chem. 2007, 17, 174-180. |
| [515] | Li, L., Liu, Y. K., Tao, J. H., Zhang, M., Pan, H. H., Xu, X. R., and Tang, R. K., Surface modification of hydroxyapatite nanocrystallite by a small amount of terbium provides a biocompatible fluorescent probe. J. Phys. Chem. C 2008, 112, 12219-12224. |
| [516] | Neumeier, M., Hails, L. A., Davis, S. A., Mann, S., and Epple, M., Synthesis of fluorescent core-shell hydroxyapatite nanoparticles. J. Mater. Chem. 2011, 21, 1250-1254. |
| [517] | Wang, W., Shi, D., Lian, J., Guo, Y., Liu, G., Wang, L., and Ewing, R. C., Luminescent hydroxylapatite nanoparticles by surface functionalization. Appl. Phys. Lett. 2006, 89, 183106 (3 pages). |
| [518] | Liu, H., Xi, P., Xie, G., Chen, F., Li, Z., Bai, D., and Zeng, Z., Biocompatible hydroxyapatite nanoparticles as a redox luminescence switch. J. Biol. Inorg. Chem. 2011;16, 1135-1140. |
| [519] | Bow, J. S., Liou, S. C., and Chen, S. Y., Structural characterization of room-temperature synthesized nanosized β-tricalcium phosphate. Biomaterials 2004, 25, 3155-3161. |
| [520] | Brunner, T. J., Bohner, M., Dora, C., Gerber, C., and Stark, W. J., Comparison of amorphous TCP nanoparticles to micron-sized α-TCP as starting materials for calcium phosphate cements. J. Biomed. Mater. Res. B (Appl. Biomater.) 2007, 83B, 400-407. |
| [521] | Brunner, T. J., Grass, R. N., Bohner, M., and Stark, W. J., Effect of particle size, crystal phase and crystallinity on the reactivity of tricalcium phosphate cements for bone reconstruction. J. Mater. Chem. 2007, 17, 4072-4078. |
| [522] | Döbelin, N., Brunner, T. J., Stark, W. J., Eggimann, M., Fisch, M., and Bohner, M., Phase evolution of thermally treated amorphous tricalcium phosphate nanoparticles. Key Eng. Mater. 2009, 396-398, 595-598. |
| [523] | Bohner, M., Brunner, T. J., Döbelin, N., Tang, R., and Stark, W. J., Effect of thermal treatments on the reactivity of nanosized tricalcium phosphate powders. J. Mater. Chem. 2008, 18, 4460-4467. |
| [524] | Liu, Y. H., Zhang, S. M., Liu, L., Zhou, W., Hu, W., Li, J., and Qiu, Z. Y., Rapid wet synthesis of nano-sized β-TCP by using dialysis. Key Eng. Mater. 2007, 330-332, 199-202. |
| [525] | Abdel-Fattah, W. I., Reicha, F. M., and Elkhooly, T. A., Nano-beta-tricalcium phosphates synthesis and biodegradation: 1. Effect of microwave and SO42- ions on β-TCP synthesis and its characterization. Biomed. Mater. 2008, 3, 034121 (13 pages). |
| [526] | Sanosh, K. P., Chu, M. C., Balakrishnan, A., Kim, T. N., and Cho, S. J., Sol-gel synthesis of pure nano sized β-tricalcium phosphate crystalline powders. Curr. Appl. Phys. 2010, 10, 68-71. |
| [527] | Dasgupta, S., Bandyopadhyay, A., and Bose, S., Reverse micelle-mediated synthesis of calcium phosphate nanocarriers for controlled release of bovine serum albumin. Acta Biomater. 2009, 5, 3112-3121. |
| [528] | Xia, C., Deng, X., Lin, Y. H., and Nan, C. W., Preparation and characterisation of nano-sized beta-tricalcium phosphate with a ps template method. Int. J. Mater. Product Technol. 2010, 37, 257-262. |
| [529] | Choi, D., and Kumta, P. N., Mechano-chemical synthesis and characterization of nanostructured β-TCP powder. Mater. Sci. Eng. C 2007, 27, 377-381. |
| [530] | Nikcević, I., Maravić, D., Ignjatović, N., Mitrić, M., Makoveć, D., and Uskoković, D., The formation and characterization of nanocrystalline phases by mechanical milling of biphasic calcium phosphate/poly-L-lactide biocomposite. Mater. Transact. 2006, 47, 2980-2986. |
| [531] | Cho, J. S., Jung, D. S., Han, J. M., and Kang, Y. C., Nano-sized α and β-TCP powders prepared by high temperature flame spray pyrolysis. Mater. Sci. Eng. C 2009, 29, 1288-1292. |
| [532] | Boutinguiza, M., Pou, J., Lusquiños, F., Comesaña, R., and Riveiro, A., Laser-assisted production of tricalcium phosphate nanoparticles from biological and synthetic hydroxyapatite in aqueous medium. Appl. Surf. Sci. 2011, 257, 5195-5199. |
| [533] | Jalota, S., Tas, A. C., and Bhaduri, S. B., Microwave-assisted synthesis of calcium phosphate nanowhiskers. J. Mater. Res. 2004, 19, 1876-1881. |
| [534] | Rameshbabu, N., and Rao, K. P., Microwave synthesis, characterization and in-vitro evaluation of nanostructured biphasic calcium phosphates. Curr. Appl. Phys. 2009, 9, S29-S31. |
| [535] | Li, B., Chen, X., Guo, B., Wang, X., Fan, H., and Zhang, X., Fabrication and cellular biocompatibility of porous carbonated biphasic calcium phosphate ceramics with a nanostructure. Acta Biomater. 2009, 5, 134-143. |
| [536] | Guha, A. K., Singh, S., Kumaresan, R., Nayar, S., and Sinha, A., Mesenchymal cell response to nanosized biphasic calcium phosphate composites. Colloids Surf. B 2009, 73, 146-151. |
| [537] | Layrolle, P., and Lebugle, A., Synthesis in pure ethanol and characterization of nanosized calcium phosphate fluoroapatite. Chem. Mater. 1996, 8, 134-144. |
| [538] | Andres, C., Sinani, V., Lee, D., Gun’ko, Y., and Kotov, N., Anisotropic calcium phosphate nanoparticles coated with 2-carboxyethylphosphonic acid. J. Mater. Chem. 2006, 16, 3964-3968. |
| [539] | Lim, H., Kassim, A., Huang, N., Hashim, R., Radiman, S., Khiew, P., and Chiu, W., Fabrication and characterization of 1D brushite nanomaterials via sucrose ester reverse microemulsion. Ceram. Int. 2009, 35, 2891-2897. |
| [540] | Shirkhanzadeh, M., and Sims, S., Immobilization of calcium phosphate nano-clusters into alkoxy-derived porous TiO2 coatings. J. Mater. Sci. Mater. Med. 1997, 8, 595-601. |
| [541] | Schmidt, H. T., and Ostafin, A. E., Liposome directed growth of calcium phosphate nanoshells. Adv. Mater. 2002, 14, 532-535. |
| [542] | Schmidt, H. T., Gray, B. L., Wingert, P. A., and Ostafin, A. E., Assembly of aqueous-cored calcium phosphate nanoparticles for drug delivery. Chem. Mater. 2004, 16, 4942-4947. |
| [543] | Yeo, C. H., Zein, S. H. S., Ahmad, A. L., and McPhail, D. S., Comparison of DOPA and DPPA liposome templates for the synthesis of calcium phosphate nanoshells. Ceram. Int. 2012, 38, 561-570. |
| [544] | Xu, H. H. K., Sun, L., Weir, M. D., Antonucci, J. M., Takagi, S., Chow, L. C., and Peltz, M., Nano DCPA – whisker composites with high strength and Ca and PO4 release. J. Dent. Res. 2006, 85, 722-727. |
| [545] | Xu, H. H. K., Weir, M. D., Sun, L., Takagi, S., and Chow, L. C., Effects of calcium phosphate nanoparticles on Ca-PO4 composite. J. Dent. Res. 2007, 86, 378-383. |
| [546] | Xu, H. H. K., Weir, M. D., and Sun, L., Nanocomposites with Ca and PO4 release: effects of reinforcement, dicalcium phosphate particle size and silanization. Dental Mater. 2007, 23, 1482-1491. |
| [547] | Singh, S., Bhardwaj, P., Singh, V., Aggarwal, S., and Mandal, U. K., Synthesis of nanocrystalline calcium phosphate in microemulsion – effect of nature of surfactants. J. Colloid Interf. Sci. 2008, 319, 322-329. |
| [548] | Wals, D., and Mann, S., Chemical synthesis of microskeletal calcium phosphate in bicontinuous microemulsions. Chem. Mater. 1996, 8, 1944-1953. |
| [549] | Ma, Z., Chen, F., Zhu, Y. J., Cui, T., and Liu, X. Y., Amorphous calcium phosphate/poly(D,L-lactic acid) composite nanofibers: electrospinning preparation and biomineralization. J. Coll. Interf. Sci. 2011, 15, 371-379. |
| [550] | Urch, H., Vallet-Regí, M., Ruiz, L., Gonzalez-Calbet, J. M., and Epple, M., Calcium phosphate nanoparticles with adjustable dispersability and crystallinity. J. Mater. Chem. 2009, 19, 2166-2171. |
| [551] | Holt, C., Wahlgren, N. M., and Drakenberg, T., Ability of a β-casein phosphopeptide to modulate the precipitation of calcium phosphate by forming amorphous dicalcium phosphate nanoclusters. Biochem. J. 1996, 314, 1035-1039. |
| [552] | Holt, C., Timmins, P. A., Errington, N., and Leaver, J., A core-shell model of calcium phosphate nanoclusters stabilized by β-casein phosphopeptides, derived from sedimentation equilibrium and small-angle X-ray and neutron-scattering measurements. Eur. J. Biochem. 1998, 252, 73-78. |
| [553] | Duan, B., Wang, M., Zhou, W. Y., and Cheung, W. L., Synthesis of Ca-P nanoparticles and fabrication of Ca-P/PHBV nanocomposite microspheres for bone tissue engineering applications. Appl. Surf. Sci. 2008, 255, 529-533. |
| [554] | Hwang, K. S., Jeon, K. O., Jeon, Y. S., and Kim, B. H., Hydroxyapatite forming ability of electrostatic spray pyrolysis derived calcium phosphate nano powder. J. Mater. Sci. 2006, 41, 4159-4162. |
| [555] | Hwang, K. S., Jeon, K. O., Jeon, Y. S., and Kim, B. H., Hydroxyapatite forming ability of electrostatic spray pyrolysis derived calcium phosphate nano powder. J. Mater. Sci. Mater. Med. 2007, 18, 619-622. |
| [556] | Perkin, K. K., Turner, J. L., Wooley, K. L., and Mann, S., Fabrication of hybrid nanocapsules by calcium phosphate mineralization of shell cross-linked polymer micelles and nanocages. Nano Lett. 2005, 5, 1457-1461. |
| [557] | Tjandra, W., Ravi, P., Yao, J., and Tam, K. C., Synthesis of hollow spherical calcium phosphate nanoparticles using polymeric nanotemplates. Nanotechnology 2006, 17, 5988-5994. |
| [558] | Sadasivan, S., Khushalani, D., and Mann, S., Synthesis of calcium phosphate nanofilaments in reverse micelles. Chem. Mater. 2005, 17, 2765-2770. |
| [559] | Morgan, T. T., Muddana, H. S., Altinoglu, E. I., Rouse, S. M., Tabakovic, A., Tabouillot, T., Russin, T. J., Butler, P. J., Eklund, P., Yun, J. K., Kester, M., and Adair, J. H., Encapsulation of organic molecules in calcium phosphate nanocomposite particles for intracellular imaging and drug delivery. Nano Lett. 2008, 8, 4108-4115. |
| [560] | Lai, C., Wang, Y. J., and Wei, K., Nucleation kinetics of calcium phosphate nanoparticles in reverse micelle solution. Colloids Surf. A 2008, 315, 268-274. |
| [561] | Yang, X., Gao, X., Gan, Y., Gao, C., Zhang, X., Ting, K., Wu, B. M., and Gou, Z., Facile synthesis of octacalcium phosphate nanobelts: growth mechanism and surface adsorption properties. J. Phys. Chem. C 2010, 114, 6265-6271. |
| [562] | Socol, G., Torricelli, P., Bracci, B., Iliescu, M., Miroiu, F., Bigi, A., Werckmann, J., and Mihailescu, I. N., Biocompatible nanocrystalline octacalcium phosphate thin films obtained by pulsed laser deposition. Biomaterials 2004, 25, 2539-2545. |
| [563] | Cho, J. S., Ko, Y. N., Koo, H. Y., and Kang, Y. C., Synthesis of nano-sized biphasic calcium phosphate ceramics with spherical shape by flame spray pyrolysis. J. Mater. Sci. Mater. Med. 2010, 21, 1143-1149. |
| [564] | Farzadi, A., Solati-Hashjin, M., Tahmasebi-Birgani, Z., and Aminian, A., Microwave-assisted synthesis and characterization of biphasic calcium phosphate nanopowders. Ceram. Transact. 2010, 218, 59-65. |
| [565] | Farzadi, A., Solati-Hashjin, M., Bakhshi, F., and Aminian, A., Synthesis and characterization of hydroxyapatite/β-tricalcium phosphate nanocomposites using microwave irradiation. Ceram. Int. 2011, 37, 65-71. |
| [566] | Pan, L., Li, Y., Zou, C., Weng, W., Cheng, K., Song, C., Du, P., Zhao, G., Shen, G., Wang, J., and Han, G., Surface modification of nanosized biphasic α-TCP/HA powders. Key Eng. Mater. 2007, 330-332, 223-226. |
| [567] | Urch, H., Franzka, S., Dahlhaus, D., Hartmann, N., Hasselbrink, E., and Epple, M., Preparation of two-dimensionally patterned layers of functionalised calcium phosphate nanoparticles by laser direct writing. J. Mater. Chem. 2006, 16, 1798-1802. |
| [568] | Sokolova, V., Prymak, O., Meyer-Zaika, W., Cölfen, H., Rehage, H., Shukla, A., and Epple, M., Synthesis and characterization of DNA functionalized calcium phosphate nanoparticles. Mat. -Wiss. u. Werkstofftech. 2006, 37, 441-445. |
| [569] | Muddana, H. S., Morgan, T. T., Adair, J. H., and Butler, P. J., Photophysics of Cy3-encapsulated calcium phosphate nanoparticles. Nano Lett. 2009, 9, 1559-1566. |
| [570] | Altinoğlu, E. I., Russin, T. J., Kaiser, J. M., Barth, B. M., Eklund, P. C., Kester, M., and Adair, J. H., Near-infrared emitting fluorophore-doped calcium phosphate nanoparticles for in vivo imaging of human breast cancer. ACS Nano 2008, 2, 2075-2084. |
| [571] | Schwiertz, J., Wiehe, A., Gräfe, S., Gitter, B., and Epple, M., Calcium phosphate nanoparticles as efficient carriers for photodynamic therapy against cells and bacteria. Biomaterials 2009, 30, 3324-3331. |
| [572] | Chen, F., Zhu, Y. J., Zhang, K. H., Wu, J., Wang, K. W., Tang, Q. L., and Mo, X. M., Europium-doped amorphous calcium phosphate porous nanospheres: preparation and application as luminescent drug carriers. Nanoscale Res. Lett. 2011, 6, 1-9. |
| [573] | Schwiertz, J., Meyer-Zaika, W., Ruiz-Gonzalez, L., González-Calbet, J. M., Vallet-Regí, M., and Epple, M., Calcium phosphate nanoparticles as templates for nanocapsules prepared by the layer-by-layer technique. J. Mater. Chem. 2008, 18, 3831-3834. |
| [574] | Hayakawa, S., Li, Y., Tsuru, K., Osaka, A., Fujii, E., and Kawabata, K., Preparation of nanometer-scale rod array of hydroxyapatite crystal. Acta Biomater. 2009, 5, 2152-2160. |
| [575] | Liao, S. S., Cui, F. Z., Zhang, W., and Feng, Q. L., Hierarchically biomimetic bone scaffold materials: nano-HA/collagen/PLA composite. J. Biomed. Mater. Res. B (Appl. Biomater.) 2004, 69B, 158-165. |
| [576] | Thomas, V., Dean, D. R., Jose, M. V., Mathew, B., Chowdhury, S., and Vohra, Y. K., Nanostructured biocomposite scaffolds based on collagen co-electrospun with nanohydroxyapatite. Biomacromolecules 2007, 8, 631-637. |
| [577] | de Yoreo, J. J., and Vekilov, P. G., Principles of crystal nucleation and growth. Rev. Mineral. Geochem. 2003, 54, 57-93. |
| [578] | Liao, S., Xu, G., Wang, W., Watari, F., Cui, F., Ramakrishna, S., and Chan, C. K., Self-assembly of nano-hydroxyapatite on multi-walled carbon nanotubes. Acta Biomater. 2007, 3, 669-675. |
| [579] | Penn, R. L., and Banfield, J. F., Imperfect oriented attachment: dislocation generation in defect-free nanocrystals. Science 1998, 281, 969-971. |
| [580] | Tao, J., Pan, H., Zeng, Y., Xu, X., and Tang, R., Roles of amorphous calcium phosphate and biological additives in the assembly of hydroxyapatite nanoparticles. J. Phys. Chem. B 2007, 111, 13410-13418. |
| [581] | Hing, K. A., Bone repair in the twenty-first century: biology, chemistry or engineering? Philos. Trans. R. Soc. Lond. A 2004, 362, 2821-2850. |
| [582] | Kokubo, T., Kim, H. M., and Kawashita, M., Novel bioactive materials with different mechanical properties. Biomaterials 2003, 24, 2161-2175. |
| [583] | Fu, J. M., Miao, B., Jia, L. H., and Lü, K. L., Nano-hydroxyapatite for repair of rabbit jaw bone defect: bone mineral density analysis. J. Clin. Rehabil. Tissue Eng. Res. 2009, 13, 2387-2390. |
| [584] | Zhou, H., and Lee, J., Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769-2781. |
| [585] | Barralet, J. E., Lilley, K. J., Grover, L. M., Farrar, D. F., Ansell, C., and Gbureck, U., Cements from nanocrystalline hydroxyapatite. J. Mater. Sci. Mater. Med. 2004, 15, 407-411. |
| [586] | Lilley, K. J., Gbureck, U., Wright, A. J., Farrar, D. F., and Barralet, J. E., Cement from nanocrystalline hydroxyapatite: effect of calcium phosphate ratio. J. Mater. Sci. Mater. Med. 2005, 16, 1185-1190. |
| [587] | Neira, I. S., Kolen’ko, Y. V., Lebedev, O. I., van Tendeloo, G., Gupta, H. S., Matsushita, N., Yoshimura, M., and Guitián, F., Rational synthesis of a nanocrystalline calcium phosphate cement exhibiting rapid conversion to hydroxyapatite. Mater. Sci. Eng. C 2009, 29, 2124-2132. |
| [588] | Dorozhkin, S. V., Calcium orthophosphate cements for biomedical application. J. Mater. Sci. 2008, 43, 3028-3057. |
| [589] | Dorozhkin, S. V., Calcium orthophosphate cements and concretes. Materials 2009, 2, 221-291. |
| [590] | Fu, Q., Zhou, N., Huang, W., Wang, D., Zhang, L., and Li, H., Effects of nano HAP on biological and structural properties of glass bone cement. J. Biomed. Mater. Res. A 2005, 74A, 156-163. |
| [591] | Strnadova, M., Protivinsky, J., Strnad, J., and Vejsicka, Z., Preparation of porous synthetic nanostructured HA scaffold. Key Eng. Mater. 2008, 361-363, 211-214. |
| [592] | Kim, J. Y., Lee, J. W., Lee, S. J., Park, E. K., S. Y. Kim, and Cho, D. W., Development of a bone scaffold using HA nanopowder and micro-stereolithography technology. Microelectronic Engineering 2007, 84, 1762-1765. |
| [593] | Severin, A. V., Komarov, V. F., Bozhevol’nov, V. E., and Melikhov, I. V., Morphological selection in suspensions of nanocrystalline hydroxylapatite leading to spheroidal aggregates. Russ. J. Inorg. Chem. 2005, 50, 72-77. |
| [594] | Krylova, I. V., Ivanov, L. N., Bozhevol’nov, V. E., and Severin, A. V., Self-organization processes and phase transitions in nanocrystalline hydroxyapatite according to exoemission data. Russ. J. Phys. Chem. A 2007, 81, 241-245. |
| [595] | Veljovic, D., Jokic, B., Jankovic-Castvan, I., Smiciklas, I., Petrovic, R., and Janackovic, D., Sintering behaviour of nanosized HAP powder. Key Eng. Mater. 2007, 330-332, 259-262. |
| [596] | Zhang, F., Lin, K., Chang, J., Lu, J., and Ning, C., Spark plasma sintering of macroporous calcium phosphate scaffolds from nanocrystalline powders. J. Eur. Ceram. Soc. 2008, 28, 539-545. |
| [597] | Grossin, D., Banu, M., Sarda, S., Martinet-Rollin, S., Drouet, C., Estournès, C., Champion, E., Rossignol, F., Combes, C., and Rey, C., Low temperature consolidation of nanocrystalline apatites toward a new generation of calcium phosphate ceramics. Ceram. Eng. Sci. Proc. 2010, 30, 113-126. |
| [598] | Chaudhry, A. A., Yan, H., Gong, K., Inam, F., Viola, G., Reece, M. J., Goodall, J. B. M., ur Rehman, I., McNeil-Watson, F. K., Corbett, J. C. W., Knowles, J. C, and Darr, J. A., High-strength nanograined and translucent hydroxyapatite monoliths via continuous hydrothermal synthesis and optimized spark plasma sintering. Acta Biomater. 2011, 7, 791-799. |
| [599] | Eriksson, M., Liu, Y., Hu, J., Gao, L., Nygren, M., and Shen, Z., Transparent hydroxyapatite ceramics with nanograin structure prepared by high pressure spark plasma sintering at the minimized sintering temperature. J. Eur. Ceram. Soc. 2011, 31, 1533-1540. |
| [600] | Kutty, M. G., Loertscher, J., Bhaduri, S., Bhaduri, S. B., and Tinga, W. R., Microwave sintering of nanocrystalline hydroxyapatite. Ceram. Eng. Sci. Proc. 2001, 22, 3-10. |
| [601] | Vijayan, S., and Varma, H., Microwave sintering of nanosized hydroxyapatite powder compacts. Mater. Lett. 2002, 56, 827-831. |
| [602] | Ramesh, S., Tan, C. Y., Bhaduri, S. B., and Teng, W. D., Rapid densification of nanocrystalline hydroxyapatite for biomedical applications. Ceram. Int. 2007, 33, 1363-1367. |
| [603] | Okada, M., and Furuzono, T., Fabrication of high-dispersibility nanocrystals of calcined hydroxyapatite. J. Mater. Sci. 2006, 41, 6134-6137. |
| [604] | Okada, M., and Furuzono, T., Nanosized ceramic particles of hydroxyapatite calcined with an anti-sintering agent. J. Nanosci. Nanotechnol. 2007, 7, 848-851. |
| [605] | Okada, M., and Furuzono, T., Calcination of rod-like hydroxyapatite nanocrystals with an anti-sintering agent surrounding the crystals. J. Nanopart. Res. 2007, 9, 807-815. |
| [606] | Müller-Mai, C. M., Stupp, S. I., Voigt, C., and Gross, U., Nanoapatite and organoapatite implants in bone: histology and ultrastructure of the interface. J. Biomed. Mater. Res. 1995, 29, 9-18. |
| [607] | Du, C., Cui, F. Z., Feng, Q. L., Zhu, X. D., and de Groot, K., Tissue response to nano-hydroxyapatite/collagen composite implants in marrow cavity. J. Biomed. Mater. Res. 1998, 42, 540-548. |
| [608] | Du, C., Cui, F. Z., Zhu, X. D., and de Groot, K., Three-dimensional nano-HAp/collagen matrix loading with osteogenic cells in organ culture. J. Biomed. Mater. Res. 1999, 44, 407-415. |
| [609] | Paul, W., and Sharma, C. P., Nanoceramic matrices: biomedical applications. Am. J. Biochem. Biotechnol. 2006, 2, 41-48. |
| [610] | Huber, F. X., McArthur, N., Hillmeier, J., Kock, H. J., Baier, M., Diwo, M., Berger, I., and Meeder, P. J., Void filling of tibia compression fracture zones using a novel resorbable nanocrystalline hydroxyapatite paste in combination with a hydroxyapatite ceramic core: first clinical results. Arch. Orthop. Trauma Surg. 2006, 126, 533-540. |
| [611] | Smeets, R., Jelitte, G., Heiland, M., Kasaj, A., Grosjean, M., Riediger, D., Yildirim, M., Spiekermann, H., and Maciejewski, O., Hydroxylapatit-Knochenersatzmaterial (Ostim®) bei der Sinusbodenelevation. Schweiz Monatsschr. Zahnmed. 2008, 118, 203-208. |
| [612] | Gerlach, K. L., and Niehues, D., Die Behandlung der Kieferzysten mit einem neuartigen nanopartikulären Hydroxylapatit. Mund Kiefer GesichtsChir. 2007, 11, 131-137. |
| [613] | Schwarz, F., Bieling, K., Latz, T., Nuesry, E., and Becker, J., Healing of intrabony periimplantitis defects following application of a nanocristalline hydroxyapatite (Ostim™) or a bovine-derived xenograft (Bio-Oss™) in combination with a collagen membrane (Bio-Gide™). A case series. J. Clin. Periodontol. 2006, 33, 491-499. |
| [614] | Strietzel, F. P., Reichart, P. A., and Graf, H. L., Lateral alveolar ridge augmentation using a synthetic nano-crystalline hydroxyapatite bone substitution material (Ostim®). Preliminary clinical and histological results. Clin. Oral Implants Res. 2007, 18, 743-751. |
| [615] | Spies, C., Schnürer, S., Gotterbarm, T., and Breusch, S., Tierexperimentelle Untersuchung des Knochenersatzstoffs OstimTM im knöchernen Lager des Göttinger Miniaturschweins. Z. Orthop. Unfall. 2008, 146, 64-69. |
| [616] | Thorwarth, M., Schultze-Mosgau, S., Kessler, P., Wiltfang, J., and Schlegel, K. A., Bone regeneration in osseous defects using a resorbable nanoparticular hydroxyapatite. J. Oral Maxillofac. Surg. 2005, 63, 1626-1633. |
| [617] | Brandt, J., Henning, S., Michler, G., Schulz, M., and Bernstein, A., Nanocrystalline hydroxyapatite for bone repair. Key Eng. Mater. 2008, 361-363, 35-38. |
| [618] | Huber, F. X., Hillmeier, J., Herzog, L., McArthur, N., Kock, H. J., and Meeder, P. J., Open reduction and palmar plate-osteosynthesis in combination with a nanocrystalline hydroxyapatite spacer in the treatment of comminuted fractures of the distal radius. J. Hand Surg. (Brit.) 2006, 31B, 298-303. |
| [619] | Huber, F. X., Hillmeier, J., McArthur, N., Kock, H. J., and Meeder, P. J., The use of nanocrystalline hydroxyapatite for the reconstruction of calcaneal fractures: preliminary results. J. Foot Ankle Surg. 2006, 45, 322-328. |
| [620] | Laschke, M. W., Witt, K., Pohlemann, T., and Menger, M. D., Injectable nanocrystalline hydroxyapatite paste for bone substitution: in vivo analysis of biocompatibility and vascularization. J. Biomed. Mater. Res. B (Appl. Biomater.) 2007, 82B, 494-505. |
| [621] | Spies, C. K. G., Schnürer, S., Gotterbarm, T., and Breusch, S., The efficacy of Biobon™ and Ostim™ within metaphyseal defects using the Göttinger Minipig. Arch. Orthop. Trauma Surg. 2009, 129, 979-988. |
| [622] | Chitsazi, M. T., Shirmohammadi, A., Faramarzie, M., Pourabbas, R., and Rostamzadeh, A. N., A clinical comparison of nano-crystalline hydroxyapatite (Ostim) and autogenous bone graft in the treatment of periodontal intrabony defects. Medicina Oral, Patologia Oral y Cirugia Bucal 2011, 16, 448-453. |
| [623] | Huber, F. X., Belyaev, O., Hillmeier, J., Kock, H. J., Huber, C., Meeder, P. J., and Berger, I., First histological observations on the incorporation of a novel nanocrystalline hydroxyapatite paste OSTIM® in human cancellous bone. BMC Musculoskelett. Disord. 2006, 7, 50 (14 pages). |
| [624] | Huber, F. X., Berger, I., McArthur, N., Huber, C., Kock, H. P., Hillmeier, J., and Meeder, P. J., Evaluation of a novel nanocrystalline hydroxyapatite paste and a solid hydroxyapatite ceramic for the treatment of critical size bone defects (CSD) in rabbits. J. Mater. Sci. Mater. Med. 2008, 19, 33-38. |
| [625] | Arts, J. J. C., Verdonschot, N., Schreurs, B. W., and Buma, P., The use of a bioresorbable nano-crystalline hydroxyapatite paste in acetabular bone impaction grafting. Biomaterials 2006, 27, 1110-1118. |
| [626] | Zhang, W., Liao, S. S., and Cui, F. Z., Hierarchical self-assembly of nano-fibrils in mineralized collagen. Chem. Mater. 2003, 15, 3221-3226. |
| [627] | Li, X., Huang, J., and Edirisinghe, M. J., Development of nano-hydroxyapatite coating by electrohydrodynamic atomization spraying. J. Mater. Sci. Mater. Med. 2008, 19, 1545-1551. |
| [628] | Guo, L., and Li, H., Fabrication and characterization of thin nano-hydroxyapatite coatings on titanium. Surf. Coat. Technol. 2004, 185, 268-274. |
| [629] | Thian, E. S., Ahmad, Z., Huang, J., Edirisinghe, M. J., Jayasinghe, S. N., Ireland, D. C., Brooks, R. A., Rushton, N., Bonfield, W., and Best, S. M., Electrosprayed nanoapatite: a new generation of bioactive material. Key Eng. Mater. 2008, 361-363, 597-600. |
| [630] | Han, Y., Xu, K., Montay, G., Fu, T., and Lu, J., Evaluation of nanostructured carbonated hydroxyapatite coatings formed by a hybrid process of plasma spraying and hydrothermal synthesis. J. Biomed. Mater. Res. 2002, 60, 511-516. |
| [631] | Li, P., Biomimetic nano-apatite coating capable of promoting bone ingrowth. J. Biomed. Mater. Res. A 2003, 66A, 79-85. |
| [632] | Mendes, V. C., Moineddin, R., and Davies, J. E., The effect of discrete calcium phosphate nanocrystals on bone-bonding to titanium surfaces. Biomaterials 2007, 28, 4748-4755. |
| [633] | Oh, S. H., Finõnes, R. R., Daraio, C., Chen, L. H., and Jin, S., Growth of nano-scale hydroxyapatite using chemically treated titanium oxide nanotubes. Biomaterials 2005, 26, 4938-4943. |
| [634] | Ma, J., Wong, H., Kong, L. B., and Peng, K. W., Biomimetic processing of nanocrystallite bioactive apatite coating on titanium. Nanotechnology 2003, 14, 619-623. |
| [635] | Gu, Y. W., Tay, B. Y., Lim, C. S., and Yong, M. S., Nanocrystallite apatite formation and its growth kinetics on chemically treated porous NiTi. Nanotechnology 2006, 17, 2212-2218. |
| [636] | Hu, R., Lin, C. J., and Shi, H. Y., A novel ordered nano hydroxyapatite coating electrochemically deposited on titanium substrate. J. Biomed. Mater. Res. A 2007, 80A, 687-692. |
| [637] | Bigi, A., Boanini, E., Bracci, B., Facchini, A., Panzavolta, S., Segatti, F., and Sturba, L., Nanocrystalline hydroxyapatite coatings on titanium: a new fast biomimetic method. Biomaterials 2005, 26, 4085-4089. |
| [638] | Narayanan, R., Seshadri, S. K., Kwon, T. Y., and Kim, K. H., Electrochemical nano-grained calcium phosphate coatings on Ti-6Al-4V for biomaterial applications. Scripta Mater. 2007, 56, 229-232. |
| [639] | Thian, E. S., Huang, J., Best, S. M., Barber, Z. H., and Bonfield, W., Nanostructured apatite coatings for rapid bone repair. Key Eng. Mater. 2006, 309-311, 519-522. |
| [640] | Cai, X., Gong, P., Man, Y., Chen, Z., and He, G., The construction and characterization of nano-FHA bioceramic coating on titanium surface. Key Eng. Mater. 2007, 330-332, 333-336. |
| [641] | Citterio, H., Jakani, S., Benmarouane, A., Millet, P., and Lodini, A., Nano-hydroxyapatite coatings on titanium substrates. Finite element analysis of process and experimental plasma thermal sprayed coatings. Key Eng. Mater. 2008, 361-363, 745-748. |
| [642] | Lee, S. H., Kim, H. E., and Kim, H. W., Nanosized hydroxyapatite coatings on Ti substrate with TiO2 buffer layer by e-beam deposition. J. Am. Ceram. Soc. 2007, 90, 50-56. |
| [643] | Nishimura, I., Huang, Y., Butz, F., Ogawa, T., Lin, A., and Wang, C. J., Discrete deposition of hydroxyapatite nanoparticles on a titanium implant with predisposing substrate microtopography accelerated osseointegration. Nanotechnology 2007, 18, 245101 (9 pages). |
| [644] | Narayanan, R., Kwon, T. Y., and Kim, K. H., Preparation and characteristics of nano-grained calcium phosphate coatings on titanium from ultrasonated bath at acidic pH. J. Biomed. Mater. Res. B (Appl. Biomater.) 2008, 85B, 231-239. |
| [645] | Hahn, B. D., Park, D. S., Choi, J. J., Ryu, J., Yoon, W. H., Kim, K. H., Park, C., and Kim, H. E., Dense nanostructured hydroxyapatite coating on titanium by aerosol deposition. J. Am. Ceram. Soc. 2009, 92, 683-687. |
| [646] | Narayanan, R., Kwon, T. Y., and Kim, K. H., Direct nanocrystalline hydroxyapatite formation on titanium from ultrasonated electrochemical bath at physiological pH. Mater. Sci. Eng. C 2008, 28, 1265-1270. |
| [647] | Yousefpour, M., Afshar, A., Yang, X., Li, X., Yang, B., Wu, Y., Chen, J., and Zhang, X., Nano-crystalline growth of electrochemically deposited apatite coating on pure titanium. J. Electroanal. Chem. 2006, 589, 96-105. |
| [648] | Mendes, V. C., Moineddin, R., and Davies, J. E., Discrete calcium phosphate nanocrystalline deposition enhances osteoconduction on titanium-based implant surfaces. J. Biomed. Mater. Res. A 2009, 90A, 577-585. |
| [649] | Yang, Y., Kim, K. H., and Ong, J. L., A review on calcium phosphate coatings produced using a sputtering process – an alternative to plasma spraying. Biomaterials 2005, 26, 327-337. |
| [650] | Nies, B., Rößler, S., and Reinstorf, A., Formation of nano hydroxyapatite – a straightforward way to bioactivate bone implant surfaces. Int. J. Mat. Res. (formerly Z. Metallkd.) 2007, 98, 630-636. |
| [651] | Jalota, S., Bhaduri, S. B., and Tas, A. C., Effect of carbonate content and buffer type on calcium phosphate formation in SBF solutions. J. Mater. Sci. Mater. Med. 2006, 17, 697-707. |
| [652] | Chen, F., Lam, W. M., Lin, C. J., Qiu, G. X., Wu, Z. H., Luk, K. D. K., and Lu, W. W., Biocompatibility of electrophoretical deposition of nanostructured hydroxyapatite coating on roughen titanium surface: in vitro evaluation using mesenchymal stem cells. J. Biomed. Mater. Res. B (Appl. Biomater.) 2007, 82B, 183-191. |
| [653] | Thian, E. S., Ahmad, Z., Huang, J., Edirisinghe, M. J., Jayasinghe, S. N., Ireland, D. C., Brooks, R. A., Rushton, N., Bonfield, W., and Best, S. M., The role of electrosprayed nanoapatites in guiding osteoblast behaviour. Biomaterials 2008, 29, 1833-1843. |
| [654] | Bigi, A., Nicoli-Aldini, N., Bracci, B., Zavan, B., Boanini, E., Sbaiz, F., Panzavolta, S., Zorzato, G., Giardino, R., Facchini, A., Abatangelo, G., and Cortivo, R., In vitro culture of mesenchymal cells onto nanocrystalline hydroxyapatite coated Ti13Nb13Zr alloy. J. Biomed. Mater. Res. A 2007, 82A, 213-221. |
| [655] | Bigi, A., Fini, M., Bracci, B., Boanini, E., Torricelli, P., Giavaresi, G., Aldini, N. N., Facchini, A., Sbaiz, F., and Giardino, R., The response of bone to nanocrystalline hydroxyapatite-coated Ti13Nb11Zr alloy in an animal model. Biomaterials 2008, 29, 1730-1736. |
| [656] | Thian, E. S., Huang, J., Ahmad, Z., Edirisinghe, M. J., Jayasinghe, S. N., Ireland, D. C., Brooks, R. A., Rushton, N., Best, S. M., and Bonfield, W., Influence of nanohydroxyapatite patterns deposited by electrohydrodynamic spraying on osteoblast response. J. Biomed. Mater. Res. A 2008, 85A, 188-194. |
| [657] | Furuzono, T., Masuda, M., Okada, M., Yasuda, S., Kadono, H., Tanaka, R., and Miyatake, K., Increase in cell adhesiveness on a poly(ethylene terephthalate) fabric by sintered hydroxyapatite nanocrystal coating in the development of an artificial blood vessel. ASAIO J. 2006, 52, 315-320. |
| [658] | Yanagida, H., Okada, M., Masuda, M., Ueki, M., Narama, I., Kitao, S., Koyama, Y., Furuzono, T., and Takakuda, K., Cell adhesion and tissue response to hydroxyapatite nanocrystal-coated poly(L-lactic acid) fabric. J. Biosci. Bioeng. 2009, 108, 235-243. |
| [659] | Li, X., Huang, J., and Edirisinghe, M. J., Development of template-assisted electrohydrodynamic atomization spraying for nanoHA patterning. Key Eng. Mater. 2008, 361-363, 585-588. |
| [660] | Shi, Z. L., Huang, X., Cai, Y. R., Tang, R. K., and Yang, D. S., Size effect of hydroxyapatite nanoparticles on proliferation and apoptosis of osteoblast-like cells. Acta Biomater. 2009, 5, 338-345. |
| [661] | Liu, Y., Wang, G., Cai, Y., Ji, H., Zhou, G., Zhao, X., Tang, R., and Zhang, M., In vitro effects of nanophase hydroxyapatite particles on proliferation and osteogenic differentiation of bone marrow-derived mesenchymal stem cells. J. Biomed. Mater. Res. A 2009, 15, 1083-1091. |
| [662] | Zhu, X., Eibl, O., Scheideler, L., and Geis-Gerstorfer, J., Characterization of nano hydroxyapatite/collagen surfaces and cellular behaviors. J. Biomed. Mater. Res. A 2006, 79A, 114-127. |
| [663] | Zhu, W., Zhang, X., Wang, D., Lu, W., Ou, Y., Han, Y., Zhou, K., Liu, H., Fen, W., Peng, L., He, C., and Zeng, Y., Experimental study on the conduction function of nano-hydroxyapatite artificial bone. Micro Nano Lett. 2010, 5, 19-27. |
| [664] | Wang, H., Li, Y., Zuo, Y., Li, J., Ma, S., and Cheng, L., Biocompatibility and osteogenesis of biomimetic nano-hydroxyapatite/polyamide composite scaffolds for bone tissue engineering. Biomaterials 2007, 28, 3338-3348. |
| [665] | Zhang, Y. F., Cheng, X. R., Chen, Y., Shi, B., Chen, X. H., Xu, D. X., and Ke, J., Three-dimensional nanohydroxyapatite / chitosan scaffolds as potential tissue engineered periodontal tissue. J. Biomater. Appl. 2007, 21, 333-349. |
| [666] | Huang, Y. X., Ren, J., Chen, C., Ren, T. B., and Zhou, X. Y., Preparation and properties of poly(lactide-co-glycolide) (PLGA) / nano-hydroxyapatite (NHA) scaffolds by thermally induced phase separation and rabbit mscs culture on scaffolds. J. Biomater. Appl. 2008, 22, 409-432. |
| [667] | Thian, E. S., Ahmad, Z., Huang, J., Edirisinghe, M. J., Jayasinghe, S. N., Ireland, D. C., Brooks, R. A., Rushton, N., Bonfield, W., and Best, S. M., Bioactivity of nanoapatite produced by electrohydrodynamic atomization. J. Bionanosci. 2007, 1, 60-63. |
| [668] | Pezzatini, S., Solito, R., Morbidelli, L., Lamponi, S., Boanini, E., Bigi, A., and Ziche, M., The effect of hydroxyapatite nanocrystals on microvascular endothelial cell viability and functions. J. Biomed. Mater. Res. A 2006, 76A, 656-663. |
| [669] | Pezzatini, S., Morbidelli, L., Solito, R., Paccagnini, E., Boanini, E., Bigi, A., and Ziche, M., Nanostructured HA crystals up-regulate FGF-2 expression and activity in microvascular endothelium promoting angiogenesis. Bone 2007, 41, 523-534. |
| [670] | Hu, Q., Tan, Z., Liu, Y., Tao, J., Cai, Y., Zhang, M., Pan, H., Xu, X., and Tang, R., Effect of crystallinity of calcium phosphate nanoparticles on adhesion, proliferation, and differentiation of bone marrow mesenchymal stem cells. J. Mater. Chem. 2007, 17, 4690-4698. |
| [671] | Kim, K., Dean, D., Lu, A., Mikos, A. G., and Fisher, J. P., Early osteogenic signal expression of rat bone marrow stromal cells is influenced by both hydroxyapatite nanoparticle content and initial cell seeding density in biodegradable nanocomposite scaffolds. Acta Biomater. 2011, 7, 1249-1264. |
| [672] | Svanborg, L. M., Hoffman, M., Andersson, M., Currie, F., Kjellin, P., and Wennerberg, A., The effect of hydroxyapatite nanocrystals on early bone formation surrounding dental implants. Int. J. Oral Maxillofac. Surg. 2011, 40, 308-315. |
| [673] | Balasundaram, G., Sato, M., and Webster, T. J., Using hydroxyapatite nanoparticles and decreased crystallinity to promote osteoblast adhesion similar to functionalizing with RGD. Biomaterials 2006, 27, 2798-2805. |
| [674] | Detsch, R., Hagmeyer, D., Neumann, M., Schaefer, S., Vortkamp, A., Wuelling, M., Ziegler, G., and Epple, M., The resorption of nanocrystalline calcium phosphates by osteoclast-like cells. Acta Biomater. 2010, 6, 3223-3233. |
| [675] | Stevens, M. M., and George, J. H., Exploring and engineering the cell surface interface. Science 2005, 310, 1135-1138. |
| [676] | Martínez, E., Engel, E., Planell, J. A., and Samitier, J., Effects of artificial micro- and nano-structured surfaces on cell behaviour. Annals Anat. 2009, 191, 126-135. |
| [677] | Lee, D. H., Han, J. S., Yang, J. H., and Lee, J. B., MC3T3-E1 cell response to pure titanium, zirconia and nano-hydroxyapatite. Int. J. Modern Phys. B 2009, 23, 1535-1540. |
| [678] | Onuma, K., Yamagishi, K., and Oyane, A., Nucleation and growth of hydroxyapatite nanocrystals for nondestructive repair of early caries lesions. J. Cryst. Growth 2005, 282, 199-207. |
| [679] | Huang, S., Gao, S., Cheng, L., and Yu, H., Remineralization potential of nano-hydroxyapatite on initial enamel lesions: an in vitro study. Caries Res. 2011, 45, 460-468. |
| [680] | Roveri, N., Battistella, E., Bianchi, C. L., Foltran, I., Foresti, E., Iafisco, M., Lelli, M., Naldoni, A., Palazzo, B., and Rimondini, L., Surface enamel remineralization: biomimetic apatite nanocrystals and fluoride ions different effects. J. Nanomater. 2009, 746383 (9 pages). |
| [681] | Lv, K., Zhang, J., Meng, X., and Li, X., Remineralization effect of the nano-HA toothpaste on artificial caries. Key Eng. Mater. 2007, 330-332, 267-270. |
| [682] | Jeong, S. H., Jang, S. O., Kim, K. N., Kwon, H. K., Park, Y. D., and Kim, B. I., Remineralization potential of new toothpaste containing nano-hydroxyapatite. Key Eng. Mater. 2006, 309-311, 537-540. |
| [683] | Tschoppe, P., Zandim, D. L., Martus, P., and Kielbassa, A. M., Enamel and dentine remineralization by nano-hydroxyapatite toothpastes. J. Dent. 2011, 39, 430-437. |
| [684] | Wang, C. J., Zhang, Y. F., Wei, J., and Wei, S. C., Repair of artificial enamel lesions by nano fluorapatite paste containing fluorin. J. Clin. Rehabil. Tiss. Eng. Res. 2011, 15, 6346-6350. |
| [685] | Kim, B. I., Jeong, S. H., Jang, S. O., Kim, K. N., Kwon, H. K., and Park, Y. D., Tooth whitening effect of toothpastes containing nano-hydroxyapatite. Key Eng. Mater. 2006, 309-311, 541-544. |
| [686] | Collares, F. M., Leitune, V. C. B., Rostirolla, F. V., Trommer, R. M., Bergmann, C. P., and Samuel, S. M. W., Nanostructured hydroxyapatite as filler for methacrylate-based root canal sealers. Int. Endodontic 2012, 45, 63-67. |
| [687] | Kim, M. Y., Kwon, H. K., Choi, C. H., and Kim, B. I., Combined effects of nano-hydroxyapatite and NaF on remineralization of early caries lesion. Key Eng. Mater. 2007, 330-332, 1347-1350. |
| [688] | Lee, H. J., Min, J. H., Choi, C. H., Kwon, H. G., and Kim, B. I., Remineralization potential of sports drink containing nano-sized hydroxyapatite. Key Eng. Mater. 2007, 330-332, 275-278. |
| [689] | Min, J. H., Kwon, H. K., and Kim, B. I., The addition of nano-sized hydroxyapatite to a sports drink to inhibit dental erosion – in vitro study using bovine enamel. J. Dent. 2011, 39, 629-635. |
| [690] | Hong, Y. W., Kim, J. H., Lee, B. H., Lee, Y. K., Choi, B. J., Lee, J. H., and Choi, H. J., The effect of nano-sized β-tricalcium phosphate on remineralization in glass ionomer dental luting cement. Key Eng. Mater. 2008, 361-363, 861-864. |
| [691] | Li, L., Pan, H. H., Tao, J. H., Xu, X. R., Mao, C. Y., Gu, X. H., and Tang, R. K., Repair of enamel by using hydroxyapatite nanoparticles as the building blocks. J. Mater. Chem. 2008, 18, 4079-4084. |
| [692] | Meng, X., Lv, K., Zhang, J., and Qu, D., Caries inhibitory activity of the nano-HA in vitro. Key Eng. Mater. 2007, 330-332, 251-254. |
| [693] | Li, B. G., Wang, J. P., Zhao, Z. Y., Sui, Y. F., and Zhang, Y. X., Mineralizing of nano-hydroxyapatite powders on artificial caries. Rare Metal. Mat. Eng. 2007, 36, 128-130. |
| [694] | Ashokan, A., Menon, D., Nair, S., and Koyakutty, M., A molecular receptor targeted, hydroxyapatite nanocrystal based multi-modal contrast agent. Biomaterials 2010, 31, 2606-2616. |
| [695] | Bauer, I. W., Li, S. P., Han, Y. C., Yuan, L., and Yin, M. Z., Internalization of hydroxyapatite nanoparticles in liver cancer cells. J. Mater. Sci. Mater. Med. 2008, 19, 1091-1095. |
| [696] | Liu, T., Tang, A., Zhang, G. Y., Chen, Y. X., Zhang, J. Y., Peng, S. S., and Cai, Z. M., Calcium phosphate nanoparticles as a novel nonviral vector for efficient transfection of DNA in cancer gene therapy. Cancer Biother. Radiopharm. 2005, 20, 141-149. |
| [697] | Czupryna, J., and Tsourkas, A., Suicide gene delivery by calcium phosphate nanoparticles. A novel method of targeted therapy for gastric cancer. Cancer Biol. Ther. 2006, 5, 1691-1692. |
| [698] | Li, B., Guo, B., Fan, H., and Zhang, X., Preparation of nano-hydroxyapatite particles with different morphology and their response to highly malignant melanoma cells in vitro. Appl. Surf. Sci. 2008, 255, 357-360. |
| [699] | Dai, H., Pei, C., Han, Y., Xinyu, W., and Li, S., Inhibitory effect of hydroxyapatite nanoparticles on K562 cells. Mater. Sci. Forum 2011, 685, 352-356. |
| [700] | Borum, L., and Wilson, O. C., Surface modification of hydroxyapatite. Part II. Silica. Biomaterials 2003, 24, 3681-3688. |
| [701] | Lee, H. J., Kim, S. E., Choi, H. W., Kim, C. W., Kim, K. J., and Lee, S. C., The effect of surface-modified nano-hydroxyapatite on biocompatibility of poly(ε-caprolactone)/hydroxyapatite nanocomposites. Eur. Polym. J. 2007, 43, 1602-1608. |
| [702] | Wilson, O. C., and Hull, J. R., Surface modification of nanophase hydroxyapatite with chitosan. Mater. Sci. Eng. C 2008, 28, 434-437. |
| [703] | Liao, J. G., Wang, X. J., Zuo, Y., Zhang, L., Wen, J. Q., and Li, Y. B., Surface modification of nano-hydroxyapatite with silane agent. J. Inorg. Mater. 2008, 23, 145-149. |
| [704] | Wang, Y., Xiao, Y., Huang, X., and Lang, M., Preparation of poly(methyl methacrylate) grafted hydroxyapatite nanoparticles via reverse ATRP. J. Coll. Interf. Sci. 2011, 15, 415-421. |
| [705] | Deng, C., Xiao, X., Yao, N., Yang, X. B., and Weng, J., Effect of surface modification of nano-hydroxyapatite particles on in vitro biocompatibility of poly (ε-caprolactone)-matrix composite biomaterials. Int. J. Polym. Mater. 2011, 60, 969-978. |
| [706] | Jensen, T., Baas, J., Dolathshahi-Pirouz, A., Jacobsen, T., Singh, G., Nygaard, J. V., Foss, M., Bechtold, J., Bünger, C., Besenbacher, F., and Søballe, K., Osteopontin functionalization of hydroxyapatite nanoparticles in a PDLLA matrix promotes bone formation. J. Biomed. Mater. Res. A 2011, 99A, 94-101. |
| [707] | Ramachandran, R., Paul, W., and Sharma, C. P., Synthesis and characterization of PEGylated calcium phosphate nanoparticles for oral insulin delivery. J. Biomed. Mater. Res. B (Appl. Biomater.) 2009, 88B, 41-48. |
| [708] | Uskoković, V., and Uskoković, D. P., Nanosized hydroxyapatite and other calcium phosphates: chemistry of formation and application as drug and gene delivery agents. J. Biomed. Mater. Res. B (Appl. Biomater.) 2010, 96B, 152-191. |
| [709] | Loo, S. C., Moore, T., Banik, B., and Alexis, F., Biomedical applications of hydroxyapatite nanoparticles. Curr. Pharm. Biotechnol. 2010, 11, 333-342. |
| [710] | Fu, H., Hu, Y., McNelis, T., and Hollinger, J. O., A calcium phosphate-based gene delivery system. J. Biomed. Mater. Res. A 2005, 74A, 40-48. |
| [711] | Liu, T. Y., Chen, S. Y., Liu, D. M., and Liou, S. C., On the study of BSA-loaded calcium-deficient hydroxyapatite nano-carriers for controlled drug delivery. J. Control. Release 2005, 107, 112-121. |
| [712] | Barroug, A., Kuhn, L. T., Gerstenfeld, L. C., and Glimcher, M. J., Interactions of cisplatin with calcium phosphate nanoparticles: in vitro controlled adsorption and release. J. Orthop. Res. 2004, 22, 703-708. |
| [713] | Cheng, X. G., and Kuhn, L. T., Chemotherapy drug delivery from calcium phosphate nanoparticles. Int. J. Nanomed. 2007, 2, 667-674. |
| [714] | Maitra, A., Calcium phosphate nanoparticles: second-generation nonviral vectors in gene therapy. Expert Rev. Mol. Diagn. 2005, 5, 893-905. |
| [715] | Yang, X. C., Walboomers, X. F., van den Dolder, J., Yang, F., Bian, Z., Fan, M. W., and Jansen, J. A., Non-viral bone morphogenetic protein 2 transfection of rat dental pulp stem cells using calcium phosphate nanoparticles as carriers. Tissue Eng. A 2008, 14, 71-81. |
| [716] | Altinoğlu, E. I., and Adair, J. H., Calcium phosphate nanocomposite particles: a safer and more effective alternative to conventional chemotherapy? Future Oncology 2009, 5, 279-281. |
| [717] | Joyappa, D. H., Kumar, C. A., Banumathi, N., Reddy, G. R., and Suryanarayana, V. V. S., Calcium phosphate nanoparticle prepared with foot and mouth disease virus P1-3CD gene construct protects mice and guinea pigs against the challenge virus. Veter. Microbiol. 2009, 139, 58-66. |
| [718] | Dreesen, I. A. J., Lüchinger, N. A., Stark, W. J., and Fussenegger, M., Tricalcium phosphate nanoparticles enable rapid purification, increase transduction kinetics, and modify the tropism of mammalian viruses. Biotechnol. Bioeng. 2009, 102, 1197-1208. |
| [719] | Tang, Q. L., Zhu, Y. J., Wu, J., Chen, F., and Cao, S. W., Calcium phosphate drug nanocarriers with ultrahigh and adjustable drug-loading capacity: one-step synthesis, in situ drug loading and prolonged drug release. Nanomedicine 2011, 7, 428-434. |
| [720] | Pittella, F., Zhang, M., Lee, Y., Kim, H. J., Tockary, T., Osada, K., Ishii, T., Miyata, K., Nishiyama, N., and Kataoka, K., Enhanced endosomal escape of siRNA-incorporating hybrid nanoparticles from calcium phosphate and PEG-block charge-conversional polymer for efficient gene knockdown with negligible cytotoxicity. Biomaterials 2011, 32, 3106-3114. |
| [721] | Behera, T., and Swain, P., Antigen adsorbed calcium phosphate nanoparticles stimulate both innate and adaptive immune response in fish, Labeo rohita H. Cell. Immunol. 2011, 271, 350-359. |
| [722] | Chu, T. C., He, Q., and Potter, D. E., Biodegradable calcium phosphate nanoparticlesas a new vehicle for delivery of a potential ocular hypotensive agent. J. Ocular Pharmacol. Therapeutics 2002, 18, 507-514. |
| [723] | Paul, W., and Sharma, C. P., Porous hydroxyapatite nanoparticles for intestinal delivery of insulin. Trends Biomater. Artif. Organs 2001, 14, 37-38. |
| [724] | Victor, S. P., and Kumar, T. S. S., Tailoring calcium-deficient hydroxyapatite nanocarriers for enhanced release of antibiotics. J. Biomed. Nanotechnol. 2008, 4, 203-209. |
| [725] | Kilian, O., Alt, V., Heiss, C., Jonuleit, T., Dingeldein, E., Flesch, I., Fidorra, U., Wenisch, S., and Schnettler, R., New blood vessel formation and expression of VEGF receptors after implantation of platelet growth factor-enriched biodegradable nanocrystalline hydroxyapatite. Growth Factors 2005, 23, 125-133. |
| [726] | Klesing, J., Wiehe, A., Gitter, B., Grafe, S., and Epple, M., Positively charged calcium phosphate/polymer nanoparticles fro photodynamic therapy. J. Mater. Sci. Mater. Med. 2010, 21, 887-892. |
| [727] | Ling, J. Y., Loo, S. C., Phung, N. T., Boey, F., and Ma, J., Controlled size and morphology of EDTMP-doped hydroxyapatite nanoparticles as model for 153Samarium-EDTMP doping. J. Mater. Sci. Mater. Med. 2008, 19, 2993-3003. |
| [728] | Jordan, M., Schallhorn, A., and Wurm, F. M., Transfecting mammalian cells: optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic Acids Res. 1996, 24, 596-601. |
| [729] | Sokolova, V. V., and Epple, M., Inorganic nanoparticles as carriers of nucleic acids into cells. Angew. Chem. Int. Ed. 2008, 47, 1382-1395. |
| [730] | Olton, D., Li, J., Wilson, M. E., Rogers, T., Close, J., Huang, L., Kumta, P. N., and Sfeir, C., Nanostructured calcium phosphates (NanoCaPs) for non-viral gene delivery: influence of the synthesis parameters on transfection efficiency. Biomaterials 2007, 28, 1267-1279. |
| [731] | Bisht, S., Bhakta, G., Mitra, S., and Maitra, A., pDNA loaded calcium phosphate nanoparticles: highly efficient non-viral vector for gene delivery. Int. J. Pharm. 2005, 288, 157-168. |
| [732] | Chowdhury, E. H., and Akaike, T., A bio-recognition device developed onto nano-crystals of carbonate apatite for cell-targeted gene delivery. Biotechnol. Bioeng. 2005, 90, 414-421. |
| [733] | Bisht, S., Chattopadhyay, D., and Maitra, A., Intraperitoneal administration of calcium phosphate nanoparticles encapsulating pSVβgal elicits immune response to encoded protein. J. Biomed. Nanotechnol. 2006, 2, 229-238. |
| [734] | Zhu, S. H., Huang, B. Y., Zhou, K. C., Huang, S. P., Liu, F., Li, Y. M., Xue, Z. G., and Long, Z. G., Hydroxyapatite nanoparticles as a novel gene carrier. J. Nanopart. Res. 2004, 6, 307-311. |
| [735] | Chowdhury, E. H., Kutsuzawa, K., and Akaike, T., Designing smart nano-apatite composites: the emerging era of non-viral gene delivery. Gene Ther. Mol. Biol. 2005, 9, 301-316. |
| [736] | Chowdhury, E. H., Maruyama, A., Kano, A., Nagaoka, M., Kotaka, M., Hirose, S., Kunou, M., and Akaike, T., pH-sensing nano-crystals of carbonate apatite: effects on intracellular delivery and release of DNA for efficient expression into mammalian cells. Gene 2006, 376, 87-94. |
| [737] | Chowdhury, E. H., pH-sensitive nano-crystals of carbonate apatite for smart and cell-specific transgene delivery. Expert Opin. Drug Deliv. 2007, 4, 193-196. |
| [738] | Chowdhury, E. H., and Akaike, T., High performance DNA nano-carriers of carbonate apatite: multiple factors in regulation of particle synthesis and transfection efficiency. Int. J. Nanomed. 2007, 2, 101-106. |
| [739] | Pedraza, C. E., Bassett, D. C., McKee, M. D., Nelea, V., Gbureck, U., and Barralet, J. E., The importance of particle size and DNA condensation salt for calcium phosphate nanoparticle transfection. Biomaterials 2008, 29, 3384-3392. |
| [740] | Zhou, C., Yu, B., Yang, X., Huo, T., Lee, L. J., Barth, R. F., and Lee, R. J., Lipid-coated nano-calcium-phosphate (LNCP) for gene delivery. Int. J. Pharm. 2010, 392, 201-208. |
| [741] | Giger, E. V., Puigmartí-Luis, J., Schlatter, R., Castagner, B., Dittrich, P. S., and Leroux, J. C., Gene delivery with bisphosphonate-stabilized calcium phosphate nanoparticles. J. Control. Release 2011, 28, 87-93. |
| [742] | Olton, D. Y., Close, J. M., Sfeir, C. S., and Kumta, P. N., Intracellular trafficking pathways involved in the gene transfer of nano-structured calcium phosphate-DNA particles. Biomaterials. 2011, 32, 7662-7670. |
| [743] | Welzel, T., Radtke, I., Meyer-Zaika, W., Heumann, R., and Epple, M., Transfection of cells with custom-made calcium phosphate nanoparticles coated with DNA. J. Mater. Chem. 2004, 14, 2213-2217. |
| [744] | Sokolova, V. V., Radtke, I., Heumann, R., and Epple, M., Effective transfection of cells with multi-shell calcium phosphate-DNA nanoparticles. Biomaterials 2006, 27, 3147-3153. |
| [745] | Sokolova, V. V., Kovtun, A., Heumann, R., and Epple, M., Tracking the pathway of calcium phosphate/DNA nanoparticles during cell transfection by incorporation of red-fluorescing tetramethylrhodamine isothiocyanate-bovine serum albumin into these nanoparticles. J. Biol. Inorg. Chem. 2007, 12, 174-179. |
| [746] | Sokolova, V. V., Kovtun, A., Prymak, O., Meyer-Zaika, W., Kubareva, E. A., Romanova, E. A., Oretskaya, T. S., Heumann, R., and Epple, M., Functionalisation of calcium phosphate nanoparticles by oligonucleotides and their application for gene silencing. J. Mater. Chem. 2007, 17, 721-727. |
| [747] | Neumann, S., Kovtun, A., Dietzel, I. D., Epple, M., and Heumann, R., The use of size-defined DNA-functionalized calcium phosphate nanoparticles to minimise intracellular calcium disturbance during transfection. Biomaterials 2009, 30, 6794-6802. |
| [748] | Graham, F. L., and van der Eb, A. J., A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology 1973, 52, 456-467. |
| [749] | Kovtun, A., Heumann, R., and Epple, M., Calcium phosphate nanoparticles for the transfection of cells. Bio-Med. Mater. Eng. 2009, 19, 241-247. |
| [750] | Roy, I., Mitra, S., Maitra, A., and Mozumdar, S., Calcium phosphate nanoparticles as novel non-viral vectors for targeted gene delivery. Int. J. Pharm. 2003, 250, 25-33. |
| [751] | Li, J., Chen, Y. C., Tseng, Y. C., Mozumdar, S., and Huang, L., Biodegradable calcium phosphate nanoparticle with lipid coating for systemic siRNA delivery. J. Controlled Release 2010, 142, 416-421. |
| [752] | Kakizawa, Y., and Kataoka, K., Block copolymer self-assembly into monodispersive nanoparticles with hybrid core of antisense DNA and calcium phosphate. Langmuir 2002, 18, 4539-4543. |
| [753] | Wang, K. W., Zhou, L. Z., Sun, Y., Wu, G. J., Gu, H. C., Duan, Y. R., Chen, F., and Zhu, Y. J., Calcium phosphate/PLGA-mPEG hybrid porous nanospheres: a promising vector with ultrahigh gene loading and transfection efficiency. J. Mater. Chem. 2010, 20, 1161-1166. |
| [754] | Epple, M., and Kovtun, A., Functionalized calcium phosphate nanoparticles for biomedical application. Key Eng. Mater. 2010, 441, 299-305. |
| [755] | Sokolova, V., Neumann, S., Kovtun, A., Chernousova, S., Heumann, R., and Epple, M., An outer shell of positively charged poly(ethyleneimine) strongly increases the transfection efficiency of calcium phosphate/DNA nanoparticles. J. Mater. Sci. 2010, 45, 4952-4957. |
| [756] | Epple, M., Ganesan, K., Heumann, R., Klesing, J., Kovtun, A., Neumann, S., and Sokolova, V., Application of calcium phosphate nanoparticles in biomedicine. J. Mater. Chem. 2010, 20, 18-23. |
| [757] | He, Q., Mitchell, A. R., Johnson, S. L., Wagner-Bartak, C., Morcol, T., and Bell, S. J. D., Calcium phosphate nanoparticle adjuvant. Clin. Diagn. Lab. Immunol. 2000, 7, 899-903. |
| [758] | He, Q., Mitchell, A. R., Morcol, T., and Bell, S. J. D., Calcium phosphate nanoparticles induce mucosal immunity and protection against herpes simplex virus type 2. Clin. Diagn. Lab. Immunol. 2002, 9, 1021-1024. |
| [759] | Liu, Z. S., Tang, S. L., and Ai, Z. L., Effects of hydroxyapatite nanoparticles on proliferation and apoptosis of human hepatoma BEL-7402 cells. World J. Gastroenterol. 2003, 9, 1968-1971. |
| [760] | Sun, J., and Ding, T., P53 reaction to apoptosis induced by hydroxyapatite nanoparticles in rat macrophages. J. Biomed. Mater. Res. A 2009, 88A, 673-679. |
| [761] | Yuan, Y., Liu, C., Qian, J., Wang, J., and Zhang, Y., Size-mediated cytotoxicity and apoptosis of hydroxyapatite nanoparticles in human hepatoma HepG2 cells. Biomaterials 2010, 31, 730-740. |
| [762] | Allen, T. M., and Cullis, P. R., Drug delivery systems: entering the mainstream. Science 2004, 303, 1818-1822. |
| [763] | Schmidt, H. T., Kroczynski, M., Maddox, J., Chen, Y., Josephs, R., and Ostafin, A. E. J., Antibody-conjugated soybean oil-filled calcium phosphate nanoshells for targeted delivery of hydrophobic molecules. Microencapsulation 2006, 23, 769-781. |
| [764] | Ferraz, M. P., Mateus, A. Y., Sousa, J. C., and Monteiro, F. J., Nanohydroxyapatite microspheres as delivery system for antibiotics: release kinetics, antimicrobial activity, and interaction with osteoblasts. J. Biomed. Mater. Res. A 2007, 81A, 994-1004. |
| [765] | Cai, Y., Pan, H., Xu, X., Hu, Q., Li, L., and Tang, R., Ultrasonic controlled morphology transformation of hollow calcium phosphate nanospheres: a smart and biocompatible drug release system. Chem. Mater. 2007, 19, 3081-3083. |
| [766] | Zhou, W. Y., Wang, M., Cheung, W. L., Guo, B. C., and Jia, D. M., Synthesis of carbonated hydroxyapatite nanospheres through nanoemulsion. J. Mater. Sci. Mater. Med. 2008, 19, 103-110. |
| [767] | Wingert, P. A., Mizukami, H., and Ostafin, A. E., Enhanced chemiluminescent resonance energy transfer in hollow calcium phosphate nanoreactors and the detection of hydrogen peroxide. Nanotechnology 2007, 18, 295707 (7 pages). |
| [768] | Kottegoda, N., Munaweera, I., Madusanka, N., and Karunaratne, V., A green slow-release fertilizer composition based on urea-modified hydroxyapatite nanoparticles encapsulated wood. Curr. Sci. 2011, 101, 73-78. |
| [769] | Chen, J. H., Wang, Y. J., Zhou, D. M., Cui, Y. X., Wang, S. Q., and Chen, Y. C., Adsorption and desorption of Cu(II), Zn(II), Pb(II), and Cd(II) on the soils amended with nanoscale hydroxyapatite. Environ. Prog. Sustain. Energy 2010, 29, 233-241. |
| [770] | Wang, D., Chu, L., Paradelo, M., Peijnenburg, W. J., Wang, Y., and Zhou, D., Transport behavior of humic acid-modified nano-hydroxyapatite in saturated packed column: effects of Cu, ionic strength, and ionic composition. J. Coll. Interf. Sci. 2011, 15, 398-407. |
| [771] | Mobasherpour, I., Salahi, E., and Pazouki, M., Comparative of the removal of Pb2+, Cd2+ and Ni2+ by nano crystallite hydroxyapatite from aqueous solutions: adsorption isotherm study. Arab. J. Chem. 2012, (early view). |
| [772] | Handley-Sidhu, S., Renshaw, J. C., Yong, P., Kerley, R., and Macaskie, L. E., Nano-crystalline hydroxyapatite bio-mineral for the treatment of strontium from aqueous solutions. Biotechnol. Lett. 2011, 33, 79-87. |
| [773] | Gandhi, R. M., Kousalya, G. N., and Meenakshi, S., Removal of copper(II) using chitin/chitosan nano-hydroxyapatite composite. Int. J. Biol. Macromolecules 2011, 48, 119-124. |
| [774] | Manocha, L. M., Disher, I. A., and Manocha, S., Sorption of cadmium ions on (AB-type) carbonated hydroxyapatite nanoparticles. Adv. Sci. Lett. 2011, 4, 44-50. |
| [775] | Ma’mani, L., Heydari, A., and Shiroodi, R. K., Nanohydroxyapatite microspheres as a biocompatible and recoverable catalyst for synthesis of carbon -phosphorous bond formation. Curr. Org. Chem. 2009, 13, 758-762. |
| [776] | Liu, Y., Zhong, H., Li, L., and Zhang, C., Temperature dependence of magnetic property and photocatalytic activity of Fe3O4/hydroxyapatite nanoparticles. Mater. Res. Bull. 2010, 45, 2036-2039. |
| [777] | Yih, T. C., and Al-Fandi, M., Engineered nanoparticles as precise drug delivery systems. J. Cell. Biochem. 2006, 97, 1184-1190. |
| [778] | Celotti, G., Tampieri, A., Sprio, S., Landi, E., Bertinetti, L., Martra, G., and Ducati, C., Crystallinity in apatites: how can a truly disordered fraction be distinguished from nanosize crystalline domains? J. Mater. Sci. Mater. Med. 2006, 17, 1079-1087. |
| [779] | Christenson, E. M., Anseth, K. S., van den Beucken, J. J. J. P., Chan, C. K., Ercan, B., Jansen, J. A., Laurencin, C. T., Li, W. J., Murugan, R., Nair, L. S., Ramakrishna, S., Tuan, R. S., Webster, T. J., and Mikos, A. G., Nanobiomaterial applications in orthopedics. J. Orthop. Res. 2007, 25, 11-22. |
| [780] | Schmidt, S. M., Moran, K. A., Kent, A. M. T., Slosar, J. L., Webber, M. J., McCready, M. J., Deering, C., Veranth, J. M., and Ostafin, A., Uptake of calcium phosphate nanoshells by osteoblasts and their effect on growth and differentiation. J. Biomed. Mater. Res. A 2008, 87A, 418-428. |
| [781] | Motskin, M., Müller, K. H., Genoud, C., Monteith, A. G., and Skepper, J. N., The sequestration of hydroxyapatite nanoparticles by human monocyte-macrophages in a compartment that allows free diffusion with the extracellular environment. Biomaterials 2011, 32, 9470-9482. |
| [782] | Powell, M. C., and Kanarek, M. S., Nanomaterials health effects – Part 1: background and current knowledge. Wisconsin Med. J. 2006, 105, 16-20. |
| [783] | Powell, M. C., and Kanarek, M. S., Nanomaterials health effects – Part 2: uncertainties and recommendations for the future. Wisconsin Med. J. 2006, 105, 18-23. |
| [784] | Motskin, M., Wright, D. M., Muller, K., Kyle, N., Gard, T. G., Porter, A. E., and Skepper, J. N., Hydroxyapatite nano and microparticles: correlation of particle properties with cytotoxicity and biostability. Biomaterials 2009, 30, 3307-3317. |
| [785] | Liu, X., Qin, D., Cui, Y., Chen, L., Li, H., Chen, Z., Gao, L., Li, Y., and Liu, J., The effect of calcium phosphate nanoparticles on hormone production and apoptosis in human granulosa cells. Reproductive Biology and Endocrinology 2010, 8, 32 (8 pages). |
| [786] | Li, S., and Huang, L., Pharmacokinetics and biodistribution of nanoparticles. Mol. Pharm. 2008, 5, 496-504. |
| [787] | Bionanotechnologies are modeled after biological substances and structures, or combine nanomaterials with biological substances. They include materials such as biochips, drug release systems, nanofibers, hybrid nanobiodevices, molecular electronics and biomimetics (synthetic genes, proteins and viruses)[788]. |
| [788] | Moghimi, S. J., Hunter, A. C., and Murray, J. C., Nanomedicine: current status and future prospects. FASEB J. 2005, 19, 311-330. |
| [789] | Xu, H. H. K., Weir, M. D., and Simon, C. G., Jr., Injectable and strong nano-apatite scaffolds for cell/growth factor delivery and bone regeneration. Dental Mater. 2008, 24, 1212-1222. |
| [790] | Watari, F., Abe, S., Tamura, K., Uo, M., Yokoyama, A., and Totsuka, Y., Internal diffusion of micro/nanoparticles inside body. Key Eng. Mater. 2008, 361-363, 95-98. |
| [791] | Oberdorster, G., Oberdorster, E., and Oberdorster, J., Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823-839. |
| [792] | Nel, A., Xia, T., Mädler, L., and Li, N., Toxic potential of materials at the nanolevel. Science 2006, 311, 622-627. |
| [793] | Jahnen-Dechent, W., and Simon, U., Function follows form: shape complementarity and nanoparticle toxicity. Nanomedicine 2008, 3, 601-603. |
| [794] | Singh, N., Manshian, B., Jenkins, G. J. S., Griffiths, S. M., Williams, P. M., Maffeis, T. G. G., Wright, C. J., and Doak, S. H., NanoGenotoxicology: the DNA damaging potential of engineered nanomaterials. Biomaterials 2009, 30, 3891-3914. |
| [795] | Dhawan, A., Sharma, V., and Parmar, D., Nanomaterials: a challenge for toxicologists. Nanotoxicology 2009, 3, 1-9. |
| [796] | Dwivedi, P. D., Misra, A., Shanker, R., and Das, M., Are nanomaterials a threat to the immune system? Nanotoxicology 2009, 3, 19-26. |

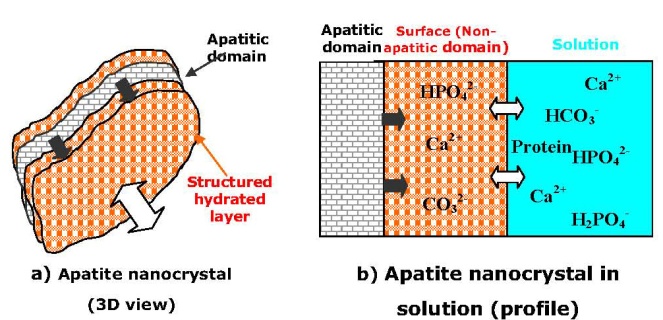
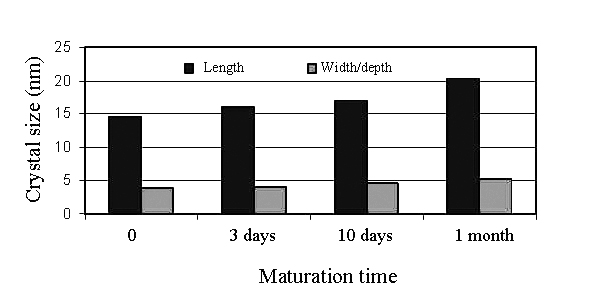
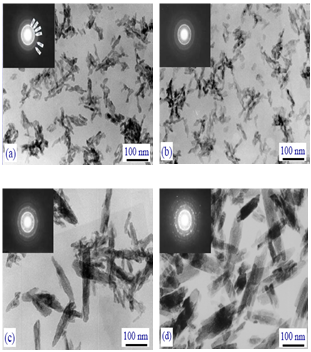

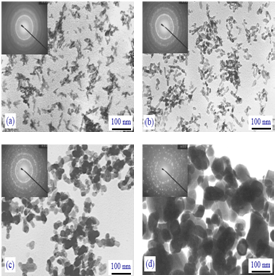
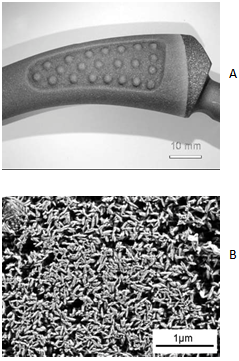
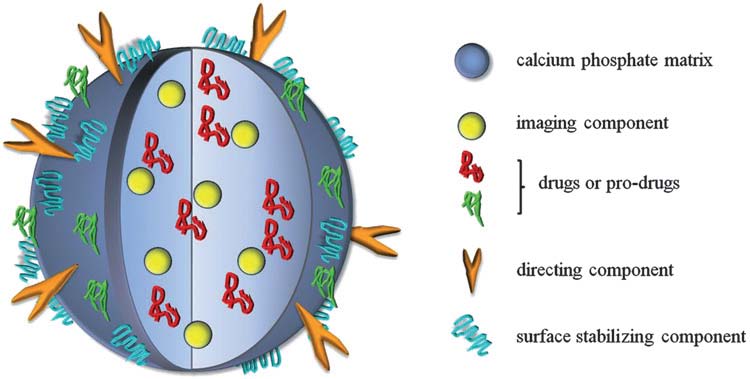
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML