-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biomedical Engineering
p-ISSN: 2163-1050 e-ISSN: 2163-1077
2012; 2(2): 41-47
doi: 10.5923/j.ajbe.20120202.08
Synthesis and Characterization of Hydroxyapatite/TiO2n Nanocomposites for Bone Tissue Regeneration
Nelson H. A. Camargo , Sarah A. de Lima, Enori Gemelli
Department Mechanical Engineering, Santa Catarina State University - UDESC, Joinville, 89.223-100, Brazil
Correspondence to: Nelson H. A. Camargo , Department Mechanical Engineering, Santa Catarina State University - UDESC, Joinville, 89.223-100, Brazil.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The development of nanostructured powders and materials is an innovative alternative that promises to replace many conventional autogenous, halogenous and exogenous biomaterials in the near future. Nanostructured biomaterials stand out as a highly current research topic and appear auspicious for biomedical applications, implant fixation and bone tissue reconstruction because their features differ from conventional biomaterials in terms of wettability, granule, grains surface area, and microporosity, which are favorable for new bone formation. The hydroxyapatite used as bone matrix in this study was produced by the Biomaterials Group of the Santa Catarina State University – UDESC (Brazil). The hydroxyapatite (HA) powder was obtained from synthesis by the dissolution-precipitation reaction of solid/liquid phase of CaO and phosphoric acid to form a composition with a Ca/P molar ratio of 1.67. The powder resulting from the synthesis was calcined at 900ºC/2h, generating the HA phase with a low content of tricalcium phosphate -TCP (whitlockite). The aim of this study was to prepare and characterize four HA/TiO2n nanocomposite powder compositions in the form of microporous granules in concentrations of 1, 2, 3 and 5 vol.% of TiO2n. The phase morphology, powder and granule surface area, and bonding bands in different powder and granule compositions were analyzed by X-ray diffraction (XRD), scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR) and gas absorption (BET).
Keywords: Hydroxyapatite, Nanocomposites, Nanostructure, Bone Regeneration
Article Outline
1. Introduction
- Nanotechnology, also known as Molecular Nanotechnology or Molecular Engineering, involves the synthesis and characterization of functional biomaterials with nanoscale features and particle sizes smaller than 100nm. Nanostructured biomaterials are an innovation and can offer mechanical, electric, magnetic, chemical, optical and biological features superior than conventional biomaterials with micrometric microstructures[1]. Nanostructured biomaterials composed of Ca/P, calcium phosphates and nanocomposites, and mixtures of calcium phosphates with Al2O3, SiO2 and TiO2n, are being widely researched and show promising traumatologic, orthopedic and dental applications, e.g., for defect repair, bone tissue regeneration, implant fixation and tooth remineralization[2-8]. The literature describes different techniques and methods used in the synthesis of nanoparticulate powders and nanocomposite biomaterials[9-14]. Nanomaterials stand out for their new surface characteristics of nanoparticles, granules and microporosity, which render them favorable in terms of cell functions such as migration, adhesion, proliferation, osseointegration and biodegradation of the biomaterial when applied in vivo[11, 15-19]. Among calcium phosphates, we highlight the medical applications of HA, -TCP and biphasic compositions of HA/-TCP, because of the chemical and crystallographic similarity of these biomaterials to those of bone apatite. Ceramic-matrix nanocomposites containing HA and/or -TCP are a new generation of biomaterials with new nanoscale microstructural features and an interface with granules and micropores, which are promising for biomedical applications in defect and bone tissue repair[1, 20-23].Titanium oxide (TiO2) has various applications in the food, cosmetics, aerospatial and biomedical areas because it contains a bioinert phase and can be osteo-integratable to the adjacent tissue[8, 24-27]. TiO2 in nanoparticle form can reach good levels of bioactivity, promoting bone tissue proliferation on nanoparticles and favoring osseointegration, osteoinduction and tissue attachment to biomaterial interface[11, 28-31]. Ceramic-matrix nanocomposites of HA/TiO2n are a current research topic and look promising for medical applications. These materials feature interconnected microporous microstructures and nanostructures, with a modified surface area of granules and micropores, which can stimulate cell proliferation in bone tissue repair processes[29, 32-34].This paper describes and characterizes the nanocomposite powders of a HA ceramic-matrix containing 1, 2, 3 and 5 vol.% of added titanium oxide. The addition of small amounts of TiO2n to the HA ceramic-matrix aimed to improve the dispersion of secondary phase in the HA matrix during mechanical fragmentation by attrition milling. The HA employed for the preparation of nanocomposite powders, which was supplied by the Biomaterials Group of UDESC – Santa Catarina State University (Brazil), was obtained by the wet chemical method, as described in[4]. The low intensity peaks observed by an X-ray technique represent the -TCP phase in the HA composition, which is related to the method of synthesis, as observed by other authors who used other methods of synthesis to produce the hydroxyapatite phase[4, 9, 35-36]. The nanocomposite powders were obtained by mixing the phases in a high energy attrition mill using distilled water and zirconia spheres. The X-ray diffraction (XRD) patterns of the attrition milled powders showed the presence of stoichiometric HA phases, low intensity peaks of -TCP phase and titanium oxide in the anatase phase. HA, -TCP and calcium titanate (CaTiO3) phases were observed in granular powders obtained after heat treatment. The nanoparticles and microstructural morphology of the granular biomaterials were analyzed by scanning electron microscopy (SEM), while the morphology of the HA matrix was examined by transmission electron microscopy (TEM). The results of the BET analysis indicated very similar surface areas in the compositions of milled nanocomposite powders. The heat-treated powders showed smaller surface areas in various compositions. The FTIR spectra revealed typical PO43- and OH- bands, indicating the presence of HA phases in all the nanocomposite powder compositions.
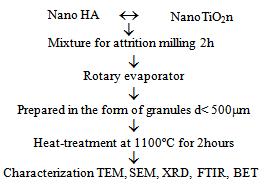 | Figure 1. Preparation and characterization of HA/TiO2n nanocomposites |
2. Materials and Methods
- Five nanostructured powder compositions were prepared: a HA (bone matrix) bioceramic composition; and nanocomposite powders containing 1, 2, 3 and 5 vol.% of nanometric TiO2n with an average nanoparticle diameter of about 20nm. Nanometric HA was supplied by the Biomaterials Group of UDESC – Santa Catarina State University (Brazil), while nanometric TiO2n powder was supplied by the VETEC Laboratory in Brazil. The nanocomposites were prepared by mechanical fragmentation in a high energy attrition mill (NETZSCH do Brasil). The phases were mixed in a solid/liquid concentration of 50 vol.%, using 2.0mm diameter zircon spheres and distilled water, as illustrated in the flowchart in Figure 1. The colloidal suspension resulting from attrition milling was dried in a rotary evaporator, yielding the nanocomposite powders. Nanocomposite powders were prepared in the form of granules by sifting through an ABNT 500µm mesh screen, followed by heat treatment at 1100ºC/2h. The resulting microporous granules were then characterized by different techniques.The material was characterized mineralogically in an X-ray diffractometer (SHIMADZU, Japan) equipped with a copper counter-cathode, operating at 40kV and 30mA with an angular scan interval of 5° to 80° and a goniometric speed of 2º/min at 2. An analysis was made of the nanocomposite powders prepared by mechanical fragmentation in the attrition mill and the granular material resulting from the heat treatment. The attrition-milled nanocomposite powders and the granular material produced by heat treatment at 1100ºC/2h were subjected to morphological, microstructural and nanostructural characterization using a SEM (ZEISS DSM 940A) operating in the secondary electron (SE) emission mode. The morphology of the hydroxyapatite ceramic matrix was examined by TEM (JEOL 2000 FX, 200Kv). Surface area and microporosity were determined by the BET technique, and the milled powder and granules heat-treated at 1100ºC/2h were characterized by several methods.PO43- and OH- bonding bands of hydroxyapatite and milled nanocomposite powders were examined by FTIR, using a Perkin Elmer spectrometer with attenuated total reflectance operating in an interval of 4000cm-1 to 650cm-1 and with a precision of 4.00cm-1.
3. Results and Discussion
- Nanomaterials are usually prepared by mechanical fragmentation through attrition milling, which allows for good secondary phase dispersion in nanocomposite powders.
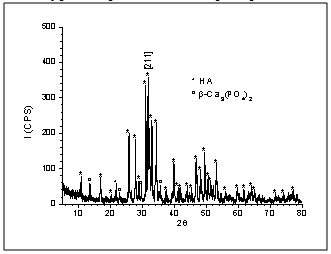 | Figure 2. XRD patterns of hydroxyapatite powder attrttion-milled for 2h |
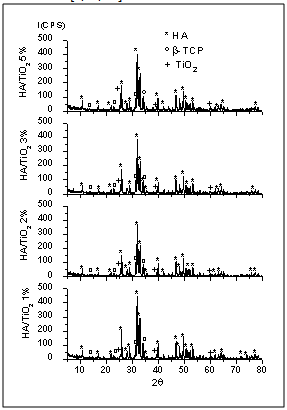 | Figure 3. XRD patterns of nanocomposite powders attrition-milled for 2h |
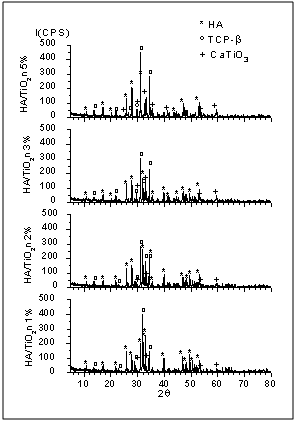 | Figure 4. XRD patterns of nanocomposite powders heat-treated at 1100ºC for 2 h |
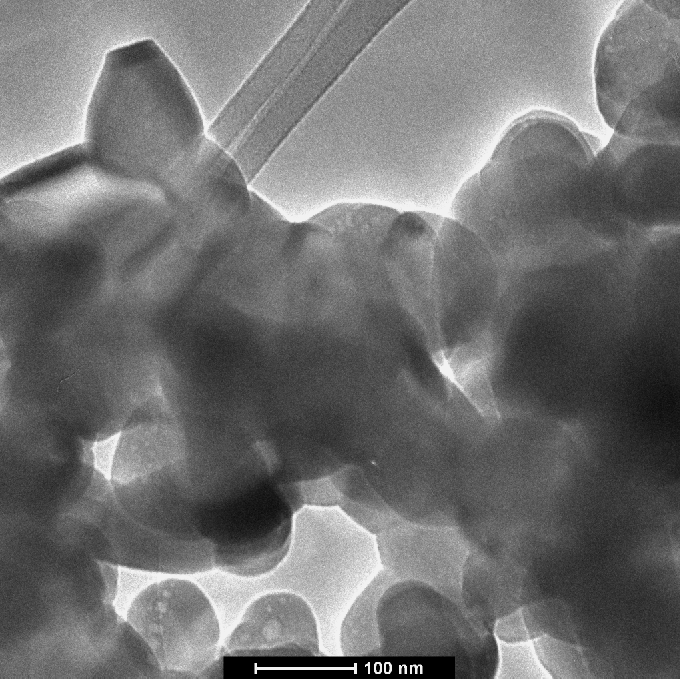 | Figure 5. TEM micrograph of the morphology of hydroxyapatite powder milled for 2h |
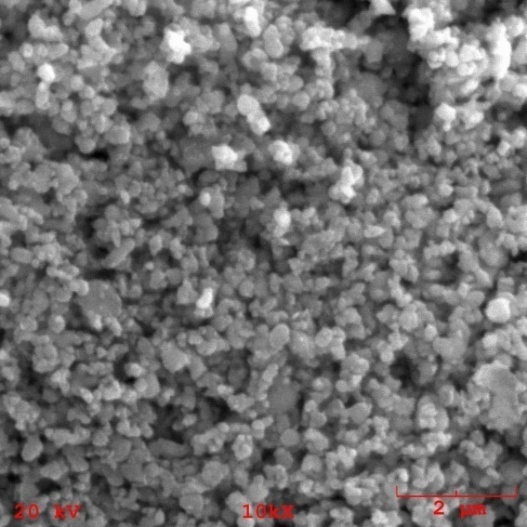 | Figure 6. SEM micrograph of the morphology of hydroxyapatite powder milled for 2h |
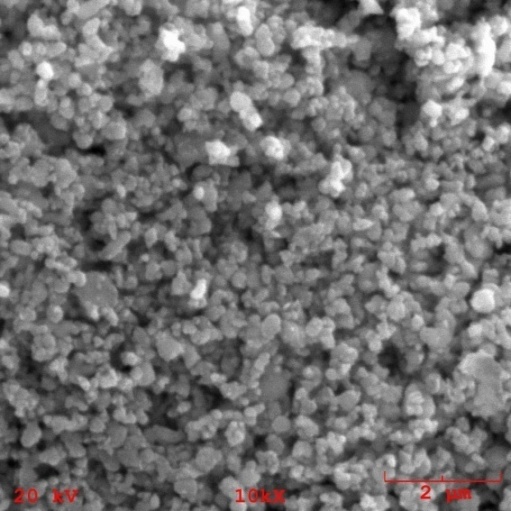 | Figure 7. Morphology of the nanocomposite powder containing 1% of TiO2n |
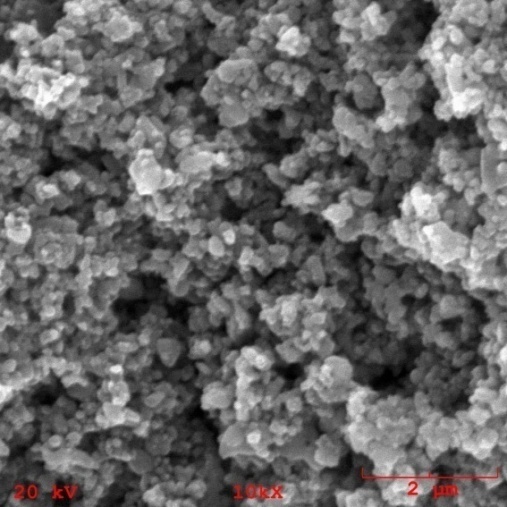 | Figure 8. Morphology of the nanocomposite powder containing 5% of TiO2n |
 | Figure 9. Morphology of nanocomposite granules containing 2% of TiO2n |
 | Figure 10. Morphology of nanocomposite granules containing 2% of TiO2n |
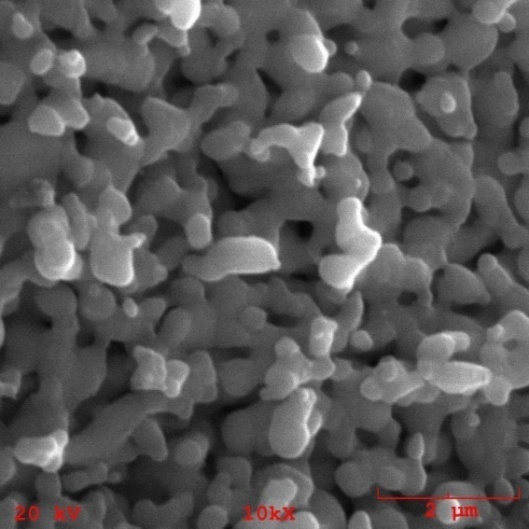 | Figure 11. Microporosity of nanocomposite granules containing 1% of TiO2n |
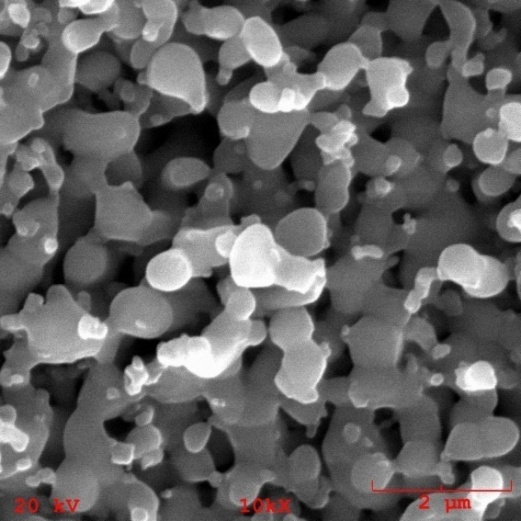 | Figure 12. Microporosity of nanocomposite granules containing 5% of TiO2n |
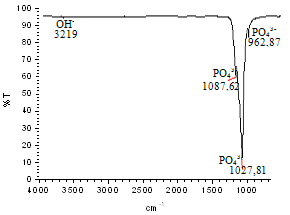 | Figure 13. FTIR spectrum of the milled hydroxyapatite nanostructured powder |
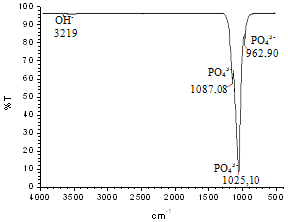 | Figure 14. FTIR spectrum of the milled nanocomposite HA powder containing 1 vol.% of TiO2n |
4. Conclusions
- Nanostructured calcium phosphate biomaterials and nanocomposites are current research topics, which show promising biomedical uses in orthopedic, trauma treatment, and dental applications. In orthopedics, they serve as matrix elements in bone tissue repair through the preventive treatment of bone structures; in traumatology, to recover and repair parts of traumatized and/or lost bone tissue; and in dentistry, to treat cavities, make direct restorations, as fillings and for reconstitution of dental enamel. This new generation of biomaterials presents new morphological characteristics in terms of granules, grians and macropores, providing favorable conditions for bone neoformation and reconstruction of bone tissue. Mixing the phases by attrition milling improved the dispersion of the secondary nanometric phase (TiO2n) in the hydroxyapatite matrix. The XRD diffractograms of the attrition-milled nanocomposite powders showed the presence of peaks corresponding to the stoichiometric HA phase, and low intensity peaks of TCP-β and titanium dioxide in the anatase phase.The nanocomposite powders heat-treated at 1100ºC/2h showed a reduction in the peak of the HA phase and an increase in that of the -TCP phase, as well as the presence of low intensity peaks corresponding to the CaTiO3 phase, resulting from increasing temperatures during the heat treatment.The micrographs of milled powders showed agglomerated nanoparticle morphologies. The secondary nanometric TiO2n phase also found to be well dispersed in the hydroxyapatite matrix. The micrographs of the granular biomaterial clearly showed interconnected micropores, grains sizes of less than 1m, and high granular interface quality, which are factors that favor cell attachment to granules and porous surfaces, ensuring good hydrophilic capacity and capillarity. This can contribute significantly to osseointegration, osteoinduction and bone tissue formation processes. The BET analysis highlighted the relevance of surface area values in different conditions of powders and granular biomaterials as factors in bone neoformation. The FTIR analyses clearly showed PO43- bands and hydrogen (OH-) bonds in all the nanostructured powder compositions.
ACKNOWLEDGMENTS
- The authors acknowledge the support of UDESC – Santa Catarina State University, and that of the company DENTSCARE for its assistance with the BET analyses. We are also indebted to Sarah Amin de Lima for her contribution to this research project.
References
| [1] | K. Anil, B.D. Narenda,. Surface Modification forBioimplants: The Role of Laser Surface Engineering. Journal of Biomaterials Applications, vol. 20, (2005), p. 5-50. |
| [2] | N.H.A. Camargo, S.A. Delima, J.F. Aguiar, E. Gemelli, M. Tomiyama, Synthesis and Characterization ofNanostructures Calcium Phosphates Powders and Calcium Phosphates/-Al2O3 Nanocomposites. Journal of Advanced Materials, vol. 41, nº3, (2009), p. 33-43. |
| [3] | N. H.A. Camargo, S.A. Delima, J.C.P. Souza, J.F. Deaguiar, E. Gemelli, M. M. Meier, S. V.E. Vanessa F. G. Mittestadt, Synthesis and Characterization of Nanostructured Ceramic Powders of Calcium Phosphate and Hydroxyapatite for Dental Applications. Key Engineering Materials Vol. 396-398, (2009), p. 619-622. |
| [4] | S. A. Delima, J. C. P. Souza, N.H. A. Camargo,. F. Pupio, R. B. M. Santos, E. Gemelli, Síntese e Caracterização de Pós Nanoestruturados de Hydroxyapatite. 5º Congresso Latino Americano de Órgãos Artificiais e Biomateriais, Ouro Preto, (2008), p. 1- 6. |
| [5] | N. H. A. Camargo, O. J. Bellini, E. Gemelli, M. Tomiyama, Synthesis and Characterization of Nanostructured Ceramic Powders for Biomedical Applications. Revista Matéria, vol. 12, nº 4, (2007), p. 574-582. |
| [6] | O. J., Bellini, Síntese e caracterização de uma matriz óssea de fosfato de cálcio e nanocompósitos fosfato de cálcio/SiO2 gel para substituição e regeneração óssea. Dissertação de Mestrado/UDESC-Joinville, (2007), p. 97. |
| [7] | M. F. Burrow, U.Nopnakeepong, S. Phrukkanon, A comparison of microtensile bond strengths of several dentin bonding systems to primary and permanent dentin. Dental Materials vol. 18, (2002), p. 239-245. |
| [8] | F. Caroff, K. Sik Oh, R. Famery, P. Boch, Sintering of TCP-TiO2 Biocomposites: Influence of secondary phase, Biomaterials vol. 19, (1998), p. 1451-1454. |
| [9] | J. C. P. Souza, Estudo de caracterização de pósnanoestruturados de fosfatos de cálcio e nanocompósitos fosfato de cálcio/Al2O3-sol-gel para aplicações biomédicas. Dissertação de Mestrado/UDESC-Joinville, (2009), p. 102. |
| [10] | R. B. M. Santos, Síntese e caracterização de pósnanoestruturados de fosfatos de cálcio e nanocompósitos hydroxyapatite/Ssilica-gel. Dissertação deMestrado/UDESC-Joinville, (2009), p. 91. |
| [11] | S.A., Delima, N.H.A.Camargo, J.C.P. Souza, E. Gemelli, Synthesis and Characterization of Nanocomposites Powders Calcium Phosphate/Titanium Oxide for Biomedical Applications. Congresso Internacional PTECH 2009, São Paulo, vol. 1, (2009), p. 913-918. |
| [12] | P.N., Kumta, C., Sfeir, D. H., Lee, D. E. Olton, D. Choi, Nanostructured calcium phosphates for biomedical applications: novel synthesis and characterization, Acta Biomatarialia, vol. 1, (2005),p. 65-83. |
| [13] | T. A. Vu, R. B. Heimann, Influence of the CaO/TiO2 ratio on thermal stability of hydroxyapatite in system Ca5(PO4)3OH-CaO-TiO2, Journal of materials Science Letters, vol.16, (1997), p. 1680-1682. |
| [14] | L. S. Passoni, N. H. A. Camargo, G. M. L. Dalmômico, P. Bonatto, E. Gemelli. Elaboration and Characterization of a Hydroxyapatite Matrix and NanocompositesHydroxyapatite/SiO2n, Latin-American conference of Powder Technology PTECH, 2011, Florianóplis SC, vol. 1 (2011), p. 1-14. |
| [15] | H. Liu, H. Yazici., E. Celaletdin, T. J. Webster, H. Bermek, An in vitro evaluation of the Ca/P ratio for the cytocompatibility of nano-to-micron particulate calcium phosphates for bone regeneration. Acta Biomaterialia vol. 4, (2008), p.1472–1479. |
| [16] | W. C. Brian, T. J. Webster. The effect of nanotopography on calcium and phosphorus deposition on metallic materials in vitro. Biomaterials, vol. 27, (2006), 3064–3074. |
| [17] | J. Tan, W. M. Saltzman. Biometerials with hierarchically defined micro- and nanoscale structure. Biomaterials, vol. 25, (2004), p. 3593-3601. |
| [18] | T. J. Webster, Improved bone tissue engineering materials, American Ceramic Society Bulletin, vol. 82, nº 6, (2003), p. 23-28. |
| [19] | T. J. Webster, R. W. Siegel, R. Bizios, Osteoblast adhesion on nanophase ceramics, Biomaterials, vol. 20, (1999), p. 1221-1227. |
| [20] | N. Levandwski, JR, Biocerâmicas nanoestruturadas para aumento ósseo guiado: Um estudo comparativo in vivo, Dissertação de mestrado em Odontologia, Universidade de Santo Amaro – São Paulo, (2009), p. 108. |
| [21] | Q. fu, M.N. Rahaman, N. Zhou, W. Huang, D. Wang, L. Zhang, H. Li, In Vitro Study on Different Cell Response to Spherical Hydroxyapatite Nanoparticles. Journal of Biomaterials Applications vol. 23, (2008), p. 37-50.. |
| [22] | M. Sato., M. A. Sambito, A. Aslani, N. M. alkhoran, E. B. Slamovich., T. J. Webster. Increased osteoblasts functions on undoped and yttrium-doped nanocrystalline hydroxyapatite coatings on titanium. Biomaterials, vol. 27, (2006), p. 2358–2369. |
| [23] | N. Y. Mostafa. Characterization, thermal stability and sintering of hydroxyapatite powders prepared by different routes. Materials Chemistry and Physics, vol. 94, (2005), p. 333-341. |
| [24] | T. Kolodiazhnyi, G. Annino, M. Spreitzer , T. Taniguchi, R. Freer, F. Azough, A. Panariello, W. Fitzpatrick,Development of Al2O3–TiO2 composite ceramics for high-power millimeter-wave applications”. Acta Materialia vol. 57, (2009),. P. 3402–3409. |
| [25] | N. Juha-Pekka, K. Tomi, M. Tapio, The effect of acidity in low-temperature synthesis of titanium dioxide. Journal of Crystal Growth vol. 304, (2007), p. 179-183. |
| [26] | M. C. Advincula, F. G. Rahemtulla, R. C. Advencula., E. T. Ada, J. E. Lemons, S. L. Bellis. Osteoblasts adhesion and matrix mineralization on sol–gel-derived titanium oxide. Biomaterials vol. 27, (2006), p. 2201–2212. |
| [27] | H.-W. Kim., Y.-H. Koh, L.-H. Li, S. Lee, H.-E. Kim, Hydroxyapatite coating on titanium substrate with titania buffer layer processed by sol–gel method. Biomaterials, vol. 25, (2004), p. 2533–2538. |
| [28] | D. Khang, J. Lu, C. Yao, K. M. Haberstroh, T. J. Webster. The role of nanometer and sub-micron surface features on vascular and bone cell adhesion on titanium” Biomaterials, vol. 29, (2008),p. 970–983. |
| [29] | E. A. A. Neel, T. Mizoguchi, M. Ito, M. Bitar, V. Salih, J. C. Knowles, In vivo bioactivityand gene expression by cells cultured on titanium dioxide doped phosphate-based glasses, Biomaterials, vol. 28, (2007), p. 2967-2977. |
| [30] | K. Goto, J. Tamura, S. Shinzato, S. Fujibayashi, M. Hashimoto, M. Kawashita, T. Kokubo, T. Nakamura, Bioactive bone cements containing nano-sized titania particles for use as bone substitute. Biomaterials, vol. 26, (2005), p. 6496–6505. |
| [31] | X. Liu, X. Zhao, R.K.Y. Fu, J. P.Y. Ho, C. Ding, P. K. Chu, Plasma-treated nanostructured TiO2 surface supporting biomimetic growth of apatite. Biomaterials, vol. 26, (2005), p. 6143–6150. |
| [32] | J. X. Wang, Y. B. Fan, Y. Ggao, Q. H. Hu, T.-C. Wang, TiO2 nanoparticles translocation and potential toxicological effect in rats after intraarticular injection”. Biomaterials, vol. 30, (2009), p. 4590–4600. |
| [33] | K. C. Popat, L. Leoni, C. A. Grimes, T. A. Desai. Influence of engineered titania nanotubular surfaces on bone cells. Biomaterials, vol. 28, (2007), p. 3188-3197. |
| [34] | F. Caroff, K. S. Oh, R. Famery, P. Boch, Sintering of TCP-TiO2 biocomposites: influence of secondary phases. Biomaterials, vol. 19, (1998), p. 1451-1454. |
| [35] | S. Raynaud, E. Champion, D. Bernache-Assollant, P. Thomas, Calcium phosphate apatites with variable Ac/P atomic ratio I. Synthesis, characterization and thermal stability of powders, Biomaterials, vol. 23, (2002), p. 1065-1072. |
| [36] | E. Gemelli, J.de Jesus, N.H.A.Camargo, G.D. de Almeida Soares, V.A.R. Henriques, F. Nery. Microestrutural study of a titanium-based biocomposite produced by the powder metallurgy process with TiH2 and nanometric β-TCP powders. Materials Science and Engineering C, vol. 32, (2012), p. 1011-1015. |
| [37] | U. Gbureck, O. Grolms, J. e> Barralet, L. M. Grover, R. Thull, Mechanical activation and cement formation of β-tricalcium phosphate, Biomaterials, vol. 24, (2003) p. 4123-4131. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML