-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biomedical Engineering
p-ISSN: 2163-1050 e-ISSN: 2163-1077
2012; 2(2): 1-6
doi: 10.5923/j.ajbe.20120202.01
Detection and Quantification of Coronary Atherosclerotic Plaque Using Different Imaging Modalities
Mohammad Karimi Moridani
Biomedical Engineering Department, Science and Research Branch, Islamic Azad University, Tehran, Iran
Correspondence to: Mohammad Karimi Moridani , Biomedical Engineering Department, Science and Research Branch, Islamic Azad University, Tehran, Iran.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The behavior and composition of coronary atherosclerotic plaques are ultimately responsible for the threat of acute ischemic events in patients with coronary artery disease. Different imaging modalities have been developed over the last several years in order to better characterize the atherosclerotic plaque and attempt to predict those in peril of complication. Recent advances in imaging modalities, including invasive and non-invasive studies, have allowed us to examine the histological components that comprise these plaques. Specific information such as variations in temperature, plaque stiffness and calcification level is currently being researched as well as biological and chemical markers. Since vulnerable plaques cannot be identified by stress testing or angiography, new modalities such as intravascular ultrasound, intracoronary thermography, intravascular palpography, optical coherence tomography, intravascular radiation detection, magnetic resonance imaging, radionucleotide imaging, and spectroscopy are under investigation. In this paper we consider to analyze and compare the Atherosclerotic Plaque detection methods.
Keywords: Atherosclerotic Plaque, Comparison, Optical Coherence, Tomography (OCT), Ultrasound, Spectroscopy
Article Outline
1. Introduction
- Atherosclerosis is a progressive disease where the lesion starts as a symptom less fatty streak in the intimal layer of blood vessels, and develops into a fibrous plaque due to the accumulation of cells, lipids, connective tissue, calcium, and pultaceous debris. The lesion preferentially develops at places of complex geometry like bifurcations, occurring in the coronary arteries, abdominal aorta, iliac, thoracic aorta, femoral, popliteals, carotids, and cerebrals. Atherosclerosis is the cause for myocardial infarction, ischemic heart disease, stroke and gangrene in the extremities and ranks number one among the causes of death in the United States. Thus, the need to study the disease cannot be overemphasized[10]. The American Heart Association categorized atherosclerotic plaques into 6 different types (Table 1) of lesions based on the characteristic components and pathogenic mechanisms of the various advanced atherosclerotic lesions [10].Acute coronary syndrome (ACS–acute myocardial infarction (AMI) or unstable angina) occurs when the myocardial demand for oxygen exceeds the supply from the coronary arteries.Typically, this condition is due to atherosclerotic coronary artery disease (CAD). Asatheromatous plaque builds up on the wall of the coronary arteries; it compromises the lumen of the artery.
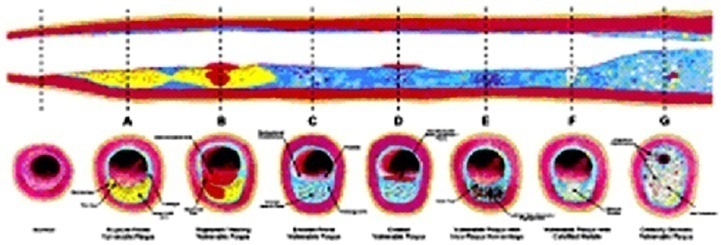 | Figure 1. Different types of vulnerable plaque as underlying cause of acute coronary events (ACS) and sudden cardiac death (SCD). A. Rupture-prone plaque with large lipid core and thin fibrous cap infiltrated by macrophages. B. Ruptured plaque with subocclusive thrombus and early organization. C. Erosion-prone plaque with proteoglycan matrix in a smooth muscle cell-rich plaque. D. Eroded plaque with subocclusive thrombus. E. Intraplaque hemorrhage secondary to leaking vasa vasorum. F. Calcific nodule protruding into the vessel lumen. G. Chronically stenotic plaque with severe calcification, old thrombus, and eccentric lumen (from Naghavi M, et al. Circulation 2003; 108:1664-1672 |
2. Detection of Soft Rupture-Prone Lipid Plaque Using Various Modalities
- Since vulnerable plaques are often asymptomatic and cannot be reliably visualized by current routinely used modalities, new methods for detecting such lesions are being developed. Today, no technology has been shown in a prospective manner to identify vulnerable plaques, but using knowledge of the composition and location of atherothrombotic plaques, several invasive and non invasive imaging modalities hold promise and are currently under investigation.
2.1. Ultrasound
- Researchers have been trying to identify plaque contents and ulceration based on plaque size, cap size, and wall thickness using ultrasound since 1975, when the quantitative examination protocol, now universally accepted, was developed along with the parallel development of two-dimensional B mode Ultrasound (US). Only one B-mode technique has gained widespread acceptance: measurement of the intima-media thickness in the common carotid artery 5. By using ultrasound wavelengths of 200μm (7.5MHz), thicknesses from 200 to 2000μm can be measured. This gives an indication of coronary artery disease but is not very useful for detecting carotid atherosclerosis[10]. Carotid atherosclerosis stents are evaluated by ultrasound images and echoes, which are used to differentiate ‘unstable plaque’, associated with a patient’s past neurologic symptoms from ‘stable plaque’. Plaque stability is associated with plaque composition or to the plaque motion during the cardiac cycle. The components of plaque are differentiated based on the echogenicity of the plaque as determined with mean pixel brightness on B mode US images of the carotid artery median pixel brightness; integrated backscatter of the radiofrequency echo; and the echo attenuation rate at different frequencies in endarterectomy samples and pathologic samples and on carotid artery images[10]. Calcium in plaques causes “bright” (hyperechoic) echoes with shadowing of echoes deep into the plaque, which could markedly limit the detection of plaque components. Fibrous plaques were associated with strong echoes and hemorrhage was associated with weak echoes. Fibrous plaques exhibit higher echogenecity dependent in the incident angle of the US beam, implying an ordered structure in such tissues. Tissues with higher elastin content produce strong echoes. Investigators agree that unstable plaques are associated with hypoechoic regions or low ultrasound echogenicity.Visual analysis of the appearance of plaque on B mode US images has been used as a basis of plaque characterization. Intra and inter observer variability has been large in studies with this method, which makes establishing standardized methods between centers difficult[10].
2.2. Intravascular Ultrasound
- Intravascular ultrasound (IVUS) is a tomographic technique that permits two-dimensional visualization of the arterial wall and allows further characterization of its individual layers[7,15]. IVUS is performed by inserting a catheter that contains a miniaturized ultrasound transducer into the vessel of study, which is then displayed in real time on a monitor. Most clinical centers use a pullback system to withdraw the catheter at a constant rate of 0.5 mm/s following its initial deployment distal to the area of interest[1]. The frequency emitted from the transducer usually ranges from 20 to 40MHz, providing an axial resolution of about 100–200μm. Lateral resolution of the ultrasonic waves is less specific and may vary depending on imaging depth and beam width, averaging around 250μm. The resolution capacity of IVUS, although much higher than non-invasive image modalities, still does not allow a precise assessment of structures smaller than 100μm, therefore hindering its ability to assess the fibrous cap that is prone to rupture, since this is usually thinner than 80μm[5].Nevertheless, the information obtained through IVUS imaging depicts the morphological characteristics of the atheromatous plaque and is used to illustrate the geometrical configuration of its layers and architecture. In addition, IVUS provides other detailed information like the presence of lipid lakes, commonly associated to vulnerable plaques and positive remodeling[5,7, and 15]. Hereby, patients with a ‘‘normal’’ coronary angiography may have subclinical involvement of their arterial wall, therefore predisposing them to an acute event. Often times the culprit lesion is not highly stenotic and may be located distal to areas of luminal narrowing. This information previously undetectable by angiography is now the hallmark for evaluating a vulnerable plaque. The predictive value of this intervention has yet to be clearly defined[1]. Quantitative IVUS analysis can be performed following the procedure in order to more accurately evaluate the characteristics of the vessel. Three dimensional IVUS image reconstruction is essential for proper assessment of the longitudinal distribution of the plaque, because multiple plaque morphologies varying from a fibrotic stable plaque to sites containing large lipids/necrotic cores can be found in a single arterial segment[6]. The utility of IVUS as a screening tool to detect sub clinical high-risk atherosclerosis disease is limited by its invasive nature and inability to assess multiple vascular beds. However, IVUS remains the most validated and utilized image modality to assess vessel wall structures and is readily available in many catheterization laboratories worldwide. Thus, IVUS will play an important role to clinical validation of new imaging modalities[5].
2.3. Spectroscopy
- Using fiberoptic technology, coronary plaques can be illuminated in-situ and the reflected light can be collected and launched into a spectrometer. Spectroscopy is based on the property that different chemical compounds absorb and scatter different amounts of energy at different wavelengths, so each tissue, due to its chemical composition (lipid, collagen, calcium, etc.), has a unique pattern of light absorbance, leaving a unique chemical (molecular) fingerprint.Different approaches are under development. Raman spectroscopy uses high-energy laser light, it has a high molecular sensitivity but its tissue penetration is as low as 0.3 mm. Near-infrared (NIR) spectroscopy (with wavelengths from 750 to 2500 nm) has greater penetration (2 mm) but lower molecular sensitivity and therefore relies on pattern recognition for plaque typing. Intracoronary spectroscopy has not yet been tested clinically[3].
2.4. Elastography
- Ultrasound elastography is an additional approach to characterize the mechanical properties of the endoluminal surface of the atherosclerotic plaque[5,8].Schaar[14] Show the correspondence of plaque morphology with strain patterns. The micro anatomic features of the atherosclerotic plaque at risk of disruption include a large lipid core, high macrophage content, and a thin cap. In elastography, a high strain region on the surface of the plaque with an adjacent low strain region represents a vulnerable plaque. The cap in vulnerable plaque is most prone to rupture due to increased strain. Intravascular elastography detects high strain regions in vulnerable plaques, which correlate with an infiltration of macrophages in caps and a decreased number of smooth muscle cells[10]. The framework of different tissues is dictated by their structural components, therefore plaques that have a high lipid content are described as ‘‘soft’’ plaques, whereas other harder tissues such as collagen and calcium makeup the constituents for ‘‘hard’’ plaques. Since these different tissues respond differently when compressed, one could extrapolate the mechanical properties from this evaluation. The basics behind this process are the foundation for IVUS elastography[4]. Calculations are made based on different strain images obtained from IVUS taking into account the degree of displacement according to variations in blood pressure[12]. It has been noted that there is an inverse relationship between the thickness of the fibrous cap and the physical strain. A cap of 250 lm is described as the physiological threshold for dramatic increases in tensile stress[12]. Also of note, these areas of thin fibrous caps have been found to have a significant amount of macrophages, which secrete metalloproteinases that break down the architecture of the cap. Data processing remains time consuming and represents a limitation for everyday use[4].
2.5. Magnetic Resonance Imaging
- The signal intensities on an MR image represent the biochemical environment of protons in the tissue being imaged. This modality offers flexibility in the design of imaging sequences and parameters, which can be adjusted to enhance the contrast between specific tissue types. MRI provides good contrast between the vessel wall and adjacent lumen by using flow sensitive pulse sequences. Thus, MR can be used to image the vessel lumen (flowing blood) and, at the same time, produce tissue information that describes the vessel wall. With MR, it is possible to acquire three-dimensional images that provide reproducible quantitative tissue information from isotropic voxels[10,16]. Results of clinical studies show that multi-contrast- weighted MR techniques can identify major components of human carotid plaques and characterize morphological features associated with vulnerable lesions[10]. MRI is likely the most promising technique for cardiovascular imaging, since it does not require ionizing radiation, has a high spatial resolution and is non- invasive. Cardiac MRI (CMRI) is performed through techniques and principles that are used in body MRI[4,5,9]. Current applications of CMRI are to detect and monitor the development of atherosclerotic plaques in peripheral vessels. The limitations encountered with CMRI are largely due to motion artifacts by both respiratory and cardiac movement. The size and tortuosity of the coronary vasculature has limited the use of CMRI especially when compared to the higher-quality images obtained of large vessel studies such as the aorta and carotid arteries. Several modifications, including the ‘‘black-blood’’ and free breathing techniques, have been implemented to enhance the quality of CMRI. MRI with contrast is, however, enhancing the characterization of the atherosclerotic plaque. A chelated version of Gadolinium further enhances the signal intensity when administered intravenously to the patient. Very little toxicity is associated with the use of small volumes of this agent. Newer venues with MRI include aggregating super paramagnetic nanoparticles of iron oxide (SPIO). Uptake of SPIO into the vessel wall would be indicative of macrophage activity and inflammation suggestive of a vulnerable plaque[4]. Newer contrast agents are being investigated which will further enhancement the ability of CMRI to delineate and identify plaque components. Gadofluorine has been shown to have high affinity for lipid-rich plaques and enhance MRI detection of soft plaques in experimental studies. The use of intravascular MRI imaging techniques may further enhance image resolution. Recent advances that allow image acquisition of multiple vascular beds, full body MRI, during a single procedure will further enhance the screening ability of MRI to detect atherosclerosis (Figure 2). Finally, newer 3 Tesla MRI scanners are expected to provide increased signal-to-noise ratio for better images and improve image resolution and speed the scanning time [4].
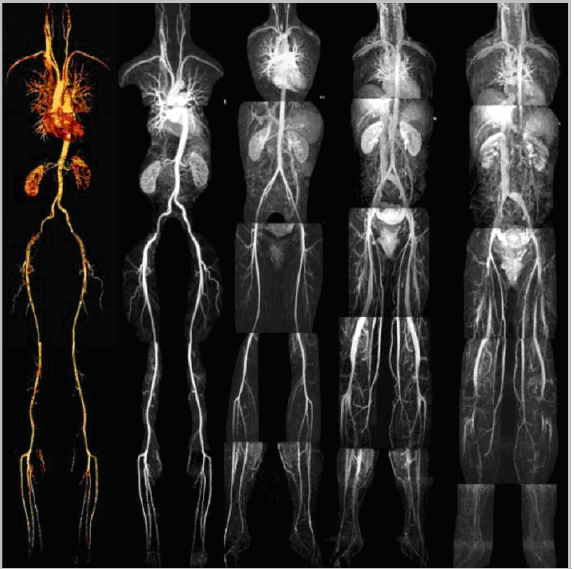 | Figure 2. Whole body magnetic resonance imaging: magnetic resonance angiography and magnetic resonance venography. |
2.6. Optical Coherence Tomography
- Optical coherence tomography (OCT) uses low coherent or broadband infrared light to generate tomographic images with a resolution of 4-20μm, depending on correlating excised coronary and aortic specimens with plaques. Yabushita et al[1999] studied 357 (diseased) atherosclerotic arterial segments with histopathological consensus diagnosis as the “gold standard”. A sensitivity and specificity ranging from 70% to 79% and 97% to 98% for fibrous plaques, 95% to 96% and 97% for fibrocalcific plaques, and 90%to 94% and 90% to 92% for lipid rich plaques, respectively, were demonstrated[10].The interobserver and intraobserver reliabilities were high. This study shows that using objective OCT image criteria, atherosclerotic plaque can be characterized in vitro with a high degree of sensitivity and specificity[9]. In recent studies comparing OCT findings with histology from cadaver vessels there was a high degree of correlation between the plaque characteristics of both assessments. Limitations of OCT include the blood absorption of light resulting in amplified interference, which has yet to be resolved for clinical application[4].
2.7. Thermography
- Thermography, performed using a thermistor catheter, is targeted at detecting specific temperature changes of the vessel wall. The variation demonstrated between atherosclerotic plaques and healthy tissue may be as high as 2C°. These measurements are reflective of the increased tissue metabolism that occurs secondary to increase monocyte migration and activation as well as an increase in proteolytic enzyme release[4].Other factors influencing local temperature are complications such as intraplaque thrombosis, hemorrhage and neovascularization. Increased local temperature variation is predictive of a further degree of instability and a risk factor for acute coronary event[7]. This technique has been tested in humans and temperature heterogeneity showed good association with repeat clinical events. Further clinical studies of this technique are required to determine its validity as a screening tool, which is expected to be limited by its invasive nature[5].
2.8. Multislice Spiral Computed Tomography
- Multislice spiral computed tomography (MSCT) consists of the simultaneous acquisition of submillimeter cuts in concordance with ECG monitoring[5,8]. In doing so, images are obtained throughout a complete diastolic and systolic phase. The diastolic images are reflective of coronary filling, while the systolic phase can be used to assess contractility. This data are subsequently reconstructed along with the ECG, in efforts to obtain the best diastolic images that have the least amount of motion. Since diastolic time increases as heart rate decreases, these patients are commonly given pharmacological intervention (metoprolol 100 mg) to reduce their resting heart rate (preferably <65 bpm). Patients with irregular heart rhythms, such as arterial fibrillation, may obscure the images or result in discontinuity throughout the serial slices resulting in a misinterpretation of the coronary vasculature. The presence of calcification, stents, surgical clips, surgical wires, pacemaker leads and other metal objects represent significant impedances in the overall diagnostic application of this technique. Benefits include precise images in ideal patients and its noninvasive characteristic[4]. High resolution 16-slice CT is currently utilized, however, 64 and 128-slice scanners are being developed which decrease acquisition time and potentially improve image quality[5]. The image resolution of CT remains limited to define histological structures of an atherosclerotic plaque. Coronary plaques have been found to have a unique density expressed as Hounsfield units (HU) for different compositions, which has been correlated with IVUS images. Soft plaques were found to have low density, usually below 50 HU. On the other hand, calcified plaques have high density, usually above 200HU (Figure 3)[5,8]. The association between these plaque characteristics, as defined by MSCT and clinical events and its predictive value to define the site of plaque rupture remain to be determined[4].
2.9. Comparison of OCT with Other Imaging Modalities
- As described above the various imaging modalities used for intravascular imaging have been pivotal in reducing the mortality associated with coronary artery disease over the last 50 years. However several areas have been identified where detection of atherosclerosis could benefit more from high-resolution imaging. Many investigators believe micron scale intravascular imaging facilitated by OCT plays a potential role in the development of clinical cardiology. The major benefits of OCT include:Resolution: 4 to 20 pm, which is higher than anyexisting imaging modality[10].Acquisition rates: OCT offers a high-speed acquisition imaging modality, with acquisition rates near video rates.Inexpensive and small system components: Unlike ultrasonography, OCT catheters consist of simple fiber optics and contain no transducers within their frame, which makes them small and inexpensive. The current smallest cross-sectional diameter being 0.014 inches (355μm).
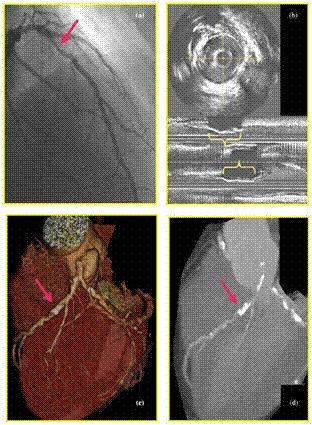 | Figure 3. Significant stenosis in the left anterior descending artery visualized by different imaging techniques: (a). Coronary angiogram revealing (arrow). (b). Intravascular ultrasound with three-dimensional reconstruction showing severe calcification (upper quadrant of transverse image; brackets in longitudinal image). (c). Three dimensional cardiac imaging using multislice computed tomography and (d). Maximum intensity projection of three-dimensional cardiac multislice computed tomography showing severe calcification (arrow). |
3. Conclusions
- Assessment of atherosclerosis by imaging techniques is essential for in-vivo identification of vulnerable plaques. The ideal technique would provide morphological, mechanical and biochemical information, however, despite several imaging techniques are currently under development, none of them provides alone such all-embracing assessment. IVUS is easy to perform and assess morphology and mechanical in stability, Elastography detects high strain regions in vulnerable plaques, which correlate with an infiltration of macrophages in caps and a decreased number of smooth muscle cells and Spectroscopy obtains information on chemical components. MRI provides good contrast between the vessel wall and adjacent lumen by using flow sensitive pulse sequences, OCT has the advantage of high resolution, Thermography has the potential to measure metabolism and MSCT and clinical events and its predictive value to define the site of plaque rupture remain to be determined. Nevertheless all techniques are still under investigation and at present, none of them can completely identify a vulnerable plaque and, most importantly, predict its further development. This is related to fundamental methodological insufficiencies that may be resolved in the future. From a clinical point of view, most techniques currently assess only one feature of the vulnerable plaque. Thus the combination of several modalities will be of importance in the future to ensure a high sensitivity and specificity in detecting vulnerable plaques.
References
| [1] | K"nig,M. Oepke,M. Leibig,V. Klauss.Coronary plaque classification using intravascular ultrasound; Clin Res Cardiol96:514– 518,2007. |
| [2] | Joseph Lau, MD.David Kent, MD.Athina Tatsioni, MD.Yannan Sun, MS.Chenchen Wang, MD, MS. Priscilla Chew, MPH.Bruce Kupelnick, BA.Harmon Jordan, ScD. Vulnerable Plaques: A Brief Review of the Concept and Proposed Approaches to Diagnosis and Treatment; January 22, 2004 |
| [3] | Pierfrancesco Agostoni, Johannes A. Schaar, Patrick W. Serruys.The challenge of vulnerable plaque detection in the cardiac catheterization laboratory; Kardiovaskuläre Medizin 2004;7:349.358 |
| [4] | Mario A. Pulido, Dominick J. Angiolillo & Marco A. Costa.Imaging of atherosclerotic plaque Mario A. Pulido, Dominick J. Angiolillo & Marco A. Costa Division of Cardiology and Cardiovascular Imaging Core Laboratories, University of Florida, Shands Jacksonville, FL, USA 553–559, 2004. |
| [5] | Sousa JE, Costa MA, Murat TE, Yadav S, Stephen E. New frontiers in interventional cardiology. Circulation 2004 (in press). |
| [6] | Panse N, Sasseen B, Prasad P, Kiran K, DeAnna R, Gilmore P, et al. Multiple plaque morphologies in a single Coronary artery. Insights from volumetric intravascular ultrasound. Catheter Cardiovasc Interv 2004; 61: 376 -380. |
| [7] | Angiolillo DJ, Alfonso F. Intravascular ultrasound. In:Cardiac Catheterisation and Percutaneous Intervention. Costa MA, Kay IP, Sabate M editors. Martin Dunitz, London 2004; 259–281. |
| [8] | Caussin C, Ohanessian A, Ghostine S, Jacq L, Lancelin B,Bambrin G, et al. Characterization of vulnerable nonstenosis plaque with 16-Slice computed tomography compared with intravascular ultrasound. Am J Cardiol 2004; 94: 99–104. |
| [9] | Nikolaou K, Becker CR, Muders M, Babaryka G, Scheidler J, Flohr T. Multidetector-row computed tomography and magnetic resonance imaging of atherosclerotic lesions in human ex vivo coronary arteries. Atherosclerosis 2004; 174:243–252. |
| [10] | Natasha F. Homji, B. E. Detection of atherosclerotic plaque: A theoretical model of the laser- tissue interaction2003; 1-86. |
| [11] | Funabashi N, Misumi K, Ohnishi H, Watanabe M, Suzuki Y, Imai N, et al. Endoluminal perspective volume rendering of coronary arteries using electron-beam computed tomography. Circ J 2003; 67: 1064– 1067. |
| [12] | Schaar JA., de Korte CL, Mastik F, Strijder C, Pasterkamp G, Boersma E, et al. Characterizing Vulnerable Plaque Features with Intravascular Elastography. Circulation 2003; 108: 2636–2641. |
| [13] | Yabushita H, Bouma BE, Houser SL, et al., Charaterisation of human atherosclerosis by optical coherence tomography, Circulation 2003;106:1640-1645. |
| [14] | Schaar JA, de Korte CL, Mastik F, Vulnerable plaque characterization with intravascular elastography, J. of the American College of Cardiology 2002, 39:1. |
| [15] | Costa MA, Kozuma K, Gaster AL, van Der Giessen WJ,Sabate M, Foley DP, et al. Three dimensional intravascular ultrasonic assessment of the local mechanism of restenosis after balloon angioplasty. Heart 2001; 85: 73–79. |
| [16] | Yuan C, Mitsumori LM, Beach KW, et al., Carotid ArterioscleroticPlaque: Noninvasive MR: Characterization and Identification of Vulnerable Lesions, Radiology 2001; 221:285-299. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML