-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biomedical Engineering
p-ISSN: 2163-1050 e-ISSN: 2163-1077
2012; 2(1): 32-38
doi: 10.5923/j.ajbe.20120201.05
From Carbides to Co5 and Co13 Metallofullerenes: First-Principles Study and Design
Andrew Kuznetsov
ATG: Biosynthetics, Weberstraße 40, 79249 Merzhausen, Germany
Correspondence to: Andrew Kuznetsov , ATG: Biosynthetics, Weberstraße 40, 79249 Merzhausen, Germany.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Trigonal-bipyramidal Co5 and icosahedral Co13 magnetic clusters were investigated in various organic environments by the first principle calculations based on the density functional theory (DFT). Adjacent carbon atoms decreased the total spin magnetic moment of the metallic clusters, such that Co5C5 and C13C12 carbides demonstrated 23.1 and 22.6 %, as well as Co5@C60 and Co13@C80 endohedral matallofullerenes showed 80.5 and 87.1 % of magnetic activities of pure cobalt clusters, respectively. As a result of increasing the number of carbon atoms on the surface, reduction of magnetism and saturation of suppression of magnetic activity was clearly shown in the case of Co13Cn carbide particles. An asymmetric impact of carbon atoms on magnetism of cobalt clusters was revealed and the screen effect depending on the size of fullerenes was described. The endohedral fullerene Co5@C80 demonstrated improved characteristics, such as 90.6 % remaining magnetic activity of pure Co5 cluster, highest magnetic moment at 2.39 µB/Co-atom, and a size of 8.5 Å. Since a variety of materials can be nanosized, the scope and limitations of an ab initio approach for scaling design is discussed.
Keywords: DFT, Cobalt, D-Metal Carbides, Fullerenes, Nanomagnets, Design Principles
Cite this paper: Andrew Kuznetsov , "From Carbides to Co5 and Co13 Metallofullerenes: First-Principles Study and Design", American Journal of Biomedical Engineering, Vol. 2 No. 1, 2012, pp. 32-38. doi: 10.5923/j.ajbe.20120201.05.
1. Introduction
- The development of modern society has always focused on creating new devices i.e. an assembly of components designed for a specific function. Each part performs a simple action so that the entire unit can achieve a more complex but useful purpose. The molecular-level device can be defined as an assembly of a number of molecular components[1]. As Richard Feynman stated in his talk to the American Physical Society in 1959, “there is plenty of room at the bottom.” The miniaturization of components has opened a path to new technologies in medicine. Starting from the bottom-up, there has been some progress in the construction of “smart” nanoparticles for targeted drug delivery as well as for cancer therapy[2-5].The fullerene family, and especially C60, was actively used in a biological domain. Water-soluble C60-derivatives have been actively explored. However, the application of fullerenes for drug delivery is still in infancy. The design and synthesis of multifunctionalized C60-based systems able to penetrate cell membranes and deliver active agents is an attractive challenge[6]. Gupta and Gupta demonstrated in 2005[7] that magnetic iron oxide particles may be used as a core. Following wild hype and some fantasy, a general arrangement for a therapeutic nanodevice consisting of a magnetic core, organic layer, sensors and actuators, such as affinity polypeptides was determined[8-10]. Zhao and co-workers[11] studied the structural transformations of metallofullerene Gd@C82 under various circumstances. It was found that the Gd@C82 was fully collapsed at 580°C, which was lower than that for the complete destruction of C60. The authors ascribed the easier decomposition to an encapsulated metal in the carbon cage that could induce a deformation of C–C bonds. To release drugs from a fullerene cage, an anisotropic magnetic core changing its own orientation under an external magnetic field could have been used[12]. Among the diverse features of fullerenes, redox properties, which are particularly attractive to biomedical applications, have been extensively studied[13, 14]. Various types of C60 functionalization that provide hydrophilicity of fullerenes have been reviewed[13]. Such fullerenes may accumulate in tumors[15]. Some studies also have reported that fullerenes can cross the blood-brain barrier[16, 17]. The Gd@ C82(OH)22, a water-soluble endohedral metallofullerene derivative, has been proven to possess antineoplastic activity in mice. Studies of the nanoparticles in vitro have demonstrated a low or absent toxicity in mice and C.elegans. The Gd@C82(OH)22 complex is tolerated by worms and has no toxic effects on longevity, stress resistance, growth and behavior of C.elegans[18].Like art, where aesthetic design principles discovered by Michelangelo in XVI century; the golden mean, balance, proportion, rhythm, repetition, emphasis, unity, symmetry and asymmetry, nanotechnology requires a set of basic building blocks and assembly rules based on the natural principles of the nanoworld[1]. Among carbon species, such as carbene complexes of transition metals, intermediate transition metal carbides, endohedral metal fullerenes, and graphite intercalation compounds, some structures are impossible. In this case, computer simulation gives rise to design novel biologically compatible nanomagnets. In this manuscript, an effort has been made to discover “design principles” using ab initio computer simulations to engineer carbide and metallofullerene nanoparticles as a platform for bio-compatible materials. Trigonal-bipyramidal Co5 and icosahedral Co13 magnetic clusters covered by carbon atoms in different positions, as well as the Co5 and Co13 clusters embedded into fullerenes C60 and C80 with Ih point group symmetry were used in computational experiments. The intention of this project is to improve understanding at an atomic level and as a step toward the major goal of designing nanostructures from the bottom up.
2. Computational Details
- Atomic coordinates of pre-optimized Co5 and Co13 magnetic clusters were obtained from[19]. Co5 and Co13 metallic clusters were covered by carbon atoms using Avogadro v.1.0.0 molecule editor[20] or Chem3D Ultra v.8.0.3 molecular modeller and analyzer[21]. Atomic coordinates of fullerenes were downloaded from the Mitsuho-Yoshida's Fullerene Library database[22]. Molecular structures of the fullerenes were combined with Co5 and Co13 clusters by SPDB viewer v.4.1[23]. Atomistic quantum chemistry calculations were performed employing Density Functional Theory (DFT)[24], which was implemented within the OpenMX v.3.5 Software[25-28]. The calculation of atomic structures was done using local spin density approximation of Ceperley-Alder (LSDA-CA)[29] or by the Perdew-Burke-Ernzerhof generalized gradient approximation (GGA-PBE)[30] with parameters fitted to d-orbitals of cobalt atoms. Structural relaxation was performed by stepwise decent optimization (SDO) or by direct inversion iterative sub-space optimization (DIIS)[31]. The atomic species were defined as C4.0-s1p1 and Co5.5-s2p2d2f1[32] with energy cutoff 200.0 Ryd, while the energy convergence criterion (SCF) was set at 10-4 Hartree during the fitting procedure. An electronic temperature in experiments was 300.0 K. Spin polarization was switched on and the total spin magnetic moment (µB) was calculated. The energy formation Eform of experimental complexes was estimated as described in[12].
3. Results
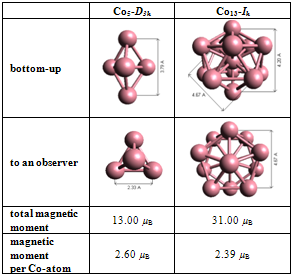
- Properties of pure Co5-D3h and Co13-Ih clustersThe Co5-D3h magnetic cluster was chosen for the experiments because it is the most stable structure with anisotropic properties and has the smallest trigonal bipyramid geometry that demonstrates the highest magnetic moment per atom, 2.6 µB, among small Com clusters (m = 2-20)[33]. The Co13-Ih cluster was selected because it is the smallest magic structure with icosahedral symmetry, including two pentagonal rings, two apex atoms and a central atom with a large total magnetic moment 31.0 µB[33, 34]. According to our calculations, the pure Co5 cluster with the average Co–Co bond length 2.33 Å and a distance of 3.80 Å between cobalt atoms on the opposite vertices of trigonal bipyramid demonstrated the total magnetic moment 13.00 µB. After structural optimization, the pure Co13 cluster showed an average bond length of 2.38 Å, as well as space up to 4.20 Å between apex atoms and a remoteness of 4.67 Å between atoms on the opposite vertices of two pentagonal rings of the icosahedron with the total magnetic moment at 31.00 µB (Table 1). Normalization that we used to express experimental data allowed comparison of results obtained by other authors. The magnetic moments for Co5 and Co13 clusters in an amount of 2.60 and 2.39 µB/atom respectively are consistent with[33, 35, 36] and higher than hcp bulk value 1.72 µB[37].
|
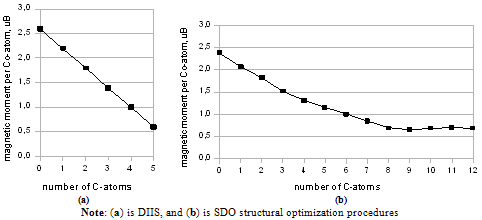 | Figure 1. Magnetic moment of carbides Co5Cn (a) and Co13Cn (b) depending on the number of carbon atoms |
|
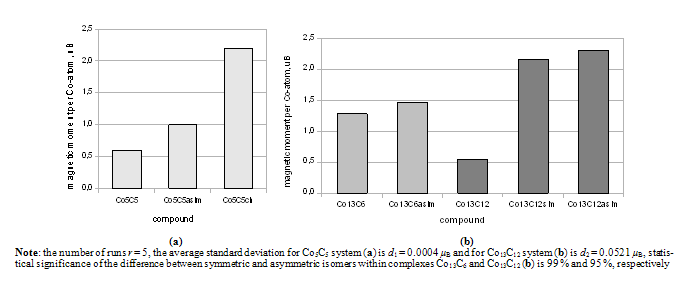 | Figure 2. Magnetic moments of isomers of Co5C5 (a) and Co13C6,12 (b) complexes |
|
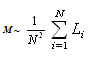 | (1) |
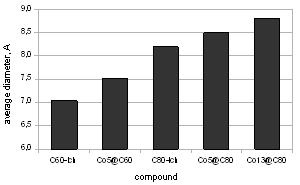 | Figure 3. The size of a fullerene cage based on the metal cluster insertion |
4. Discussion
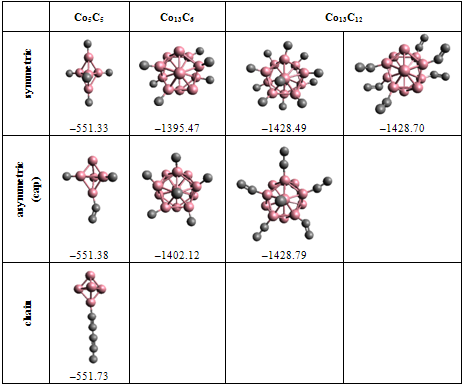
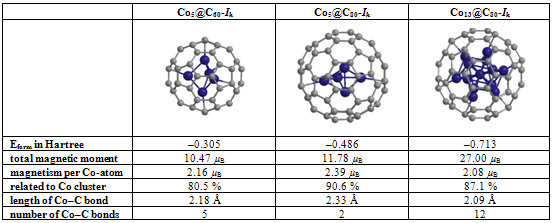
- Many scientific phenomena are investigated by computer models. Computer experiment is a number or runs of the code with various inputs[42]. We proved the utility of computational experiments to elucidate key features of interaction between Co-atoms and organic environment. Metallic clusters with a large magnetic moment were chosen because magnetic nanoparticles should be manipulated by an external magnetic field during the magneto-thermal cancer treatment[4]. We performed a study of magnetism according to the geometry of clusters and carbon shell. The properties of nanostructures depended on how atoms are organized. Asymmetric magnetic carbidesAlthough pure Co13 cluster demonstrated a higher total spin magnetic moment (31.00 µB) than a pure Co5 cluster (13.00 µB), the magnetic moment per atom was bigger for Co5 than for the Co13 cluster, i.e. 2.60 versus 2.39 µB respectively (Table 1), which might be significant for tiny magnetic devices. Here, we described a negative effect of the carbon environment on magnetism for Co clusters. Magnetic moment of the clusters decreased monotonically with an increasing number of attached C-atoms. Nevertheless, covalent binding of carbon atoms to the surface of cobalt clusters did not suppress the magnetism totally. Still Co5C5 complex showed 23.1 % of initial activity of pure Co5 cluster, and Co13C8-12 presented about 28.7 % of magnetism of Co13 cluster (Fig. 1). We used molecular complexes of cobalt carbides and their isomers with stable energetic characteristics (Table 2) in experiments on various initial conformations of the complexes; the dissimilar outputs were analyzed and a difference in magnetic moments was found. That is substantial in that the average standard deviation of magnetic moments was about 130 times larger for Co13C12 system than the deviation for Co5C5 system. This spread might have been because the number of local minima in a potential energy surface increases very rapidly with the number of atoms in a complex.Surprising effects were observed in experiments with asymmetric localization of carbon atoms on the surface of cobalt clusters. When we removed part of the carbon atoms from the surface of ComCn complexes and added them to the rest of the attached carbon atoms, actually building short carbon chains, the magnetic moment grew for both Co5C5 and Co13C12 carbides (Fig. 2). This effect could be explained by the reduction of the number of Co–C bonds and the increase in the number of unpaired d-electrons with uncoupled spins, which contribute to the total spin magnetic moment[39]. However, it is difficult to realize the arrangements of electrons in details. Why does a simple shift of carbon atoms to one side of the cobalt cluster increase magnetism? In this case, the π-electrons of double C=C bonds might interact with each other on the cap of a carbide particle. Nanomagnets based on the endohedral metallofullerenesSeveral attempts have been made to formalize aspects of interaction metallic clusters with fullerenes[40, 41]. The position effect of Co5 cluster within an elongated fullerene C70-D3h was published in[12]. Here, we interested in the future discovery of “design rules” at nanosclae. Drawing from recent data and our own results, we proposed a simple model to estimate the magnetic moment based on the length and the number of Me–C bonds (1). We suppose that magnetism of endohedral fullerenes depends directly on the average length of Me–C bond and is in reverse dependence with the number of bonds.Among investigated endohedral fullerenes, the Co5@C80 complex provided the highest magnetic moment per cobalt atom of 2.39 µB, resembling 90.6 % activity of pure Co5 cluster, with the minimal number and maximal distance of Co–C bonds (Table 3). The size of the complex was about 8.50 Å (Fig. 3), the deformations of Co core were negligible (Table 4), which will be attractive for biological applications. We also note that the hydrated derivative Gd@C82(OH)22 used in many biological studies[43, 44], resembles endohedral metallofullerene Co5@C80. Based on the computational studies, we suppose that the Co5@C80 might be a prospective platform for magneto-thermal cancer therapy and developing novel treatment approaches for humans.Remaining problemsThe current state-of-the-art technology is still not able to provide full computer added design and engineering of new functional materials from the atomic level up[45]. The lack of a broadly applicable theory across multiple scales leaves the design of magnetic nanoparticles incomplete. To date, a “designer” needs to use different and separate tools and approaches to meet a required goal. Although we studied molecular structures of growing complexity from carbides to metallofullerenes in the frame of OpenMX software, our attempt to investigate Co13 cluster in 1PRN barrel protein from the bacteria R.blastica was unsuccessful (data not present). This example calls for effective DFT/MD coupling and the search for new numerical methods such as time-dependent DFT, application of Green’s function techniques, and completely innovative approaches to soft matter systems. To answer questions about how to design, analyze, and build molecular devices, development of theoretical nanoscience is needed as well as libraries of primitives for bottom-up engineering on a nanometer scale, knowledge about building blocks, an addressable platform to combine those primitives in a desirable way[46], a proof-of-principle for much larger composed systems, and the efficient combination of experiments to prove the point of view.
5. Conclusions
- The trends obtained from our DFT study give a qualitative understanding of magnetism at the atomistic level. It has been shown that carbon atoms decreased the magnetic moment of cobalt clusters; the pattern of distribution of C-atoms on the surface of Co clusters played an important role in the magnetic properties of a whole cobalt-carbon system, depending generally on the number of metal-carbon bonds and the length of interatomic distances that also possibly provided a charge transfer from metallic cluster to carbon cage. As we have seen, magnetism at nanoscale is very sensitive to an atomic environment. The more crowded the carbon environment for a metallic core, the less the magnetic moment of the metal-carbon complex.
References
| [1] | Balzani V., Venturi M., Credi A. Molecular devices and machines - a journey into the nano world. Wiley-VCH: , 511 pages (2003). ISBN-10: 3527305068 |
| [2] | Goya G.F., Grazu V., Ibarra M.R. // Current Nanoscience 4(1), 1-16 (2008) |
| [3] | Huis R., Storm G., Hennink W.E., Kiessling F., Lammers T. // Nanoscale 3, 4022 (2011) |
| [4] | Jurgons R., Seliger C., Hilpert A., Trahms L., Odenbach S., Alexiou C. // J. Phys.: Condens. Matter. 18(38), S2893 (2006) |
| [5] | Mornet S., Vasseur S., Grasset F., Duguet E. // J. Mater. Chem. 14, 2161 (2004) |
| [6] | Montellano A., Da Ros T., Bianco A., M. // Nanoscale 3, 4035 (2011) |
| [7] | Gupta AK, Gupta M. // Biomaterials 26(18), 3995 (2005) |
| [8] | Kuznetsov A., Korvink J. From DNA-structures to a NanoSwarm // DECOI 2006: Design of Collective Intelligence, International Summer School on Collective Intelligence and Evolution, Amsterdam, Holland, 7-11 August (2006) |
| [9] | Yezhelyev M.V., Gao X., Xing Y., Al-Hajj A., Nie S., O'Regan R.M. // The Lancet Oncology 7(8), 657 (2006) |
| [10] | Varadan V.K., Chen L., Xie J. Nanomedicine: Design and Applications of Magnetic Nanomaterials, Nanosensors and Nanosystems. John Wiley & Sons Ltd, Medical, 484 pages (2008). ISBN-10: 0470033517 |
| [11] | Zhao S., Zhang J., Guo X., Qiu X., Dong J., Yuan B., Ibrahim K., Wang J., Qian H., Zhao Y., Yang S., Hao J., Zhang H., Yuan H., Xing G., Sun B. // Nanoscale 3, 4130 (2011) |
| [12] | Kuznetsov A. // Comp. Mater. Sci. 54, 204 (2012) |
| [13] | Bosi S., Da Ros T., Spalluto G., M. // European Journal of Medicinal Chemistry 38, 913 (2003) |
| [14] | Jensen A.W., Maru B.S., Zhang X., Mohanty D.K., Fahlman B.D., Swanson D.R., Tomalia D.A. // Nano Letters 5, 1171 (2005) |
| [15] | Tabata Y., Murakami Y., Ikada Y. Antitumor effect of poly(ethylene glycol)-modified fullerene // Fullerene Science and Technology 5(5), 989 (1997) |
| [16] | Tsao N., Kanakamma P.P., Luh T-Y., Chou C-K., Lei H-Y. // Antimicrob. Agents Chemother. 43(9), 2273 (1999) |
| [17] | Yamago S., Tokuyama H., Nakamura E., Kikuchi K., Kananishi S., Sueki K., Nakahara H., Enomoto S., Ambe F. // Chem Biol. 2(6), 385 (1995) |
| [18] | Zhang W., Sun B., Zhang L., Zhao B., Nie G., Zhao Y. // Nanoscale 3, 2636 (2011) |
| [19] | Tereshchuk P.L. // Comp. Mater. Sci. 50(3), 991 (2011) |
| [20] | http://avogadro.openmolecules.net |
| [21] | http://www.cambridgesoft.com |
| [22] | http://jcrystal.com/steffenweber/gallery/Fullerenes/FullereneLib.zip |
| [23] | http://spdbv.vital-it.ch |
| [24] | Hohenberg P., Kohn W. // Phys. Rev. 136(3), B864 (1964) |
| [25] | Ozaki T. // Phys. Rev. B 67, 155108 (2003) |
| [26] | Ozaki T., Kino H. // Phys. Rev. B 69, 195113 (2004) |
| [27] | Ozaki T., Kino H. // Phys. Rev. B 72, 045121 (2005) |
| [28] | http://www.openmx-square.org |
| [29] | Ceperley D.M., Alder B.J. // Phys. Rev. Lett. 45(7), 566 (1980) |
| [30] | Perdew J.P., Burke K., Ernzerhof M. // Phys. Rev. Lett. 77, 3865 (1996) |
| [31] | Csaszar P., Pulay P.J. // Mol. Struct. (Theochem) 114, 31 (1984) |
| [32] | http://www.jaist.ac.jp/~t-ozaki/vps_pao2006/vps_pao.html |
| [33] | Datta S., Kabir M., Ganguly S., Sanyal B., Saha-Dasgupta T., Mookerjee A. // Phys. Rev. B 76, 014429(1) (2007) |
| [34] | Li Zhi-Qiang, Gu Bing-Lin // Phys. Rev. B 47(20), 13611 (1993) |
| [35] | Ma Q.M., Liu Y., Xie Z., Wang J. // J. Phys.: Conf. Ser. 29, 163 (2006) |
| [36] | Rodríguez-López J.L., Aguilera-Granja F., Michaelian K., Vega A. // Phys. Rev. B 67, 174413 (2003) |
| [37] | Kittel C. Introduction to Physics // Wiley, , 7th ed. (1996) |
| [38] | Kuznetsov A. Magnetic moments of carbides Co13C1-12: a density functional investigation // Actual Problems of Applied Physics 2011, , 18-21 October (2011) |
| [39] | Blügel S., Bihlmayer G. Magnetism of low-dimensional systems: Theory, in: Handbook of Magnetism and Advanced Magnetic Materials, H. Kronmüller and S.S.P. Parkin (eds), John Wiley & Sons Ltd, Chichester, UK, 598-640 (2006) |
| [40] | Javan M.B., Tajabor N., Roknabadi M.R., Behdani M. // Applied Surface Science 257, 7586 (2011) |
| [41] | Weck P.F., Kim E., Czerwinski K.R., Tomanek D. // Phys. Rev. B 81, 125448 (2010) |
| [42] | Sacks J., Welch W.J., Mitchell T.J., Wynn H.P. // Statist. Sci. 4(4), 409 (1989) |
| [43] | Chen C., Xing G., Wang J., Zhao Y., Li B., Tang J., Jia G., Wang T., Sun J., Xing L., Yuan H., Gao Y., Meng H., Chen Z., Zhao F., Chai Z., Fang X. // Nano Lett. 5(10), 2050 (2005) |
| [44] | Wang J., Gu F., Ding T., Liu X., Xing G., Zhao Y., Zhang N., Ma Y. // Oncology Letters 1, 771 (2010) |
| [45] | Lyubartsev A., Tu Y., Laaksonen A. // J. Computational and Theoretical Nanoscience 6(5), 951 (2009) |
| [46] | Kuznetsov A. // IET Synthetic Biology 1(1–2), 7 (2007) |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML