-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biomedical Engineering
p-ISSN: 2163-1050 e-ISSN: 2163-1077
2012; 2(1): 1-8
doi: 10.5923/j.ajbe.20120201.01
Effect of Transforming Growth Factor-β1 in Biological Regulation of Primary Chondrocyte
Seyed Ali Khaghani 1, Morgan C. T. Denyer 2, Mansour Youseffi 3
1School of Engineering, School of Life Sciences, University of Bradford, Bradford, BD7 1DP, United Kingdom
2School of Life Sciences, University of Bradford, Bradford, BD7 1DP, United Kingdom
3School of Engineering, University of Bradford, Bradford, BD7 1DP, United Kingdom
Correspondence to: Seyed Ali Khaghani , School of Engineering, School of Life Sciences, University of Bradford, Bradford, BD7 1DP, United Kingdom.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The effect of transforming growth factors-β (TGF-βs) in the regulation and control of cell growth is widely studied, but the capability of these cytokines to regulate chondrocyte cell functions such as migration, cell death, proliferation, differentiation and wound repair is not clearly understood. In this work the effect of TGF-β1 on the biological regulation of chondrocyte was evaluated using a model wound closure assay. The experiments were carried out on primary chondrocyte cells with fibroblast like and chondrocytic phenotypes. The cells were isolated from knee articular cartilage of five day old Sprague-Dawley neonate rats and seeded at low density to obtain a fibroblast like morphology. Chondrocytes with chondrocytic phenotype were derived by seeding at high density.The results revealed that TGF-β1 slowed down proliferation and migration of cells into a model wound. It was also found that cell attachment, as determined by the detachment time during trypsinization, was greater for cells with a fibroblast like morphology when compared with cells exhibiting chondrocytic morphology. Treatment with TGF-β1 was found to increase the detachment times of fibroblast like chondrocytes, indicating that TGF-β1enhanced cell attachment of this cell type, whilst treatment with TGF-β1 decreased detachment time for the chondrocytic type chondrocyte cells indicating that TGF-β1 decreased cell attachment in these cells.
Keywords: Chondrocyte cell culture, Wound repair, TGF-β1, Detachment assay, Cell adhesion
Cite this paper: Seyed Ali Khaghani , Morgan C. T. Denyer , Mansour Youseffi , "Effect of Transforming Growth Factor-β1 in Biological Regulation of Primary Chondrocyte", American Journal of Biomedical Engineering, Vol. 2 No. 1, 2012, pp. 1-8. doi: 10.5923/j.ajbe.20120201.01.
Article Outline
1. Introduction
- Cartilage is an avascular tissue[1] with oxygen and nutrients delivered via the synovial fluid[15]. This is believed to contribute to the poor repair characteristic of cartilage [10]. Small lesions in the cartilage can cause severe problems which can affect patient’s mobility[19]. In order to understand how cartilage injuries can be treated in vivo[9], chondrocyte behaviour in relation to model wounds[4] in the presence of various cell stimulators[1] such as different growth factors, can be examined in vitro.TGF-β1 is a 25kD homodimeric peptide[16] and is widely involved in the biological regulation and cellular activities[22]. The TGF-β superfamily consists of various types of polypeptides effecting up and down-regulation of membrane proteins[24] and consequently inducing cell adhesion, proliferation, differentiation, activation, migration and apoptosis[14]. Stimulation by TGF-β1 of the synthesis of cartilaginous matrix suggested[12,23] that this type of growth factor may play an important role in cartilage repair by increasing cell proliferation rates, cell migration and cellular adhesion. This may indicate how cartilage repair is initiated and how this could be accelerated by transforming growth factor-beta1[4]. It has also been reported that chondrocytes can be induced to obtain a chondrocytic morphology by seeding at high cell density[2] and a fibroblastic morphology by culturing in low density after their isolation[18]. This work aims to investigate the effect of TGF-β1 on cell length, proliferation rate, focal adhesion formation /cell surface attachment, cell motility and formation of the extracellular matrix in both fibroblastic and chondrocytic cartilage cells isolated from knee joint of rats.
2. Materials and Methods
2.1. Cell Culture
- Epiphyseal plates were carefully separated from the end of both tibias and femurs derived from 3 days old Sprague- Dawley rats. Tissue was then immersed in 4ml of 0.25% trypsin and 2g per litre EDTA (Sigma, 2007) and stirred for 15 minutes at 37℃. The supernatant was aspirated after 15 minutes and transferred into a 15ml centrifuge tube. Trypsin was deactivated by adding 4 ml of 10% FCS contained DMEM media. The process of digestion by trypsin was repeated three times. The aspirated supernatants and epiphyseal plates were centrifuged at 2000 rpm and the obtained pellet was immersed in 4ml of 0.1% collagenase type-I solution (Sigma, 2007) for 90 minutes. Following treatment with collagenase type -1 solution the supernatant was mixed with 4ml of FCS containing cell culture media and re- centrifuged at 2000 rpm. The supernatant was discarded and the obtained pellet was resuspended with 5 ml chondrocyte culture media, seeded in a 25 cm² tissue culture flask and incubated at 37℃. After 24 hours the epiphyseal plates and non-attached cells were discarded, fresh media was added to the cell culture and incubated at 37℃ until 70-80% confluency.To obtain the chondrocytes with chondrocytic morphology the cells were resuspended at cell density of 250,000 cells/ml in 10ml high glucose DMEM (Dulbecco’s modified eagles medium) with 10% FCS supplementation and 0.1mg/ml hyaluronic acid (Sigma, 2007). Five millilitres of cell suspension were transferred into a 25cm² TG grade falcon cell culture flask. To acquire chondrocytes with a fibroblast like phenotype 1ml of cell suspension was added to 4ml chondrocyte culture media to achieve a cell density of 50,000 cells/ml. Both cultures were incubated at 37℃ until 70-80% confluency.
2.2. Cell Proliferation
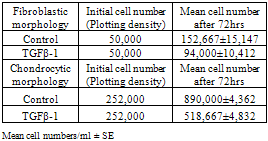
- The effect of TGF-β1 on proliferation rate of chondrocyte cells with both fibroblast like, and chondrocytic morphologies was assessed. The initial chondrocyte cell density for fibroblast like morphology was 50,000 cell/ml and for chondrocytic cells was 250,000 cell/ml. After 72 hours in culture cells were trypsinized and counted using a haemocytometer and these cell densities were compared with the initial plating densities. The experiment was repeated three times and the mean proliferation rate (±SE) were calculated.
2.3. Cell length
- From each cell culture two subcultures were provided one was supplemented with a bathing concentration of 10 ng/ml [7] TGF-β1 (Sigma, 2007). Non-attached cells were removed after 24 hours and the media was subsequently renewed every 48 hours. Time related changes in cell length were determined every two days via the acquisition of photomicrographs and measurement using Image J software (NIH) for 150 control and TGF-β1 treated cells. The experiment was repeated 3 times and the mean cell lengths at each time point (±SE) were calculated.
2.4. Immunocytochemistry
- Immunocytochemistry was used to evaluate the effect of TGF-β1 on antibody localization of collagen type-I, collagen type-II and fibronectin. Monoclonal anti-collagen type-I (mouse IgG1 isotype), monoclonal anti-collagen type-IIA1 (mouse IgG1) and monoclonal anti-fibronectin antibodies (Sigma, UK) were used as primary antibodies and goat anti-mouse IgG, conjugated to Alexa-Fluor 488 SFX Kits (Invitrogen, UK) as secondary antibody.
2.5. Wound Closure Assay
- The capacity of cartilage to repair in the presence of TGF-β1 was assessed by means of creating a wound via scratching a confluent chondrocyte monolayer. Both control and TGF-β1 supplemented monolayers were scratched using the tip of a sterilized 3ml fine tip extended transfer pipette with a 1mm tip diameter. The experiments were carried out for both fibroblast like and chondrocytic morphologies.The scratched areas were imaged in 10 different places by phase contrast microscope at 10x magnification every 2hrs to determine the average width of the wound over a period of 72 hours.
2.6. Cell Adhesion Analysis
- Chondrocyte cell surface attachment in both control and TGF-β1 contained environments was assessed at room temperature using a trypsinization assay. Trypsin is a temperature dependant enzyme which stimulates integrin dependent cell adhesion[20] and digests proteins by cleaving the peptide chains at the carboxyl side of amino acids[21]. This effect causes breakage of cell-cell and cell-extracellular environment adhesion releasing the cells from the culture flask and/or dissociation from each other. The time taken for detachment of the chondrocytes from the surface allows evaluation of the strength of cell attached to the extracellular environment.In this case primary chondrocyte cells grown in cell culture medium with and without TGF-β1 supplementation were rinsed 3 times with Hank’s Balanced Salt Solution (HBSS) prior to trypsinization and then the cells were removed by adding 2ml of 0.25% Trypsin-EDTA (Sigma, 2007) to the culture flask. As soon as trypsin was added to the culture flask, the cells started to round up. A sequence of 45 images at 10 second intervals was taken during trypsinization using a digital camera connected to the phase contrast microscope and related software. The cell detachment rate was measured by determining the time taken for all chondrocyte cells to round up.
2.7. Statistical Analyses
- Data acquired from the experiments in this study were tested for normality and then analysed by t-tests or the non-parametric equivalent tests if required. All means in this study are quoted ± standard error (SE).
3. Results and Discussion
3.1. Proliferation
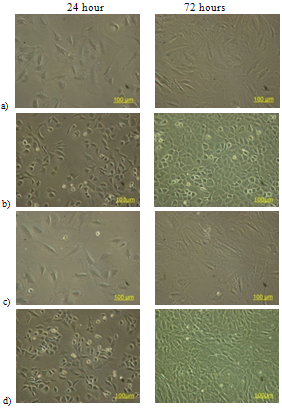
- The experiments examining the effect of TGF-β1 on chondrocyte proliferation showed that the proliferation rate of the cells in medium with TGF-β1 supplementation was noticeably lower than that seen in control cultures (See Table 1). This effect was comparable for both chondrocytic phenotypes and indicates that TGF-β1 controls the production of chondrocytes by decreasing cell proliferation in vitro. The proliferation rate of chondrocytes without TGF-β1 supplementation was higher than those cultured with TGF-β1. The average cell number after 72 hours in the control cultures of fibroblastic chondrocytes increased to 152,667±15,147 cells/ml, whereas treatment with TGF-β1 for 72 hours resulted in cell numbers increasing to only 94,000±10,412 cells/ml. It was also found that TGF-β1 treatment for 72 hours induced the fibroblastic chondrocytes to take on a much more flattened and spread morphology. This enabled the cells to cover more of the surface without large increases in cell number (See Figures 1, and 2). This change in cell morphology was also seen in the chondrocytic chondrocyte cells, where TGF-β1 also reduced cell proliferation by approximately 1/3 and promoted a de-differentiation into a more fibroblastic morphology.
|
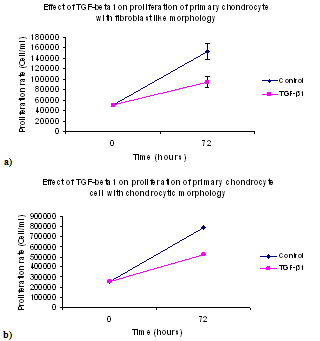 | Figure 2. Effect of TGF-β1 on the proliferation of primary chondrocyte monolayer: (a) with fibroblast like morphology, and (b) with chondrocytic morphology (mean cell numbers ± SE) |
3.2. Cell Length
- The lengths of the both fibroblastic and chondrocytic chondrocytes was influenced by treatment with TGF-β1 (See Figure 3). Cell lengths for fibroblast like chondrocytes cultured in TGF-β1 contained media were 41±0.27µm after 24 hours , 64±0.28 µm after 48 hours and 75±0.46μm after 72 hours, respectively, and for cells without TGF-β1 supplementation were 40±0.7 µm on day one, 48±0.14 µm on day two and 54±0.26 µm on day three, respectively.In contrast with fibroblastic chondrocytes TGF-β1 had a slightly converse effect on length of cells with chondrocytic phenotype. The average lengths of the chondrocytes in the medium without TGF-β1 supplementation were 22±0.07 µm on day one, 25±0.06 µm on day two and 26±0.18 µm on day three, respectively and 19±0.22 µm on day one, 16±0.32 µm on day two and 21±0.34 µm on day three in the TGF-β1 contained environment.Statistical analyses of cell length in relation to time in culture indicated that TGF-β1 significantly (p<0.05), increased cell length at the 48 hour and 72 hour time point for the fibroblastic chondrocytes. However, in the case of chondrocytic chondrocytes, TGF-β1 induced a significant decrease in cell length (p<0.05) at the 48 hour time point, but no overall increase in cell length after 72 hours in culture. It is highly likely that the decrease in cell length seen at the 48 hour time point in the chondrocytic type cells may be associated with cell division at that time.These results indicate that TGF-β1 induced a significant increase in fibroblastic cell spreading possibly associated with increased extracellular matrix protein secretion; (See Figure 3). In comparison, TGF-β1 had little effect on chondrocytic cell length after 72 hours in culture, however the cells did change their morphology, becoming more elongated and more fibroblastic.
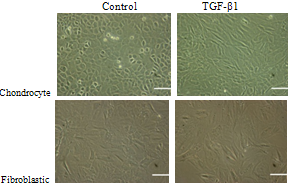 | Figure 3. Cultured chondrocyte cells after 72 hours: (left) without, and (right) with TGF-β1 supplementation (Scale bar=100 µm) |
|
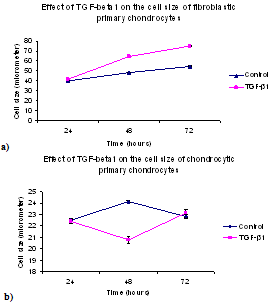 | Figure 4. Cell length of primary chondrocyte monolayers vs time: (a) Fibroblastic, and (b) Chondrocytic. The error bars represent SEM of measured cell length |
3.3. Immunostaining
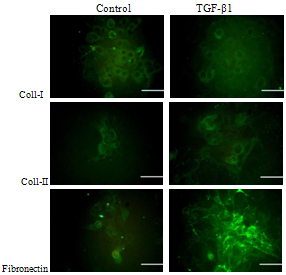
- Immunocytochemistry of monolayer cultures of chondrocytes revealed that the primary chondrocytes isolated from knee joint of neonate Sprague-Dawley rats form different type of morphologies. Immunofluorescence micrograph of chondrocytes stained for type-I collagen, type-II collagen and fibronectin showed that the chondrocytes with 10 ng/ml TGF-β1 supplementation obtain fibroblast like morphology while at the same density of chondrocytes cultured in environment without TGF-β1 exhibit a rounded shape (Figure 5). The type-II collagen and fibronectin spread around the cell membrane of chondrocytes without TGF-β1 supplementation, whereas this protein was mainly distributed between cell membrane and extracellular matrix in presence of TGF-β1. There was no significant difference between both cultures stained for collagen type-I (Figure 5).
3.4. Wound repair
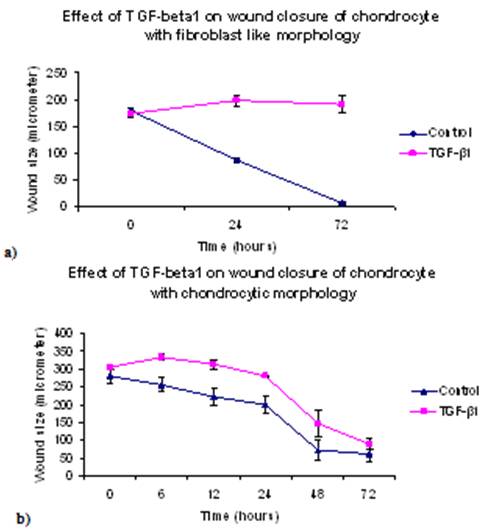
- The results for the in vitro monolayer wound repair assay of primary chondrocyte with fibroblastic and chondrocytic phenotypes showed that the process of healing for a mechanically created wound model in the presence of TGF-β1 was much slower than control cultures (Figures 6-8). Addition of TGF-β1 to fibroblastic chondrocytes caused 14.1 % increase in wound size after 24 hours, and 9.8% increase in wound size after 72 hours as compared to control which showed 52.2% wound closure after 24 hours and 96.6% wound closure after 72 hours, respectively.Statistical analyses indicated that wound widths in response to TGF-β1 treatment in fibroblastic chondrocytes cultures remained unchanged after 72 hours in culture (p=0.94). However, in controls cultures of fibroblastic chondrocytes wound size decreased significantly after 72 in culture (p<0.05).In contrast, control culture of chondrocytes with a chondrocytic phenotype showed ~ 28.3% wound closure after 24 hours and 79.1% after 72hours. Addition of TGF-β1 caused 8.4% wound closure after 24 hours and 71.2% after 72 hours (see Figure 6).This suggests that TGF-β1 down regulates cell proliferation, adhesion and cell migration which are essential in wound repair. In all experiments the wound width increased during the first 6 hours after creating the wound. Statistical analyses of wound closure in chondrocytic cell cultures indicated that although TGF-β1 seemed to decrease wound closure this decrease in wound closure as determined by comparing wound width after 72 hours was not significant (p=0.133).
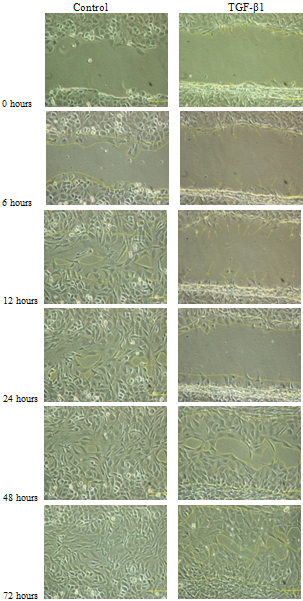 | Figure 6. An evaluation of wound closure for primary chondrocytes with chondrocytic phenotype with and without TGF-β1 supplementation (Scale bar=100μm) |
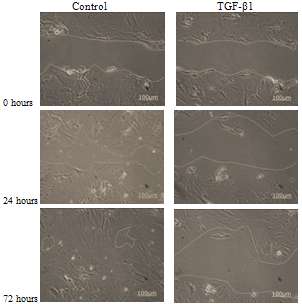 | Figure 7. A comparison in wound closure of chondrocytes with fibroblast like morphology with and without TGF-β1 addition |
3.5. Trypsinization Assay
- Trypsinization of chondrocytes monolayers revealed that the detachment time (See Table 3) from a solid surface for chondrocytes with fibroblast like morphology with TGF-β1 supplementation was longer than those cultured in medium without TGF-β1. However, this time for cells with chondrocytic morphology and TGF-β1 supplementation was relatively short when compared to the cells without TGF-β1 supplementation (See Table 3).
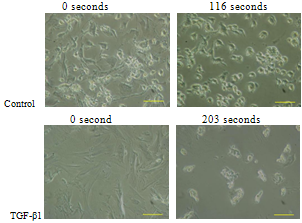 | Figure 9. Detachment of primary chondrocyte cells with fibroblast like morphology from solid surface by trypsinization (Scale bar=100μm) |
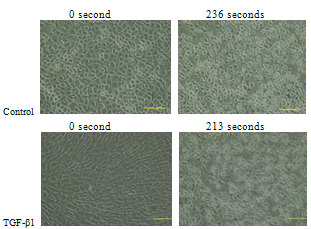 | Figure 10. Detachment of primary chondrocyte cells with chondrocytic morphology from solid surface by trypsinization (Scale bar=100μm) |
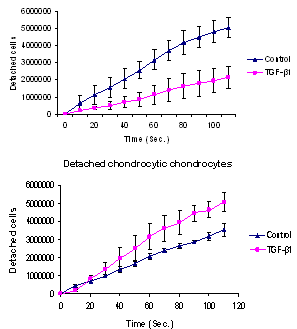 | Figure 11. Detachment of the primary chondrocytes: (above) with fibroblast like morphology, and (below) with chondrocytic morphology, with and without TGF-β1 |
4. Conclusions
- Primary chondrocyte cells acquired a fibroblast like morphology after the first subculture in the 2-dimensional cell culture system. TGF-β1 caused down regulation of chondrocyte proliferation and up-regulation of cell death.TGF-β1 caused increase in cell length of chondrocyte with fibroblastic phenotype but not with chondrocytic chondorcyte.TGF-β1 had a negative effect in wound closure process of chondrocytes in vitro suggesting that the inactive TGF-β1 down regulates cell proliferation and cell migration which are essential for tissue repair.The healing process of scratched chondrocytes monolayer started after ca. 6 hours after creating the wound model. This confirms the stress responsive nature of the cell and delay in the cell activation, particularly the down regulation of chondrocyte mitogenesis[3].TGF-β1 therefore plays a very important role in rapid formation of fibroblast like chondrocyte cells in vitro. This could be linked to an up-regulation in the production of extracellular matrix and extended collagen type-I fibres causing the cells to flatten and occupy a larger area which prohibits cells migration and consequently reduces cell proliferation[6].Results of this study also suggest that TGF-β1 stimulates chondrocyte-ECM adhesion but that this depends on the cell phenotype. Chondrocytes with a fibroblast like morphology cultured in medium with TGF-β1 supplementation displayed a greater degree of cell/surface attachment than did those those cultured in medium without TGF-β1. In contrast the degree of cell surface attachment was decreased in cells with chondrocytic morphology when cultured in TGF-β1 contained media. It is believed that TGF-β1 is involved in chondrocyte cell-ECM adhesion suggesting that TGF-β1 may cause synthesis of different type of CAMs, which may influence cell migration and proliferation in the wound site. However, the negative effect of this cytokine in wound repair of cartilage suggests the hypothesis that the inhibition or activation of TGF-β1 may induce cartilage repair[8]
References
| [1] | AREND, W. P. (2001) Cytokines and cellular interactions in inflammatory synovitis. Journal of Clinical Investigation, 107. |
| [2] | BASHEY, R. I., IANNOTTI, J. P., RAO, V. H., REGINATO, A. M. & JIMENEZ, S. A. (2006) Comparison of morphological and biochemical characteristics of cultured chondrocytes isolated from proliferative and hypertrophic zones of bovine growth plate cartilage. International Society for Differentiation, 46, 199-207. |
| [3] | BORDELEAU, F., BESSARD, J., SHENG, Y. & MARCEAU, N. (2008) Keratin contribution to cellular mechanical stress response at focal adhesions as assayed by laser tweezers. Journal of Biochemistry and Cell Biology, 86, 352-9. |
| [4] | BOS, P. K., VERHAAR, J. A. N. & OSCH, G. J. V. M. V. (2007) Age-Related Differences in Articular Cartilage Wound Healing: A Potential Role for Transforming Growth Factor β1 in Adult Cartilage Repair IN FISHER, J. P. (Ed.), Springer US. |
| [5] | CELESTE, M. N. & CHRISTOPHER, S. C. (2002) Cell-cell signaling by direct contact increases cell proliferation via a PI3K-dependent signal. Federation of European Biochemical Societies, 514, 238-242. |
| [6] | CHI, S. S., RATTNER, J. B. & MATYAS, J. R. (2004) Communication between paired chondrocytes in the superficial zone of articular cartilage. Journal of Anatomy, 205, 363-370(8). |
| [7] | DAN-NING, H. & MCCORMICK, S. (2005) Chondrocyte culture formulationns. IN FREEPATENTSONLINE (Ed.) USA, NEW YORK EYE & EAR INFIRMARY (310 East 14th Street, New York, NY, 10003-4297, US) |
| [8] | DAVIDSON, E. B., SCHARSTUHL, A., VITTERS, E., KRAAN, P. V. D. & BERG, W. V. D. (2005) Reduced transforming growth factor-beta signaling in cartilage of old mice: role in impaired repair capacity. Arthritis Research & Therapy, 7. |
| [9] | ELTAWIL, N. M., BARI, C. D., ACHAN, P., PITZALIS, C. & DELL'ACCIO, F. (2009) A novel in vivo murine model of cartilage regeneration. Age and strain-dependent outcome after joint surface injury. Osteoarthritis and Cartilage, 17, 695-704. |
| [10] | ERICKSON, G. R., GIMBLE, J. M., FRANKLIN, D. M., RICE, H. E., AWAN, H. & GUILAK, F. (2002) Chondrogenic Potential of Adipose Tissue-Derived Stromal Cells in Vitro and in Vivo. Biochemical and Biophysical Research Communications, 290, 763-769(7). |
| [11] | FOX, V., GOKHALE, P., WALSH, J., MATIN, M., JONES, M. & ANDREWS, P. (2008) Cell-cell signaling through NOTCH regulates human embryonic stem cell proliferation. Journal of Stem Cell, 26, 715-23. |
| [12] | HICKERY, M., BAYLISS, M., DUDHIA, J., LEWTHWAITE, J., EDWARDS, J. & PITSILLIDES, A. (2003) Age-related changes in the response of human articular cartilage to IL-1alpha and transforming growth factor-beta (TGF-beta): chondrocytes exhibit a diminished sensitivity to TGF-beta. The Journal of Biologycal Chemistry, 278, 53063-71. |
| [13] | IM, G.-I. & SHIN, S.-R. (2002) Changes in the production and the effect of nitric oxide with aging in articular cartilage: An experimental study in rabbits Acta Orthopaedica, 73, 6-10. |
| [14] | KRAUSS, G. (2006) Biochemistry of Signal Transduction and Regulation (Third Edition). Published by Wiley-VCH. |
| [15] | LAUGE-PEDERSEN, H. & ASPENBERG, P. (2003) Synovial fluid depletion: successful arthrodesis without operative cartilage removal Journal of Orthopaedic Science, 8, 591-595. |
| [16] | LAWRENCE, D. (2001) Latent-TGF-beta: an overview. Moelcular and Cellular Biochemistry, 219, 163-70. |
| [17] | LIN, X. & HELMKE, B. P. (2009) Cell Structure Controls Endothelial Cell Migration under Fluid Shear Stress. Journal of Cellular and Molecular Bioengineering, 2, 231-243. |
| [18] | MARTIN, I., SUETTERLIN, R., BASCHONG, W., HEBERER, M., VUNJAK-NOVAKOVIC, G. & FREED, L. E. (2001) Enhanced cartilage tissue engineering by sequential exposure of chondrocytes to FGF-2 during 2D expansion and BMP-2 during 3D cultivation. Journal of Cellular Biochemistry, 83, 121-128. |
| [19] | MITHOEFER, K., WILLIAMS, R., WARREN, R., WICKIEWICZ, T. & MARX, R. (2006) High-impact athletics after knee articular cartilage repair: a prospective evaluation of the microfracture technique. The American Journal of Sports Medicine, 34, 1413-18. |
| [20] | MIYATA, S., KOSHIKAWA, N., YASUMITSU, H. & MIYAZAKI, K. (2000) Trypsin Stimulates Integrin α5β1-dependent Adhesion to Fibronectin and Proliferation of Human Gastric Carcinoma Cells through Activation of Proteinase-activated Receptor-2. Journal of Biological Chemistry, 275, 4592-4598. |
| [21] | OLSEN, J., ONG, S. & MANN, M. (2004) Trypsin cleaves exclusively C-terminal to arginine and lysine residues. Molecular and Cell Proteomics, 3, 608-614. |
| [22] | RAFTERY, L. A. & SUTHERLAND, D. J. (2002) TGF-β Family Signal Transduction in Drosophila Development: From Mad to Smads Development Biology, 210, 251-268. |
| [23] | SHAH, M., REVIS, D., JR., HERRICK, S., BAILLIE, R., THORGEIRSON, S., FERGUSON, M. & ROBERTS, A. (1999) Role of Elevated Plasma Transforming Growth Factor-β1 Levels in Wound Healing. The American Journal of Pathology, 154. |
| [24] | SPAGNOLI, A., O'REAR, L., CHANDLER, R. L., GRANERO-MOLTO, F., MORTLOCK, D. P., GORSKA, A. E., WEIS, J. A., LONGOBARDI, L., CHYTIL, A., SHIMER, K. & MOSES, H. L. (2007) TGF-β signaling is essential for joint morphogenesis. The Journal of Cell Biology, 177. |
| [25] | VINCENT, T., HERMANSSON, M., BOLTON, M., WAIT, R. & SAKLATVALA, J. (2002) Basic FGF mediates an immediate response of articular cartilage to mechanical injury. Proceedings of the National Academy of Sciences of the United States of America, 99. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML