-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biomedical Engineering
p-ISSN: 2163-1050 e-ISSN: 2163-1077
2011; 1(1): 7-12
doi: 10.5923/j.ajbe.20110101.02
Response to Ultra-high Molecular Weight Polyethylene Particles
M. K. Musib
Department of Orthopaedic Surgery and Rehabilitation Medicine, SUNY Downstate Medical Center, Brooklyn, NY 11203, USA
Correspondence to: M. K. Musib , Department of Orthopaedic Surgery and Rehabilitation Medicine, SUNY Downstate Medical Center, Brooklyn, NY 11203, USA.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Ultrahigh molecular weight polyethylene (UHMWPE) is widely used in the field of orthopedics to fabricate various orthopedic total joint (primarily hip, knee and shoulder) device components. In spite of its biocompatibility and excellent mechanical properties, wear debris particles are released during use at the articulating surfaces, usually at the implant-implant, bone-implant and cement-bone and cement-implant interfaces. Prior studies have shown that these wear debris particles are responsible for periprosthetic bone resorption (wear-mediated osteolysis) which leads to implant loosening and eventually failure of the prosthetic device. Previously investigators have implicated the size, shape, and number of UHMWPE wear particles released from implants in vivo and in situ to the wear-mediated-osteolytic phenomenon. In this short review I will discuss some of the in vitro and in vivo studies pertaining to the response to the wear-debris particles.
Keywords: UHMWPE, Wear-Debris, Nanoparticles, Osteolysis
Cite this paper: M. K. Musib , "Response to Ultra-high Molecular Weight Polyethylene Particles", American Journal of Biomedical Engineering, Vol. 1 No. 1, 2011, pp. 7-12. doi: 10.5923/j.ajbe.20110101.02.
Article Outline
1. Cellular and Tissue Responses to UHMWPE Debris
1.1. In Vitro Studies
- In vitro techniques offer a sensitive means to investigate the responses of cells to wear particles. At present, a diverse group of cell types have been reported to release inflammatory and chemical mediators in response to particulate assault including macrophages, osteoclast, osteoblasts, giant cells, and fibroblasts. Numerous studies have been conducted to determine the effect of wear particles in vitro and in vivo1-18. Murray et al.19, used macrophage cell culture and used HDPE of approximately 1μm (1200 grit silicon carbide paper fabricated) and demonstrated that the particles were phagocytosed resulting in the release of PGE2 and osteoclastic bone resorption. Shanbhag et al.20 co-cultured fabricated and retrieved UHMWPE particles with human monocytes and demonstrated that IL-1β, IL-6 and TGF-β were produced at significantly elevated levels, with fabricated particles eliciting greater quantities of the cytokine release by cells than retrieved UHMWPE particles. The results showed that human monocytes were sensitive to different grades of polyethylene for the release of osteolytic cytokines. Ingham et al.21 determined the effect of neutralization of IL-1, IL-6, TNF-α, and PGE2 alone and in combinations on the capacity of polyethylene particle to stimulate bone resorption in vitro. They showed that only when TNF-α was neutralized along with neutralization of other mediators, there was any significant reduction in bone resorption. Several investigators have suggested that endotoxin adsorbed to the particulate surface may account for the increased release of proinflammatory mediators by activated macrophages22. Catelas et al.23 introduced HDPE (round, 4.5μm) to macrophage cell culture and found increased TNF-α release and decreased cell viability. Green et al,11 using a macrophage cell culture treated with different sizes of UHMWPE (Ceridust 3615: 0.21±0.069μm, 0.49±0.11μm, 4.3±1.89μm, 7.2±3.15μm) and UHMWPE (GUR 1120: 88±29μm and found elevated levels of TNF-α, IL-1β and IL-6. They concluded that the size and volume (number) were the critical factors in macrophage activation. Horowitz et al.24 used macrophage/osteoblasts co-culture using round HDPE particles (5.4±0.3μm) and found increased TNF-α and PGE2 release and bone resorption. In another study Martinez et al.13 used human osteoblastic cell culture and UHMWPE (Hoechst) <80μm and UHMWPE (Howmedica) <100μm and concluded that UHMWPE particles inhibited human osteoblastic cell growth. Shanbhag et al.25 used macrophage cell culture UHMWPE (GUR 415) having two different sizes, 0.47±0.2μm (retrieved) and 0.66±0.6μm (fabricated). They concluded that the increase in the surface area increased cellular response. In another study, Voronov et al.12 used in vitro macrophage cell culture, and UHMWPE: 18-20μm, HDPE: 4-10μm and UHMWPE: 18-20µm. The smaller particles were phagocytosed and released IL-1β, IL-1α, TNF-α and PGE2. Wear debris stimulate the various cell types present in the periprosthetic region resulting in the secretion of mediators of bone resorption such as eicosanoids, interleukin-1 (IL-1), tumor necrosis factor-alpha (TNF-a), and interleukin-6 (IL-6) in vitro and in vivo6,26,27. Wear mediated osteolysis has been attributed to the number, size, shape, rate of generation, time of exposure, and antigenic properties of the wear debris particles, produced at the articulating surfaces of total joint prosthesis4,28. There are numerous studies on cellular response to wear particles on various cell lineages including macrophages, osteoblasts, osteoclasts and lymphocytes29. Studies on particles from wear machines and retrieved tissues from revised arthroplasties have revealed UHMWPE particles ranging in size from 0.1 to greater than 10µm but majority of the UHMWPE particles were between 0.1–0.5µm in size30. Green et al.7,11 showed that both the volume of debris and particle size are critical factors in determining the reactivity of polyethylene particles in vitro. None the less only particles within the phagocytosable size range (0.1–10µm) and particle volume to cell number ratios greater than 10µm3:1 induced the secretion of a range of pro-inflammatory cytokines by murine macrophages. It has also been shown that particles in the 0.1–1.0µm size range are the most stimulatory for primary human mononuclear phagocytes in vitro21,31. Thus biological activity of wear debris depends not only upon the total volume of debris and the number of particles generated but also upon the proportion of those particles which are within the most biologically active size range. Other studies have also correlated the particle dose to osteolysis32. It has been estimated that when the number of UHMWPE particles exceed 1×1010 per gram of tissue there is a greater possibility of osteolysis33. This information becomes more important as crosslinked polyethylenes may produce a greater percentage of wear particles in the most biologically active size range.Sethi et al.17 studied macrophage response to cross-linked UHMWPE (XLPE), conventional UHMWPE, TiAlV and CoCr. Human peripheral blood monocytes and murine macrophages, were cultured onto disks. Culture supernatants were collected at 24 and 48 h and analyzed for cytokines such as IL-1α, IL-1β, TNF-α and IL-6. Total RNA was extracted from adherent cells and gene expression was analyzed. In both in vitro models, macrophages cultured on cross-linked and conventional polyethylene released similar levels of cytokines, which were also similar to levels on control tissue culture dishes. Macrophages cultured on TiAlV and CoCr-alloy released significantly higher levels of cytokines8,9,34,35 . Researchers in various experiment7,31,36 generated clinically relevant UHMWPE particles in a tri-pin-on-disc tribometer and co-cultured with mouse peritoneal macrophages in a agarose gel system at various cell concentration to particle volume. They found that the particles elicited the release of various cytokines and that particles within 0.2-7µm were most biologically active. Green et al. used ceridust and GUR416 with different size ranges using primary murine macrophages and human peripheral blood mononuclear phagocytes and measured IL-1β, IL-6, TNF-α, and PGE2 production. The critical size range for biological activity was 0.2– 8.0µm and the smaller particles elicited a higher response as compared to the larger ones7,11It has been demonstrated that UHMWPE particles of different sizes stimulate macrophages to produce bone resorptive cytokines and chemikines in vitro19,24,37 Green et al. and Matthews et al. found that the critical size range was higher for ceridust was higher and for working with human cells the critical size is 0.2-8µm11,36 Furthermore Catellas et al, using a macrophage cell culture line and PE particles found that cytotoxicity increased with size and dose23. Green et al.7 studied the in vitro bone resorption response of C3H murine peritoneal macrophages in response to clinically relevant GUR 1120 UHMWPE particles. They co-cultured macrophages with GUR 1120 particles with a mean size of 0.24, 0.45, 1.71 and 7.62 and GUR 1120 polyethylene resin with a mean size of 88µm at various particle volume (µm)3: macrophage ratios (0.1:1; 1:1; 10:1; and 100:1). The conditioned supernatants were incubated with 45Ca radio-labeled mouse calvariae, and bone resorption was measured as 45Ca release. The results showed that the smaller 0.24µm particles stimulated the macrophages to generate bone resorbing activity at a ratio of 10(µm)3 per macrophage. The 0.45 and 1.71µm particles were active at a ratio of 100(µm)3 per macrophage, and the 7.62 and 88 µm particles were inactive at all the doses tested. The co-culture supernatants were also assayed for TNF-α, IL-1β, IL-6, and PGE2. The results followed the same trend. Moreover Goodman and colleagues38 also showed that inhibition of tissue ingrowth and differentiation in a rabbit bone-harvest chamber model was greater with 2.7 µm particles compared to 4.7 µm particles. The reason may be attributed to the fact that more number of the smaller particles were phagocytosed by the cells and as we know phagocytosis is the hallmark phenomenon that initiates the cells to release the cytokines responsible for the wear mediated osteolytic process.
1.2. In Vivo Studies
- Animal models have contributed greatly in elucidating the tissue response to wear mediated particles. They are useful for various reasons, like standard animal models may be developed to characterize the particulate response for that particular model, the particle properties may be manipulated so that particles with known physico-chemical properties may be introduced into the animal body and end points may be predetermined. But the shortcomings include the cost involved and that the animal model may not fully mimic the human system. Moreover it may not fully take into account other factors such as human gait cycle and resulting mechanical stress. There are numerous in vivo models that have been established to study the effects of particles on various animal models. The studies include the mouse air pouch model39,40, calvarial defect models41, rabbit models5,15,38,42-45, rat models46-49, canine models50-53 and sheep models54. Allen et al.55 used an in vivo rat model. They examined the effects of HDPE, having a round morphology of about 2.03μm and found that the particles caused chronic inflammatory response with numerous foreign-body giant cells in peri-prosthetic tissues. Goodman et al.56 used rabbits and drilled a hole into the proximal tibia. HDPE particles that were round with mean diameter of (4.7±2.1)μm were tested and found that 106 to109 particles/ml evoked a histiocytic reaction. In another experiment they examined the histologic effects of different size of polyethylene particles implanted into rabbit tibia found that larger polyethylene particles elicited a higher response than smaller particles57. Howie et al.47 used a rat model to test particulate effect at the cement-bone interface. Irregular shaped UHMWPE (Howmedica) particles having diameter of 20-200μm were placed at the bone-cement interface. They found that it caused bone resorption.
1.3. Tissue Retrieval Studies
- There are numerous studies on the analysis of explanted tissue33,58,59 to characterize wear debris particles. Chiba et al.37 demonstrated that UHMWPE wear debris (0.3–15µm) isolated from explanted human tissues stimulated human peripheral blood monocytes to release elevated levels of IL-6, TNF-a and IL-8 in tissue culture.
2. Discussion and Conclusions
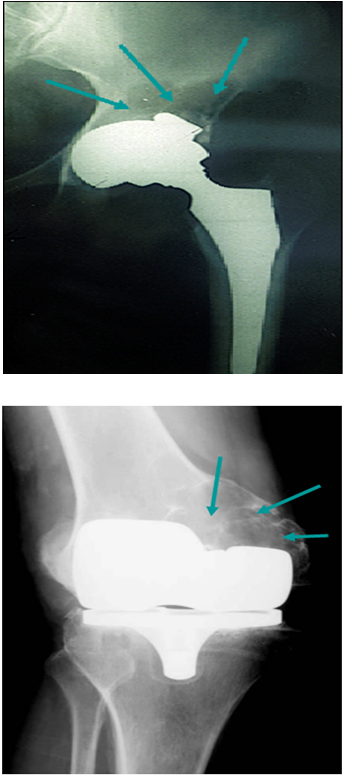
- Bone is a dynamic tissue in a state of homeostasis balanced by both formation and resorption of bone60,61. Normal turnover of bone is disrupted by either increased osteoclast activity or decreased osteoblast function. This disruption may be caused by the biological response to wear-debris. The response to wear debris depends on particle number, size, shape and time of exposure. Numerous researchers have studied the effects of phagocytosis of wear particles (including UHMWPE) on the functions of MG63 cells and found phagocytosis of particles reduced cell proliferation and procollagen gene expression. Numerous other studies have demonstrated that wear particles including metals and UHMWPE inhibit cellular differentiation and viability62-65.Figure 1-2 are X-ray images of the implants of patients who are undergoing revision of total joints showing the extensive bone resorption as a result of component wear around a hip (Figure 1) and knee implant (Figure 2). Figure 3 is a representative SEM micrograph of a retrieved tibial tray showing wear and delamination. The particulate wear debris present in failed joint replacements has been isolated and characterized66-69, the response of various cell types including macrophages, fibroblasts and osteoblasts to such debris has been explored; animal models have been developed and the molecular mechanisms of this wear mediated osteolytic process have been dissected. The information gained from these studies will help us in better understanding and discerning a comprehensive role of size and dose of UHMWPE wear particles on wear mediated osteolysis.
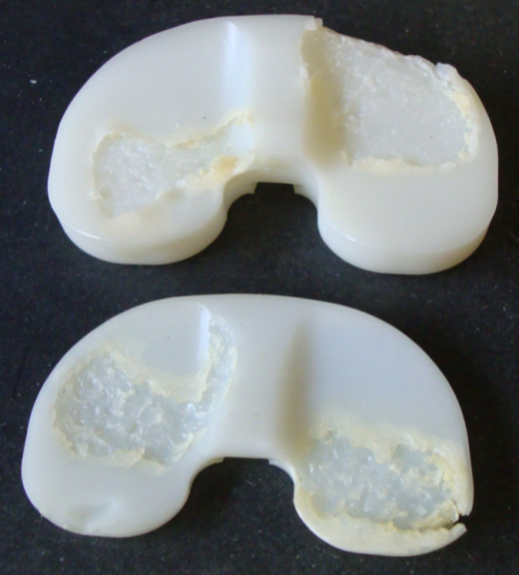 | Figure 3. Representative image of a retrieved tibial component from patient with a failed implant device. Note the extensive wear, delamination and cracks. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML