-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2025; 15(1): 8-14
doi:10.5923/j.ajb.20251501.02
Received: Mar. 22, 2025; Accepted: Apr. 12, 2025; Published: Apr. 16, 2025

Phytochemical Profile and Biological Activities of Aqueous and Hydroethanolic Extracts of the Leaves of Maerua angolensis DC (Forsk) (Capparidaceae) Used in the Treatment of Digestive Diseases in Traditional Medicine in Burkina Faso
David Bado1, Alphonsine Ramde-Tiendrebeogo1, 2, 3, Ibrahim Ouedraogo4, Benjamin Ouedraogo3, 5, Moussa Compaore1, Martin Kiendrebeogo1, Innocent Pierre Guissou6
1Laboratory of Applied Biochemistry and Chemistry/Joseph KI-ZERBO University (UJKZ), 03 BP 7021 Ouagadougou 03, Burkina Faso
2Health Sciences Research Institute / National Center for Scientific and Technological Research (IRSS/CNRST), 03 BP 7047 Ouagadougou 03, Burkina Faso
3International Research Laboratory IRL 3189 Environment, Health, Societies (CNRST/USTTB/UCAD/UGB/CNRS)
4Laboratory of Physical-Chemical, Bacteriological and Heavy Metal Analysis of Water/ AINA SARL, 01 BP 558 Ouagadougou 01, Burkina Faso
5Research and Development Laboratory (LRD)/ Ledea Bernard Ouedraogo University (ULBO), Ouahigouya, Burkina Faso
6St Thomas d’Aquin University (USTA)/ Faculty of Health Sciences, 06 BP 10212 Ouagadougou 06, Burkina Faso
Correspondence to: Alphonsine Ramde-Tiendrebeogo, Laboratory of Applied Biochemistry and Chemistry/Joseph KI-ZERBO University (UJKZ), 03 BP 7021 Ouagadougou 03, Burkina Faso.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Digestive tract diseases are various and considered as a public health issue worldwide. In Burkina Faso, they are the second leading cause of hospital consultations. The leaves of Maerua angolensis are traditionally used in the treatment of digestive diseases. The phytochemical profile was carried out using specific reagents. Aqueous and hydroethanolic decoctions of Maerua angolensis leaves were used for different tests. The antiradical activity and safety use were evaluated using the DPPH method and the acute oral toxicity test according to OECD guideline 423, respectively. The concentration (mg/mL) necessary to reduce the DPPH radical by 50% (IC50) were 0.06 mg/mL and 0.09 mg/mL for the aqueous and hydroethanolic extracts, respectively. The extracts' ability to scavenge free radicals is related to their content in total phenolic compounds (108.44 ± 0.32 mg GAE/g and 106.79 ± 0.79 mg GAE/g), flavonoids (43.04 ± 0.04 mg QE/g and 69.27 ± 0.02 mg QE/g), flavonols (12.73 ± 0.53 mg QE/g and 24.61 ± 0.01 mg QE/g), and tannins (43.18 ± 2.18 mg GAE/g and 39.37 ± 1.85 mg GAE/g) for the aqueous and hydroethanolic extracts, respectively. The essential mineral contents were: Fe (27.59 mg/100g), Ca (1.9 mg/100g), Zn (4.25 mg/100g), K (1.43 mg/100g), Mg (0.77 mg/100g), and Na (0.01 mg/100g). The acute oral toxicity test showed no signs of toxicity associated with the consumption of the leaves of this plant with the dose of 2000 mg/kg wc. The results obtained provide scientific prerequisites for the development of improved traditional medicines and functional foods for the management of digestive diseases.
Keywords: Maerua angolensis, Digestive diseases, Acute toxicity
Cite this paper: David Bado, Alphonsine Ramde-Tiendrebeogo, Ibrahim Ouedraogo, Benjamin Ouedraogo, Moussa Compaore, Martin Kiendrebeogo, Innocent Pierre Guissou, Phytochemical Profile and Biological Activities of Aqueous and Hydroethanolic Extracts of the Leaves of Maerua angolensis DC (Forsk) (Capparidaceae) Used in the Treatment of Digestive Diseases in Traditional Medicine in Burkina Faso, American Journal of Biochemistry, Vol. 15 No. 1, 2025, pp. 8-14. doi: 10.5923/j.ajb.20251501.02.
Article Outline
1. Introduction
- Digestive tract diseases are various and considered as a global public health problem [1]. In Africa, particularly in Burkina Faso, they remain the second leading cause of hospital consultations after malaria, with their prevalence varying by region [2]. These include peptic ulcers, digestive cancers (gastric cancer, colorectal cancer, and liver cancer), functional colopathies, hemorrhoids, hepatitis, chronic inflammatory bowel [3]. At least 800 000 people die each year from gastroenteritis in the world, including 500 000 children under the age of five [4,5]. Helicobacter pylori is a Gram-negative bacterium responsible for the majority of digestive diseases and is the main cause of peptic ulcers and gastric cancers [6]. Other infectious pathogens are responsible for digestive diseases, with the most frequently detected in healthcare facilities in Burkina Faso being Escherichia coli (E. coli), Staphylococcus aureus (S. aureus), Salmonella typhi, Vibrio cholerae, Shigella dysenteriae, etc. Certain parasites, as Entamoeba histolytica and Giardia intestinalis, are also responsible for digestive tract diseases. Additional factors, such as the use of nonsteroidal anti-inflammatory drugs, skipping meals, tobacco and alcohol consumption, and oxidative stress, can cause inflammation of the digestive tract [2,7,8]. Various medications, such as anti-inflammatory drugs, intestinal and gastric antisecretory drugs, gastric antacids, and antibiotics, are used in conventional medicine to treat digestive diseases [8]. However, some of these medications have side effects that can lead to further damage to the digestive tract. Additionally, the phenomenon of antibiotic resistance renders some treatments ineffecient. Previous studies have shown that certain medicinal plants are an important source of nutrition, especially in developing countries [9]. This category of plants offers dual benefits for humans, as they provide bioactive molecules such as phenolic compounds (flavonoids, tannins, saponins, coumarins) and terpenic compounds with anti-inflammatory, antibacterial, antioxidant, and antispasmodic properties, as well as nutrients through their fruits and leaves [9,10]. Consequently, the adoption of these plants in the dietary habits of populations can help to prevent many chronic diseases such as diabetes, hypertension, cardiovascular diseases, oxidative stress, and digestive tract diseases, the occurrence of which results from both diagnostic and therapeutic delay [11]. Maerua angolensis, commonly called Zilogo in the local Mooré language, is a deciduous and perennial plant belonging to the Capparidaceae family. It is a shrub that can reach up to 10 meters in height [12]. An ethnobotanical survey conducted in the Central Plateau region (Zitenga and Tanghin Dassouri) revealed that the leaves of this plant are used in traditional medicine for treating digestive disorders, bloating, and diarrhea. In addition further its medicinal uses, the leaves of Maerua angolensis are consumed as a sauce or used in the preparation of various dishes such as couscous or leaf fritters. The objective of this study was to determine the phytochemical profile, antiradical activity, and acute general toxicity of the leaves of Maerua angolensis used in the treatment of digestive diseases.
2. Materials and Methods
2.1. Plant Material
- The leaves of Maerua angolensis were collected on June, 2023 at Zitenga, located 53 km from Ouagadougou, the capital. After identification by the National Herbarium of Burkina Faso (HNBU), a specimen was deposited there under code 8769. The leaves were thoroughly washed and then dried in a well-ventilated room under continuous airflow. After one week of drying, they were ground using an electric grinder, and the obtained powder was packaged in a transparent bag and stored in the laboratory, protected from light (Figure 1).
 | Figure 1. Leaves of Maerua angolensis (Photo Bado D., 2023) |
2.2. Reagents and Solvents
- The main chemicals used are: 1,1-diphenyl-2-picrylhydrazyl (DPPH) (Sigma, St. Louis, MO, USA), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), and Folin-Ciocalteu reagent (Sigma Chemical Company, Steinheim, Germany), Neu reagent, polyethylene glycol, and 2-aminoethyl diphenylborate, Na2CO3, NaOH (VWR, France), analytical methanol, absolute ethanol, dichloromethane, ethyl acetate (Carlo Erba, France), sulfuric acid, aluminum trichloride, and acetic acid (Labosi, France), gallic acid, rutin, tannic acid, and catechin (Sigma-Aldrich, Germany).
2.3. Methods
2.3.1. Extraction of Phenolics Compounds
- The extraction of phenolic compounds was performed using aqueous and ethanolic decoction. Therefore, 50 g of dry powder was mixed with 500 mL of solvent (70% ethanol or distilled water) in a 1000 mL flask. The resulting mixture was boiled under reflux for 30 minutes and then filtered using filter paper and a funnel. The obtained filtrate was concentrated under reduced pressure using a rotary evaporator (BÜCHI) and then freeze-dried. The resulting dry extract was stored in the refrigerator at a temperature of 4°C.
2.3.2. Phytochemical Screening by High-Performance Thin-Layer Chromatography (HPTLC)
- The phytochemical screening of the extracts was carried out following the HPTLC method as described by [13]. Aluminum plates coated with Silica Gel 60 F254 were used. The deposits were made using the spraying system of a semi-automatic device, Linomat 5 (Camag, Muttenz, Switzerland). An elution system (ethyl acetate: formic acid: acetic acid: water, 100:11:11:26, v/v/v) was used for the migration of flavonoids, coumarins, and saponins. Another elution system (hexane: ethyl acetate, 20:4, v/v) was used for the migration of sterols and triterpenes. A third system (ethyl acetate: methanol: water: dichloromethane, 18:2, 4:2, 1:6, v/v/v/v) was used for the migration of tannins. Flavonoids and phenolic acids were revealed with Neu reagent under UV light (366 nm), while sterols and triterpenes were revealed with 3% H₂SO₄ in 96% ethanol, and tannins with FeCl₃ (2%). The revelation of coumarins was performed using KOH at 366 nm. Saponins were revealed using sulfuric anisaldehyde at 366 nm.
2.3.3. Total Phenolics Content Determination
- Total phenolics content were determined using the Folin-Ciocalteu reagent (FCR) according to the method described by [14]. In a test tube, a mixture consisting of 1 mL of extract and 1 mL of FCR (0.2 N) was prepared. After waiting for 8 minutes, 2 mL of 7.5% Na₂CO₃ was added. The resulting mixture was incubated at 37°C for 30 minutes and then the absorbance was measured at 760 nm against a blank consisting of distilled water. The total phenolic content was evaluated using a standard calibration curve with gallic acid as the reference substance (y = 10.459x + 0.0335; R² = 0.9993). The experiment was performed in triplicate, and the result obtained was the average of these experiments. The contents were expressed in milligrams of Gallic Acid Equivalent per gram of dry extract (mg GAE/g).
2.3.4. Determination of total Flavonoids Content
- Total flavonoids content were determined according to the method used by [15] with slight modifications. In a test tube, a mixture consisting of 3 mL of extract and 3 mL of AlCl3 was prepared. The resulting mixture was incubated for one hour at room temperature. At the end of the incubation period, the absorbance was measured at 510 nm against a blank consisting of distilled water. The total flavonoid content was evaluated using a standard calibration curve with quercetin as the reference substance (y = 10.972x - 0.2075; R² = 0.9998). The experiment was conducted in triplicate, and the result obtained was the average of these experiments. The contents were expressed in milligrams of Rutin Equivalent per gram of dry extract (mg QE/g).
2.3.5. Determination of Total Flavonols Content
- Total flavonol were estimated using the method described by [16]. A volume of 2mL of 20% AlCl3 in 96% ethanol was mixed with 2mL of 1mg/mL extract. A blank was prepared by mixing 2mL of extract and 2mL of ethanol. Optical densities were read after 10 minutes incubation at room temperature at 425 nm. Levels were assessed using a standard calibration curve with quercetin as the reference substance (y = 37.342x + 0.1494; R2 = 0.996). Values are expressed in mg Quercetin Equivalents (QE) /100 mg plant extract.
2.3.6. Total Tannins Content
- Total tannins (TT) were determined indirectly by spectrophotometric measurement of the absorbance of the solution obtained after the precipitation of insoluble tannins with polyvinyl polypyrrolidone (PVPP), as described by [17]. PVPP (110 mg of PVPP per mL of extract) was added to 10 mL of extract (1 mg/mL). The resulting mixture was vortexed and then centrifuged at 3000 g for 10 minutes at 4°C to precipitate the tannins. The phenolic content of the supernatant, corresponding to the non-precipitable phenols (NPP), was measured using the Folin–Ciocalteu method, and the total tannins were calculated using the following equation: TT = TPC − NPP. The results were expressed in mg of Gallic Acid Equivalent (mg GAE)/g of dry extract.
2.3.7. Determination of Minerals Content
- The minerals content were determined according to the method described by [18]. A mass of 5 g of dry material was digested by adding 10 mL of HNO₃ (10%). The resulting mixture was incinerated at 600°C for 6 hours in an incinerator. The ash obtained after incineration was collected and weighed using a precision balance. Then, 0.5 g of this ash was dissolved in 5 mL of HNO₃ (10%), and the supernatant was collected and diluted to 1/100 in a 50 mL volumetric flask. The diluted solutions were centrifuged at 5000 G for 5 minutes and subsequently analyzed using the Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) (PerkinElmer, Optima 800) with the winLab32 version 7.0 software for mineral determination. The contents are expressed in mg/100g.
2.3.8. Antiradical Activity by the DPPH Test
- The antiradical activity by the DPPH test was evaluated according to the method described by [19]. In ten (10) numbered tubes (1-10), a serial dilution of the extract (1 mg/mL) was prepared. Then, 2 mL of DPPH (0.04 mg/mL in methanol) was added to each tube. The resulting reaction mixture was incubated for 15 minutes at 37°C in the dark. The absorbance of the residual DPPH was read at 517 nm. Thus, the antiradical activity was assessed by determining the concentration (mg/mL) required to reduce DPPH radicals by 50% (IC50).
2.3.9. Study of Acute Toxicity
- The acute oral toxicity of each extract was determined following the OECD Test Guideline 423. Two groups of six rats were formed, one being a control group and the other a test group. The rats were Wistar (both male and female), weighing between 150 and 270 g, obtained from the animal facility of the Institute of Health Sciences Research (IRSS) in Ouagadougou, where they were fed wheat bran (29% protein). Drinking water was tap water. They were kept in air-conditioned conditions (23-25°C) with alternating light/dark cycles and 75% humidity. The rats were fasted for 4 hours before the start of the test. After weighing, each animal was administered 10 mL of the plant extract orally at a dose of 2000 mg/kg body weight using a 10 mL syringe. The animals were then fasted again for 4 hours. Following treatment, the animals were regularly observed every 24 hours. Weight changes were monitored during the test by weighing the rats on the following days: 0, 1, 2, 3, 7, and 14. Day 0 represents the beginning of weight measurements after the first fasting period. All rats were sacrificed under anesthesia on the 14th day, and organs such as the liver, lungs, spleen, kidneys, and heart were examined and weighed [20].
2.3.10. Statistical Analysis
- The results were expressed as mean ± SEM (n = 3). The data were analyzed using analysis of variance (ANOVA) with XLSTAT software. Differences were considered statistically significant for a p value <0.05.
3. Results and Discussion
3.1. Chemical Compounds of Maerua angolensis Leaves
- The determination of constituents by HPTLC and the revelation of the spots were showed in Figure 2.
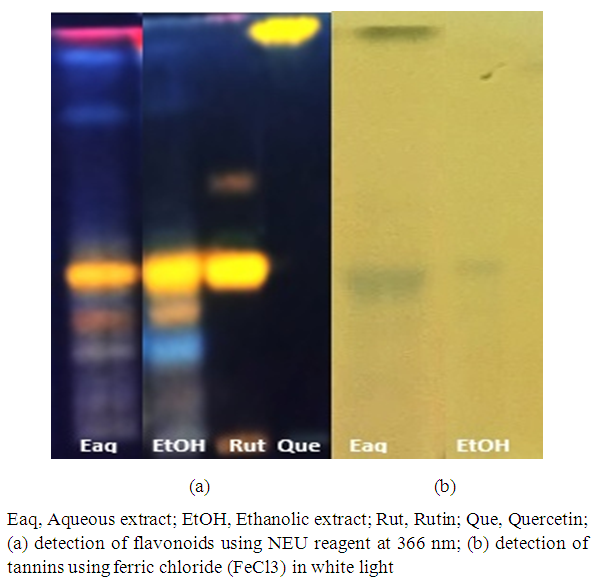 | Figure 2. Chemical compounds present in the leaves of Maerua angolensis |
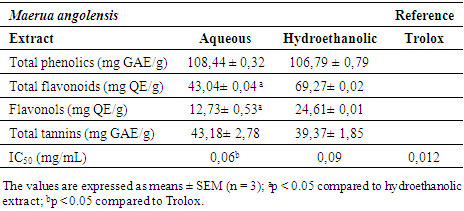
3.2. Content of Phenolic Compounds and Antioxidant Activity
- The contents of phenolic compounds and the minimum inhibitory concentration at 50% the extracts are presented in Table 1.
|
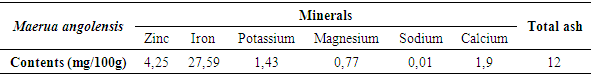
3.3. Mineral Content
- The contents of mineral are presented in Table 2.
|
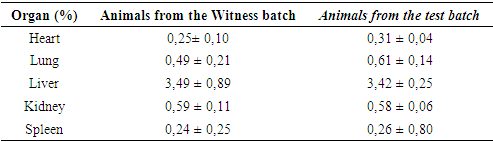
3.4. Safety of Using Leaves of Maerua angolensis
- The results show that no behavioral changes were observed in the animals, nor were there any cases of death. The weights of the organs from the test group and those from the control group were comparable (Table 3). This indicates that the extracts from the leaves of Maerua angolensis did not damage organs such as the lungs, liver, heart, spleen, and kidneys.
|
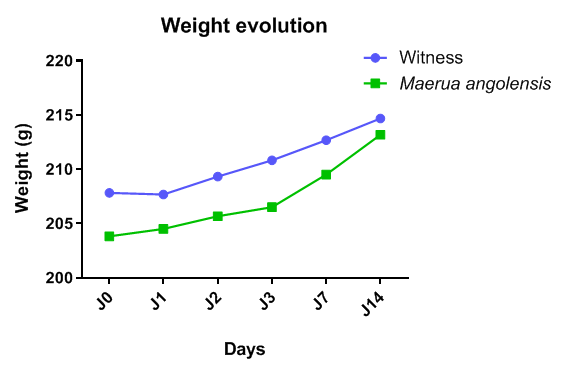 | Figure 3. Weight evolution of the animals |
4. Conclusions
- The objective of this study was to determine the phytochemical profile, radical-scavenging activity, and acute oral toxicity of the leaves of Maerua angolensis used in the treatment of digestive diseases. The results showed that the leaves of the plant contain phenolic compounds, including flavonoids, tannins, saponins, coumarins, and exhibit remarkable radical-scavenging power with high iron content. The results also showed that regular consumption of the leaves of this plant by populations is safe and can significantly reduce the risk of diseases related to oxidative stress, particularly diseases of the digestive tract. The development of improved traditional medicines or functional foods based on the leaves of Maerua angolensis could be an alternative for the treatment of digestive diseases.
ACKNOWLEDGEMENTS
- We thank the Aktionsgemeinschaft Solidarische Welt (ASW)/Berlin for financing the project.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML