-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2025; 15(1): 1-7
doi:10.5923/j.ajb.20251501.01
Received: Jan. 25, 2025; Accepted: Feb. 20, 2025; Published: Mar. 28, 2025

Spirulina Against the Risks of Exposure to Toxic Metals
Koumolou Luc Babatounde1, Elegbede Manou Bernadin2, Dah-Nouvlessounon Durand3, Sina Haziz3, Edorh Patrick1, Baba-Moussa Lamine3
1Laboratory of Research in Biochemistry and Environmental Toxicology, Department of Biochemistry and Cell Biology, University of Abomey-Calavi, Benin
2Department of Water and Sanitation, National Water Institute, University of Abomey-Calavi, Benin
3Laboratory of Biology and Molecular Typing in Microbiology, Department of Biochemistry and Cell Biology, University of Abomey-Calavi, Benin
Correspondence to: Koumolou Luc Babatounde, Laboratory of Research in Biochemistry and Environmental Toxicology, Department of Biochemistry and Cell Biology, University of Abomey-Calavi, Benin.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This study evaluated the potential of protection of a green alga used in herbal medicine, spirulina, against the toxic effects induced by the mixture of heavy metals, lead, cadmium and arsenic in Sprague Dawley. The experiment was performed during 4 weeks with 5 groups of 6 rats per group (3 males and 3 females) fed normally. Except the control group, the other groups were fed orally, simultaneously and repeatedly by a mixture of the three toxic metals with or without Spirulina: two groups of rats received different doses of toxic metals and the last two groups received, apart metals, some different concentrations of Spirulina. Haematological and biochemical parameters were analysed in parallel with the removal of metals and calcium in feces and urine of exposed rats. The results showed that exposure to heavy metals induce a decrease in number for white blood cells and red blood cells and an increase of transaminases and total cholesterol. Correcting these dysfunctions with the rats that received spirulina and metals confirms the protective role of the algae against anemia, immune deficiency and liver poisoning. These protective effects were parallel to the retention of calcium in against the elimination of lead and cadmium in the feces and urine of these rats.
Keywords: Spirulina, Heavy metals, Toxic effects, Protective effect
Cite this paper: Koumolou Luc Babatounde, Elegbede Manou Bernadin, Dah-Nouvlessounon Durand, Sina Haziz, Edorh Patrick, Baba-Moussa Lamine, Spirulina Against the Risks of Exposure to Toxic Metals, American Journal of Biochemistry, Vol. 15 No. 1, 2025, pp. 1-7. doi: 10.5923/j.ajb.20251501.01.
Article Outline
1. Introduction
- The constraints associated with social change are increasingly increasing the risk of exposure to environmental pollutants, including heavy metals, which are highly hazardous to health. Studies have highlighted human exposure to mixtures of toxic substances such as lead (Pb), cadmium (Cd) and arsenic (As) through the consumption of contaminated market garden produce in Cotonou [1]. Other studies have characterized the risk associated with this exposure [2] and assessed the danger of a few doses of the mixture of these heavy metals on health through an experimental study on rats [3]. Very few studies have been carried out on the toxicity of mixtures of heavy metals on species. According to [4], it appears that, in mixtures, simultaneous exposure to lead, cadmium and arsenic leads to an increase in urinary excretion of porphyrins in the kidney, and this has been suggested as a good biological marker of combined or mixed exposure to these metals. According to [5], exposure to these metal mixtures has also revealed clastogenic and aneugenic effects in peripheral lymphocytes. Lead is a toxic element [6]. Several studies have shown that lead can cause neurological, haematological, gastrointestinal, reproductive, immunological and apoptotic disorders according to [7] and [8]. Moreover, lead acetate and phosphate are classified as carcinogens [9]. In the United States, the International Agency for Research on Cancer has also classified this element in the list of 2B inorganic compounds (the agent is possibly carcinogenic to humans) and chemicals [10]. Recent studies have shown that lead inhibits the activity of enzymes involved in oxidative stress according to [11] and [12] and increases the production of free radicals after treatment with inorganic lead in [13] and [14]. On the other hand, cadmium is toxic to humans and excessive exposure could cause death [15]. It enters cells and accumulates in high concentrations in the cytoplasmic and nuclear space in [16] and [17]. It has a strong affinity for the liver and kidneys [18]. It is carcinogenic according to [19], [20] and [21]. Also, genotoxic and apoptotic effects have been observed in several cell types [22]; [23] and [24]. The results of studies into the hepatic effects of prolonged oral exposure to cadmium are contradictory. While the metabolic changes (increased serum concentrations of hepatic enzymes) appear to be indisputable, histological changes in the liver have only been observed in a single study [25]. Finally, acute ingestion of inorganic arsenic causes irritation of the stomach and intestine, resulting in nausea, profuse, bloody, watery diarrhoea and vomiting. The blood pressure of these patients may be low, their heart rate may be accelerated and there may be a drop in blood counts [26]. The effects of chronic exposure to inorganic arsenic in humans are marked by skin lesions, cancer, foetotoxicity, neurotoxicity, cardiovascular disease, abnormal glucose metabolism and diabetes [27]. Furthermore, the multiplicity of sources of toxic metal contamination makes it difficult to control human exposure and means that we are increasingly having to reconcile living with the presence of these contaminants in our daily lives. It is in this context that Spirulina, a green alga rich in antioxidants [28] and used in phytotherapy, is being evaluated in rats subchronically exposed to a mixture of lead, cadmium and arsenic. It is because of all the above that the objective of this study is to evaluate the potential of protection of a green alga used in herbal medicine, spirulina, against the toxic effects induced by the mixture of heavy metals, lead, cadmium and arsenic in Sprague Dawley in order to prevent health risks associated with consumer exposure to food contaminated by heavy metals. Heavy metals such as lead and cadmium were chosen because they are the most toxic to humans according to [29] and [30], and the most frequently found in the environment. Arsenic is associated with them for two reasons: firstly, chemically, it is not a metal; secondly, it is only toxic above a certain threshold and only in its inorganic form [31]. On the other hand, spirulina has been chosen for its reputation as a protector of the body according to [32], [33] and [14].
2. Materials and Methods
- The aim of this study was to assess the protective potential of spirulina against the toxic effects induced by a mixture of lead, cadmium and arsenic in Sprague Dawley rats. The spirulina used was Spirulina platensis, which is sold over the counter in pharmacies (Photo 1). The three metals lead (Pb), cadmium (Cd) and arsenic (As) were supplied by the GTVD laboratory at the University of Lomé.
 | Photo 1. Spirulina platensis |
2.1. Animal Model and Stabling
- The animal model chosen was the Rattus norvegicus rat, of the Sprague Dawley strain, from the University of Ghana. The females were nulliparous and non- pregnant. The rats were acclimatised for a fortnight to the housing conditions and placed in cages measuring 43 cm x 21.5 cm x 30 cm. The animal house was maintained at a constant ambient temperature of 24°C, a hygrometry of 60% and a 12h-12h day-night cycle, with daylight provided by electric light. Each animal has ad libitum access to food and drinking water.
2.2. Distribution and Processing of Batches
- Rats aged 10 to 12 weeks were divided into 5 batches of 6 rats each (3 males and 3 females per batch). Male rats weighed between 196 and 230 grams and female rats between 188 and 216 grams. This heterogeneity in the weight of the animals suggests an equivalent heterogeneity in their age. The metal mixtures, Pb(NO3)2 (for lead), CdCl2 (for cadmium) and As2O5 (for arsenic), supplied by MERCK in Germany, were administered daily to each rat at a rate of 10 mL/kg of weight, using a gavage probe, for 28 consecutive days. The treated batches were as follows:Batch 1 control receives distilled waterBatch 2 receives 60 mg/kg of the equimolar mixture of heavy metals and 250 mg/kg PC of Spirulina®Batch 3 receives a mixture consisting of 4 mg/kg Pb + 1 mg/kg Cd + 25 mg/kg As and 160 mg/kg PC of Spirulina®Batch 4 receives 4 mg/kg Pb + 1 mg/kg Cd + 25 mg/kg AsBatch 5 receives an equimolar mixture of 60 mg/kg of heavy metals.This mixture, which contains 4 mg/kg of lead, 1 mg/kg of cadmium and 25 mg/kg of inorganic arsenic, was chosen on the basis of the average levels of these metals found in vegetables [2] (Koumolou et al., 2013). On the other hand, the equimolar mixture dose of 60 mg/kg of each metal is 1/5th of the dose of 300 mg/kg of this mixture used during the acute toxicity phase and which caused clear hepatic toxicity acting on the biochemical parameters (LDH, ASAT and ALAT), in both males and females [3]. The doses of Spirulina correspond to 15 g of Spirulina, for an adult man weighing 60 kg, i.e. approximately 3 and 2 teaspoons, indicated by the daily dosage. In addition, information on the effects of Spirulina consumption in normal conditions is available in [34]; [35] and [36], which is why another batch of control rats fed Spirulina alone was not considered.
2.3. Obtaining Biological Material and Analyses
- After treatment, the rats were observed individually and daily for 28 days. At the end of the treatment, blood samples were taken from the orbital sinuses of the anaesthetised rats using capillary tubes in anticoagulant tubes and dry tubes following the [37] protocol modified by [38]. The haemogram was performed using a RAYTO model RT-7200 haematology machine and a SCREEN MASTER Molecular Absorption Spectrophotometer was used to measure blood glucose, creatinine, total cholesterol and ASAT and ALAT transaminases. The rats were then sacrificed after asphyxiation with ether and cervical dislocation. The organs were removed, observed, weighed and preserved in formalin. Urine and faeces from 24 hours were collected once in the last week of treatment and assayed for lead, cadmium and calcium using an atomic absorption spectrophotometer based on the method of [39]. Statistical processing presented the results in the form of averages ± standard deviations in tables and compared them using SPSS software 17.0 (T-test) p (T>t) = 0.05.
3. Results
- Three rats died towards the end of the treatment: one male in the controls, one female in batch 2 and one male in batch 3.
3.1. Hematology
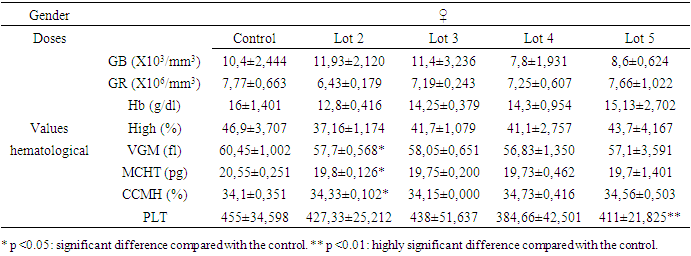
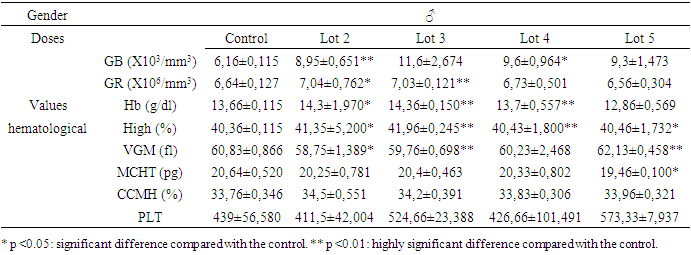
- Variations in hematological parameters are shown in tables 1 for females and 2 for males.
|
|
3.2. Clinical Biochemistry
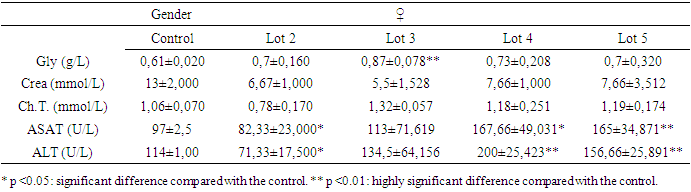
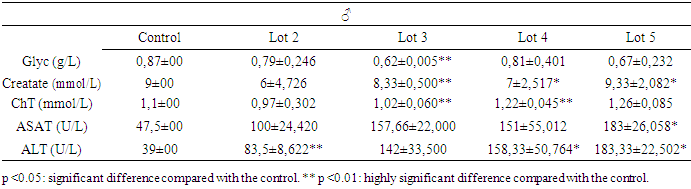
- The results of the biochemical assays are shown in tables 3 and 4.
|
|
3.2.1. Blood Glucose
- The significant drop in blood sugar levels in males and the significant increase in females at a time when transaminase levels did not vary significantly in both sexes belonging to the same batch 3 shows that variations in liver enzymes and blood sugar levels are not necessarily linked.
3.2.2. Creatinine Levels
- The reduction in creatinine in the blood is a good sign that Spirulina was taken by male rats. But a significant reduction was also observed in rats exposed only to metals (batch 4) without any obvious explanation. On the other hand, the significant increase in creatinine in batch 5 justifies the toxic nature of the mixture of heavy metals. Exposure to heavy metals can cause lead accumulation in proximal tubular cells. This may be followed by proximal tubular dysfunction, with reduced urate excretion and hyperuricemia. Uricemia is positively correlated with creatinine levels. So, its increase in the blood is a sign of heavy metal intoxication. Spirulina therefore plays a detoxifying role by lowering creatinine levels.
3.2.3. Total Cholesterol
- It was only in males, in batch 4, that a significant increase in total cholesterol levels was observed, and the only significant reduction was recorded in the corresponding group that had received Spirulina (batch 3). Spirulina could therefore prevent the accumulation of cholesterol in the body.
3.2.4. ASAT and ALAT
- The significant and simultaneous increase in both transaminases leads to the conclusion that repeated dose exposure to the mixture of heavy metals lead, cadmium and arsenic causes liver damage. But Spirulina at a dose of 250 mg/kg PC rats (about 16 grams or 3 teaspoons per day for a 60 kg man) both enzymes were significantly reduced (p<0.05). The liver remains a target organ for heavy metals, as it is responsible for detoxification by transforming toxic substances into secondary metabolites. But when the quantity of toxic metals exceeds the liver's metabolic capacity, it attacks and destroys liver cells: this is liver toxicity, resulting in increased levels of the enzymes ALAT and ASAT in the blood. But a reduction in both enzymes can be explained by a strengthening of the liver's detoxification capacity, certainly through an increase in the body's antioxidant activity. This leads to the conclusion that Spirulina plays a protective role on the liver.
3.3. Study of the Decontaminating Potential of Spirulina Eliminating Heavy Metals
- The results of lead, cadmium and calcium measurements in the faeces and urine of rats exposed to the mixture of metals with or without Spirulina are shown in Table 5.
|
4. Discussion
- This study assessed the protective potential of Spirulina against the toxic effects of mixtures of lead, cadmium and arsenic in Sprague Dawley rats exposed for 28 days to repeated doses. In terms of haematological parameters, the results showed that the relevant significant variations were concentrated in white blood cells and red blood cells. In females, there was a non-significant reduction in blood leucocyte levels when they were exposed to heavy metals alone. But, Spirulina corrected this reduction. In males, when Spirulina added, the number of white blood cells varied very significantly. It was concluded that Spirulina strengthens the immune system. This conclusion was also reached by [41], [42] and [43]. In males given Spirulina, blood erythrocyte concentration increased significantly. In females, however, there was no significant difference. It was concluded that Spirulina corrected the variation in red blood cell levels in intoxicated rats. to heavy metals. However, studies have shown that Spirulina combats anaemia and the effects of malnutrition in [36]. As far as transaminases are concerned, ASAT and ALAT changed very significantly (p <0.01) in the same direction during exposure to heavy metals in the groups that received only toxic substances. However, Spirulina at a dose of 250 mg/kg PC significantly reduced (p <0.05) the levels of both enzymes. It was concluded that simultaneous intake of Spirulina has a protective potential for the liver. These observations were confirmed by the work of [44]. There was a significant reduction in creatinine in the blood of males fed Spirulina. At a dose of 250 mg/kg PC, Spirulina reduced the concentration of total cholesterol in both females and males. The dose of 160 mg/kg PC did not have a very significant effect, but it also reduced this level in males in which the concentration of total cholesterol had risen with exposure to metals. The conclusion is that high- dose Spirulina prevents the accumulation of cholesterol in the body. This observation was also made by [35]. These authors proved that Spirulina's omega-3 and omega-6 fatty acids are responsible for this role. A study of the elimination of lead and cadmium showed that Spirulina played a detoxifying role in the rats' bodies by evacuating these pollutants through the faeces and urine. When lead is absorbed, 80% of it is eliminated via the urine, 16% via the bile, 8% via gastrointestinal secretions, sweat and dander, and the rest via the faeces according to [45]. Cadmium eliminated mainly via the faeces. Elimination via the urine is very slow and insignificant, as the metallothionein must be saturated before urinary elimination is observed according to [46] and [47]. Thus, the effectiveness of Spirulina would be found in the levels of lead and cadmium directly excreted in the faeces, which has been verified by the results. One of the trace elements that slows down the absorption of lead and cadmium is calcium, which competes with it in the intestine [48]. The unabsorbed portion of calcium is found mainly in the faeces and digestive juices in [49], but urinary calcium is the calcium that was first absorbed before being excreted [49]. The lower this urinary rate (batch 2 and batch 3), the more calcium is retained in the body and the greater the competition between calcium and heavy metals, which will be reflected in the abundant elimination of these metals in the faeces and urine. Our results also confirm this analysis, as it was in the batches where the rats were given Spirulina that the concentration of urinary calcium was lowest. Did Spirulina enable the body to save dietary calcium so that could oppose absorption of lead and cadmium, or did the calcium content of Spirulina have this protective effect? The calcium contained in the rats' croquettes could not have played this role without the addition of Spirulina. Otherwise, the same results would have been obtained with the positive controls (rats exposed only to heavy metals and not Spirulina, because they ate the same food), which would have meant that the rats would have been less likely to be exposed to heavy metals. is not the case. So calcium retention is attributed to Spirulina. So what is the mechanism that allows Spirulina to retain calcium to counter the toxic effects of heavy metals in rats? Further studies are therefore needed. But as things stand, we can conclude that Spirulina enables the body to retain calcium to combat metals effectively. These results confirm the work of [50], [51] and [52]. Because of all its benefits, the FAO has identified spirulina as the food of the future in [28].
5. Conclusions
- The results obtained in this evaluation of the protective potential of Spirulina against the effects of heavy metals seem to prove firstly that the component of the mixture of heavy metals as well the dose of each component in the mixture influence toxicity. The sex of the individual also toxicity. The toxicity of the heavy metals lead, cadmium and arsenic was verified and established in this study with regard to the toxic effects observed. Fortunately, Spirulina could prevent anaemia, has a hepatoprotective role and enables the body to retain calcium to fight effectively against lead and cadmium, which it eliminates in the faeces and urine. The work of several authors confirms these results.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML