-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2024; 14(2): 9-16
doi:10.5923/j.ajb.20241402.01
Received: Sep. 23, 2024; Accepted: Oct. 16, 2024; Published: Oct. 31, 2024

Assessment of Lipid Profile in Homozygous Sickle Cell Subjects (SS) According to Physical Activity
Thioune Ndèye Marème1, Kandji Pape Matar1, 2, Djite Moustapha1, 2, Barry Nene Oumou Kesso1, 2, Ndiougue Faye3, Ndour El Hadji Malick2, Gueye-Tall Fatou2, Mor Diaw4, Macoura Gadji3, Lopez-Sall Philomene2, Cisse Aynina2, Diop Pape Amadou2, Gueye Papa Madieye1, 2
1Laboratory of Biochemistry-Hematology, National University Hospital of Fann, Dakar, Senegal
2Laboratory of Pharmaceutical Biochemistry, Faculty of Medicine, Pharmacy, Cheikh Anta Diop University, Dakar, Senegal
3Laboratory of Hematology, Faculty of Medicine, Pharmacy of the Cheikh Anta DIOP University of Dakar
4Laboratory of Physiology and Functional Explorations of the Cheikh Anta DIOP University of Dakar
Correspondence to: Thioune Ndèye Marème, Laboratory of Biochemistry-Hematology, National University Hospital of Fann, Dakar, Senegal.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

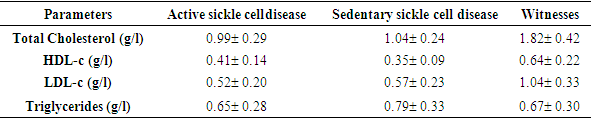
Homozygous sickle cell disease (SS) is characterized by a disturbance in lipid balance parameters and a risk of cardiovascular disease. Physical activity, which has long been considered a high-risk situation for sickle-cell patients, could be a means of preventing CVD. The overall aim of this study was to assess the impact of regular physical activity on lipid parameters in homozygous sickle cell subjects (SS). This was a case-control study; carried out at the biochemistry laboratory of CHNU/FANN in the period from 01 March 2021 to 30 June 2022. All SS sickle cell patients followed at the CNTS were included. Each subject was associated with a control of the same sex and age ±2 years. The physical activity or inactivity levels of our subjects were assessed with a questionnaire (GPAQWHO) and an accelerometer watch. Lipid parameters were determined using the ARCHITECT® ci4000 automaton, and the three ratios CT/HDL-c, LDL/HDL-c and log TG/HDL-c were calculated. The study included 55 homozygous sickle cell (SS) patients. Mean age was 29 years. Evaluation of mean lipid parameters in active and sedentary sickle cell patients revealed mean values of 0.99(g/l), 1.04; (g/l) for total cholesterol; 0.41(g/l), 0.35(g/l) for HDL-c; 0.52(g/l), 0.57(g/l) for LDL-c and 0.65(g/l), 0.79 (g/l) for triglycerides, respectively. Comparison of mean lipid parameters between these two groups showed no statistically significant difference. Comparison of the three indices showed a statistically significant difference (p = 0.025) for Log(TG/HDL-c). Atherogenic risk was higher in sedentary sickle-cell patients, with a frequency of 66%. These results suggest that regular physical activity can help prevent cardiovascular disease in SS homozygous sickle cell patients.
Keywords: Physical activity, Sickle cell disease, Lipids, Atherogenic risk
Cite this paper: Thioune Ndèye Marème, Kandji Pape Matar, Djite Moustapha, Barry Nene Oumou Kesso, Ndiougue Faye, Ndour El Hadji Malick, Gueye-Tall Fatou, Mor Diaw, Macoura Gadji, Lopez-Sall Philomene, Cisse Aynina, Diop Pape Amadou, Gueye Papa Madieye, Assessment of Lipid Profile in Homozygous Sickle Cell Subjects (SS) According to Physical Activity, American Journal of Biochemistry, Vol. 14 No. 2, 2024, pp. 9-16. doi: 10.5923/j.ajb.20241402.01.
Article Outline
1. Introduction
- Sickle cell disease is an autosomal recessive inherited disorder [1]. It is the most widespread hemoglobinopathy in the world, affecting over 270 million people, most of them in sub-Saharan Africa [2]. In Senegal, its prevalence varies from 8 to 10% [3] including 1% for homozygous forms [4]. Sickle cell disease in its homozygous form (SS) is characterized by a number of complications, including chronic hemolytic anemia, abdominal pain, vaso-occlusive crises (CVO), acute chest syndrome (ACS) and disturbances of lipid parameters, which can be disabling or even fatal [5]. Management of patients with sickle cell disease has improved steadily over the last few decades. This improvement is accompanied by a better quality of life for the patient and reduced morbidity [6]. However, exercise is considered a high-risk situation for the sickle cell patient, and clinical practice recommends reduced physical activity for these subjects. The mechanisms identified as deleterious for sickle-cell patients and which may be associated with physical exercise are those linked to a reduction in PaO2 [7]. However, in the non-sickle-cell population, regular physical activity (not necessarily intense) helps maintain efficient vascular function [8,9]. This could therefore be a means of preventing some of the cardiovascular complications observed in sickle-cell patients. Studies have already suggested assessing the level of physical activity in sickle cell patients [7,10]. However, none of these studies have examined the clinical/biological manifestations of sickle cell disease in relation to the intensity of physical activity. Thus, the general objective of this work was to evaluate the impact of physical activity on lipid balance parameters in homozygous sickle cell subjects (SS).
2. Materials and Methods
- This was a prospective, descriptive and analytical case-control study, lasting sixteen months from 01 March 2021 to 30 June 2022. Subjects were recruited at the Centre National de Transfusion Sanguine (CNTS) and biological tests were performed at the Biochemistry Laboratory of the Centre Hospitalier National Universitaire de Fann.The study involved adult male SS sickle cell patients followed at the CNTS, residing in Senegal and having given their agreement to the study through free and informed consent. The study did not include subjects who had received a transfusion in the previous three months or subjects who had suffered a vaso-occlusive crisis (VOC) or acute chest syndrome (ACS) in the previous three months. For each case, a control (same sex and age ± 2) was chosen from among the blood donors.Several types of variables were studied in our study population:- Clinical parameters: acute chest syndrome (ACS), myalgia, leg ulcer, priapism, abdominal pain and stroke. - Biological parameters: lipid balance parameters (total cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides) were assessed and plasma atherogenicity indices calculated using the following formulae: (TG/HDL-c; LDL-c/HDL-c et Log (TG/HDL-c).Blood samples were taken from these patients in the morning, after a 12-hour fast, by venipuncture at the elbow. The blood was collected in a dry tube and centrifuged at 3000 rpm for 5 minutes.Plasma concentrations of lipid balance parameters were determined using the ARCHITECT® ci4000 automated system (Abbott core laboratory, Illinois, USA). Our subjects' levels of physical activity or inactivity were assessed in two different ways:- Global Physical Activity Questionnaire (GPAQ, WHO). This questionnaire consists of 16 questions enabling us to assess our subjects' lifestyles [(sedentary or inactive) or active] in different areas of physical activity (occupation, travel, leisure).- To be much more precise, we used ‘accelerometer’ watches. These are wristwatches that measure the number of steps taken per day, the distance covered, calorie expenditure and the number of active minutes. They also assess hour-by-hour activity and inactivity. Fitbit Alta HR accelerometers from China were used. Our data was recorded using Microsoft EXCEL 2016 software. XLSTAT 2020 was used to analyse the data. The Mann Whitney test was used to compare mean values. The Chi-square test was used to compare frequencies and a p-value of less than 0.05 was considered statistically significant.
3. Results
- Our study population consisted of 55 sickle cell subjects (SS profile) and 55 control subjects. The mean age of our subjects was 29 years, with a standard deviation of ±8years and extremes of 18 and 49 years (Table 1).
|
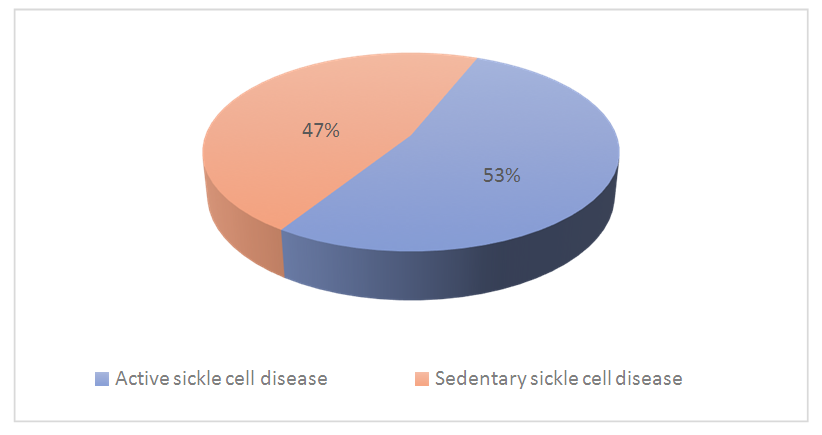 | Figure 1. Distribution of subjects with sickle cell disease according to physical activity |
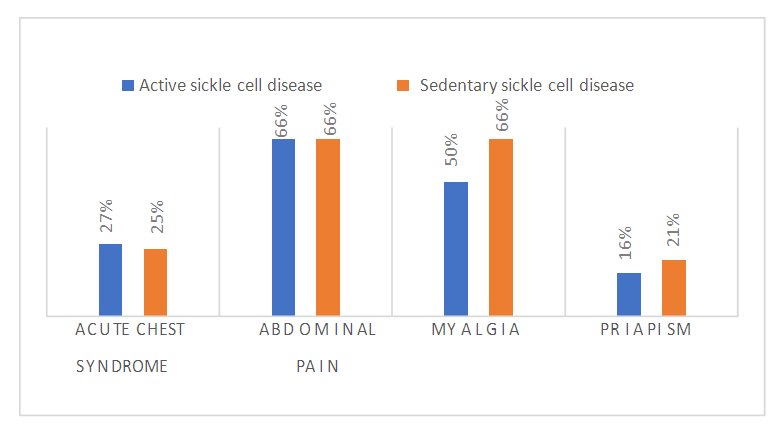 | Figure 2. Assessment of clinical characteristics according to physical activity |
|
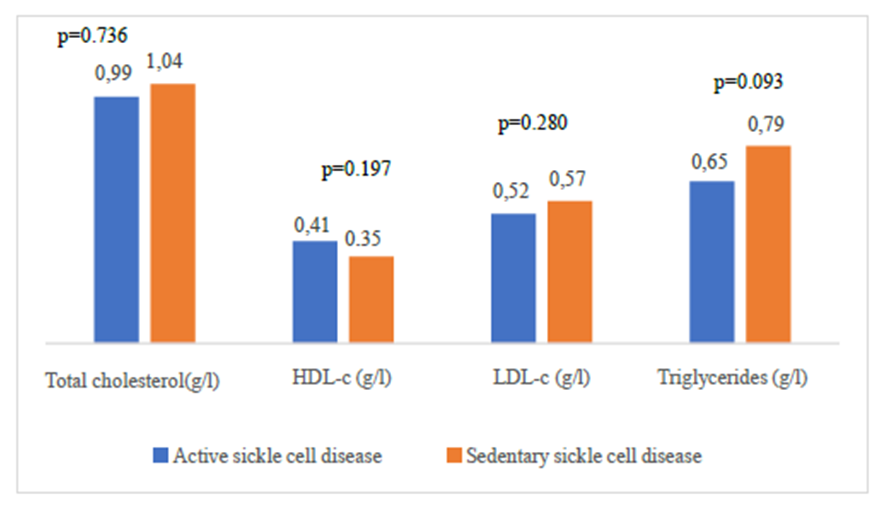 | Figure 3. Comparison of lipid balance parameters according to physical activity |
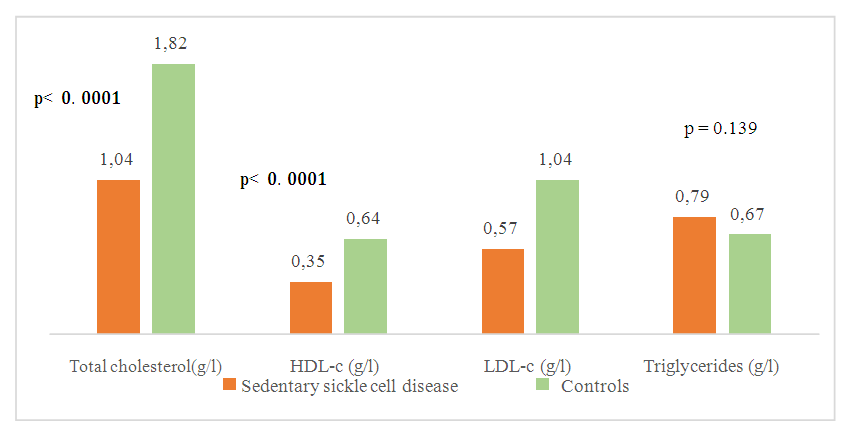 | Figure 4. Comparison of mean concentrations of lipid balance parameters between sedentary sickle-cell subjects and controls |
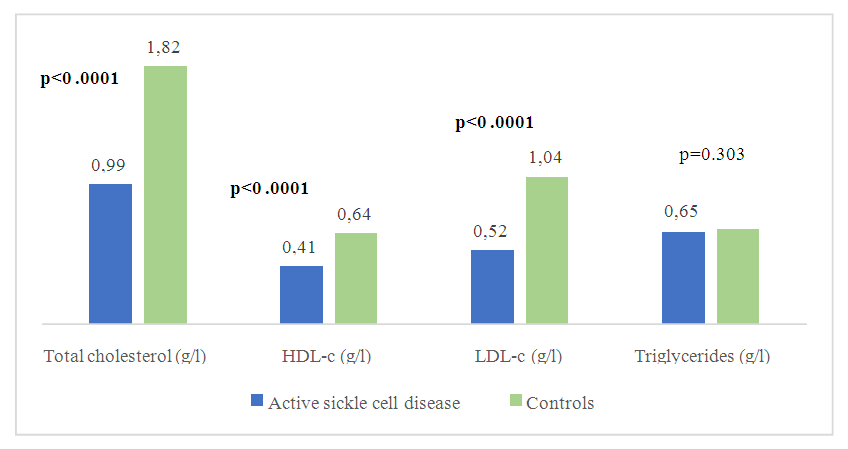 | Figure 5. Comparison of mean concentrations of lipid balance parameters between active sickle cell subjects and controls |
|
|
|
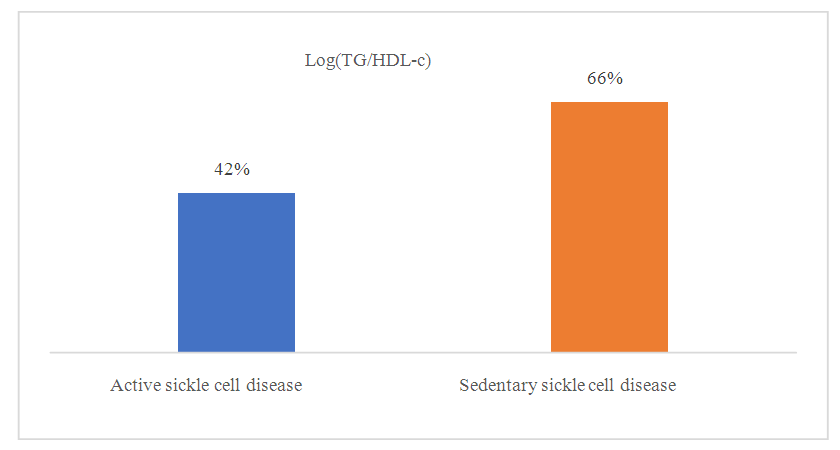 | Figure 6. Frequency of sickle cell disease patients with elevated plasma atherogenicity index (PAI) as a function of physical activity |
4. Discussion
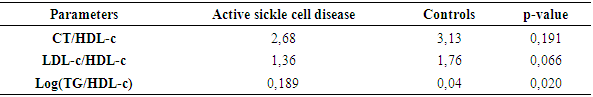
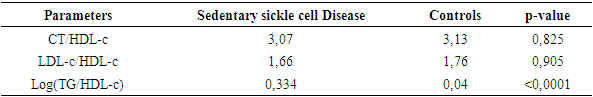
- Sickle cell disease is an autosomal recessive inherited disorder linked to a genetic mutation that results in the production of a pathological hemoglobin called hemoglobin S (HbS) [11]. Homozygous sickle cell disease SS is characterized by painful vaso-occlusive crises, an inflammatory reaction, oxidative stress and a high atherogenic risk [12].The care of sickle cell patients has steadily improved over the last few decades. This improvement is accompanied by a better quality of life for the sickle cell patient. However, exercise is considered a high-risk situation in sickle cell patients, and clinical practice recommends reduced physical activity for these patients. Yet regular physical activity is a means of preventing cardiovascular disease.Thus, the general objective of this work was to evaluate the impact of physical activity on lipid balance parameters in homozygous sickle cell subjects (SS).Analysis of the study population revealed an average age of 29, with extremes of 18 and 49. Our results are similar to those of Diop et al. in a study carried out in Senegal, who found a mean age of 27 years, with extremes of 20 and 51 years [13]. In addition, a recent study of homozygous male sickle cell patients in Senegal by Gaye et al. also found a mean age of 27.1 with a standard deviation of ± 7.1 years [14]. These results could be explained by the major efforts made in Senegal to provide early care for patients from childhood onwards [15] but also by the increased life expectancy of homozygous sickle-cell patients worldwide. Authors have also reported that in the USA, 50% of patients reach the age of 50 [16] and life expectancy for patients in Jamaica has been estimated at 53 years for men [17]. This improvement in life expectancy could be explained by several factors, including earlier diagnosis, the implementation of prophylactic measures, and the increase in the number of specific centers for the care of sickle cell patients.Clinically, abdominal pain (AP) was the most common complication, with a frequency of 66% in both active and inactive sickle-cell patients. For priapism, we found a frequency (21%) in sedentary sickle cell patients versus (16%) in active sickle cell patients, Gaye et al. had found frequencies (43%) higher than ours [14]. Other studies have shown that the prevalence of priapism in adult sickle-cell patients varies from 9.3% to 42% [18,19].The higher frequency of priapism in sedentary sickle-cell patients could be explained by a sedentary lifestyle. In most cases, priapism is due to a blood circulation disorder, and physical activity helps improve vascular function. What's more, physical activity (e.g. rapid ascent and descent of stairs, cycling, etc.) is even recommended in cases of priapism, to relieve the pain [20].Evaluation of the lipid profile in the sickle-cell subjects in the study population revealed a reduction in total cholesterol levels, as well as in HDL-c and LDL-c fractions. Comparison of mean lipid parameters between sickle cell patients and controls showed statistically significant differences (p<0.05). This hypocholesterolemia is corroborated by several studies, including that of Guarda et al. who found mean concentrations of 1.18g/l for total cholesterol, 0.40 g/l HDL-c and 0.58 LDL-c. These values are similar to ours [21]. The same is true of Gueye et al. in Dakar [22] and Mondé et al. in Côte d'Ivoire [23] who found results comparable to ours.In fact, this hypocholesterolemia could be explained by an increase in plasma volume due to a reduction in packed red blood cells. The increase in plasma volume is greater and more frequent in sickle-cell subjects than in other anemic states. Similarly, reduced endogenous cholesterol synthesis may be at the root of this hypocholesterolemia [24,25] as well as the possibility of membrane lipoperoxidation of cholesterol, with secondary mobilization of plasma cholesterol to the erythrocyte membrane, resulting in a decrease in plasma cholesterol [26,27]. On the other hand, the decrease in the LDL-c fraction could be due to inflammation associated with oxidative stress and increased lipoperoxidation. The LDL-c level would not reflect the real situation in homozygous sickle-cell patients due to oxidative stress, which would transform part of the LDL into oxidized LDL.Evaluation of triglyceridemia had shown a higher mean concentration (0.79g/l) in sedentary sickle cell patients compared with active sickle cell patients (0.65 g/l) and controls (0.67 g/l). Similar results were reported in the study by Monnet et al. [28]. This rise in triglycerides could be explained by the increased production of endogenous lipids (VLDL) and the decrease in lipoprotein lipase activity caused by oxidative stress [29].A comparative study of mean concentrations of lipid balance parameters between active and sedentary sickle cell patients showed no statistically significant difference for any of the parameters studied. On the other hand, we obtained a higher mean HDL-c concentration in active sickle-cell patients, associated with a lower mean LDL-c and triglyceride concentration. This could be explained by physical activity, which, according to several studies, helps combat cardiovascular risk factors (CRFs) by lowering LDL-cholesterol (by 5%) and triglycerides (by 3.7%), and increasing HDL-cholesterol (vasculo-protective, by 4.6%) [30,31].To assess atherogenic risk in our study population, atherogenicity indices were determined using different formulas: the TC/HDL-c ratio, the LDL-c/HDL-c ratio and the logarithm of the TG/HDL-c ratio, also known as the plasma atherogenicity index (PAI). Comparison of mean index values between active, sedentary sickle-cell patients and controls showed a significant difference with a p<0.05 only for the logarithm of the TG/HDL-c ratio. Our results are similar to those reported by Mondé et al. who also found no significant difference between stationary sickle cell patients and controls for these two indices (the TC/HDL-c ratio and the LDL-c/HDL- c ratio) [23].Comparison of the three indices used in active and sedentary sickle-cell subjects showed a statistically significant difference (p = 0.025) only for Log (TG/HDL-c), with a value of 0.189 in active sickle-cell subjects versus 0.334 in inactive sickle-cell subjects. Furthermore, atherogenic risk assessment in our study showed that 66% of sedentary sickle cell patients had a log ratio (TG/HDL-c) greater than 0.21, compared with 42% of active sickle cell patients. A study by Mudhaffar et al. found a higher frequency ((84%); IAP>0.21) than ours [32]. Other studies have been carried out along these lines, notably that of Gasiano et al. who reported that DPI is a strong predictor of cardiovascular disease and can be successfully used to assess atherogenic risk [33].In our study population, the atherogenic risk was higher in sedentary sickle cell patients, which could be explained by the lack of physical activity. Studies have reported that regular physical activity, such as slow or moderate running or, failing that, walking for a relatively long time (around 45 to 60 min), repeated three times a week, can considerably modify atherogenic blood lipid levels and reasonably increase anti-atherogenic blood lipid levels [34,35]. Regular aerobic exercise therefore theoretically has the potential to induce significant weight loss and, consequently, to favourably modify atherogenic lipid levels [36].
5. Conclusions
- Homozygous sickle cell disease SS is characterized by painful vaso-occlusive crises, an inflammatory reaction, oxidative stress, disturbed lipid balance parameters and a risk of developing cardiovascular disease.Improved management of sickle cell patients is accompanied by a better quality of life. Regular physical activity is seen as a means of preventing cardiovascular disease.Thus, the general objective of this work was to evaluate the impact of physical activity on lipid balance parameters in homozygous sickle cell subjects (SS).At the end of this study, we found a higher atherogenic risk in sedentary sickle cell patients, which could be linked to a sedentary lifestyle. These results suggest that physical activity could be a means of preventing cardiovascular disease in sickle-cell patients.A longitudinal study of sedentary sickle cell patients is currently underway, which will enable us to assess the impact of a gradual increase in the intensity of daily physical activity on clinical and biological parameters.
ACKNOWLEDGEMENTS
- We would like to thank all those who took part in this study.
CONFLICT OF INTERESTS
- The authors have not declared any conflict of interest.
CONSENT
- Written and informed consent was obtained from each participant.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML