-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2021; 11(1): 1-10
doi:10.5923/j.ajb.20211101.01
Received: Apr. 3, 2021; Accepted: Apr. 20, 2021; Published: Jun. 15, 2021

Effect of Methanol Stem Bark Extract of Detarium microcarpum on Diethylnitrosamine-Induced Hepatocellular Carcinoma in Rats
Christopher Kwansai, Umaru Hauwa Aduwamai, Gabriel Ijuptil Banga, Dahiru Daniel
Department of Biochemistry, School of Life Sciences, Modibbo Adama University of Technology Yola, Adamawa State, Nigeria
Correspondence to: Christopher Kwansai, Department of Biochemistry, School of Life Sciences, Modibbo Adama University of Technology Yola, Adamawa State, Nigeria.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The study is aimed at divulging the hepatoprotective nature of stem bark extract of Detarium microcarpum against Diethylnitrosamine (DEN)-induced hepatocellular carcinoma (HCC) in male wistar rats. Fifty-four male wistar rats g 140 ± 20 g were divided into nine groups of six rats per group. Group I, II and III served as normal, negative and standard drug control respectively while group IV to IX were treated with different doses of the extract and fractions. All treatments were carried out by oral gavages for fourteen consecutive days. Assays of liver biochemical parameters, lipid profile parameters, antioxidant enzymes/total antioxidant status, haematological profile and histopathological examination were carried out. Results obtained showed that, DEN administration induced hepatocellular damage in the rats, characterised by significant (p<0.05) elevation of aspartate transaminase, alanine transaminase, alkaline phosphatase, albumin, total cholesterol, triglycerides, and low density lipoprotein-cholesterol; decreased total bilirubin, total protein, high density lipoprotein-cholesterol; decreased antioxidant and non-antioxidant enzymes activities and haematological parameters when compared with the normal control group. However, oral post-treatment with stem bark extract, fraction-I and fraction-II of Detarium microcarpum in a dose-dependent manner compared to the negative control group restored all the parameters with significant (p<0.05) restoration observed in fraction-II. Detarium microcarpum stem bark may be effective for ameliorating DEN-induced hepatocellular damage in male Wistar rats and therefore, may be further investigated for the molecular mode of action.
Keywords: Cancer, Detarium microcarpum, Diethylnitrosamine, Hepatocellular Carcinoma, Albino Rats
Cite this paper: Christopher Kwansai, Umaru Hauwa Aduwamai, Gabriel Ijuptil Banga, Dahiru Daniel, Effect of Methanol Stem Bark Extract of Detarium microcarpum on Diethylnitrosamine-Induced Hepatocellular Carcinoma in Rats, American Journal of Biochemistry, Vol. 11 No. 1, 2021, pp. 1-10. doi: 10.5923/j.ajb.20211101.01.
Article Outline
1. Introduction
- Cancer is a group of diseases in which genetically damaged cells proliferate autonomously. Such cells cannot respond to normal regulatory mechanisms that ensure the intercellular cooperation required in multicellular organisms. Consequently, they continue to proliferate, thereby robbing nearby normal cells of nutrients and eventually crowding surrounding healthy tissue (Ferlay et al., 2013). There is a steady increase in incidence of cancers as observed in most developed and developing countries. Apart from incidence, cancer related deaths are also increasing. In 2008 alone, up to 7.6 million people died from cancers all over the world with about 70% of these deaths occurring in developing countries (Adewale, 2013). In the whole world, there were 14 million new cases and 8 million deaths in 2012, and in 2018, there were 18.1 million new cases and 9.6 million cancer-related deaths (Freddie et al., 2018). This value is projected to rise by at least 70% by the year 2030 (Ferlay et al., 2012). It is estimated that more than 250,000 new cases of cancers are diagnosed in Nigeria every year and up to 10,000 Nigerians die each year from cancer related causes (Baba and Hincal, 2016). These estimates are based largely on hospital generated data without provision for the many cases that do not present themselves in hospitals as well as the many cases of misdiagnosis in our numerous peripheral hospitals (Adewale, 2013).N–Diethylnitrosamine (DEN) is one of the most important environmental carcinogens in N-Nitrosamine class, which primarily induce liver cancer by oxidative damage in the DNA of hepatocyte cell (Gayathri et al., 2009). Hepatocellular carcinoma (HCC) is the most common primary liver cancer and is an environmentally related cancer, with hepatitis viruses (HBV or HCV), anabolic steroids, alcohol, aflatoxin, cirrhosis (of any etilogy), hemochromatosis and chemical carcinogens involved in a multistage process (Abarker et al., 2015). The incidence of HCC increases in cirrhotic patients who have undergone curative treatment for primary HCC (Strivatanakul et al., 2004; Yao et al., 2007). Medicinal plants play a key role in the human health care. Ameesh and Murugan (2016) reported that, about 80% of the world population rely on traditional medicine, predominantly plants. These practices incorporated ancient beliefs and were passed on from one generation to another by oral tradition and guarded literature. Detarium microcarpum Guill and Perr is an African tree belonging to the Fabaceae family (legumes) (Abdalbasit et al. 2009) and is widely distributed in dry savannah areas of Africa. Different parts of the plant have been reported to possess a lot of medicinal activities (Abreu et al., 1998). For instances, the stem bark extract have been reported to possess antimicrobial, antitumor, antileishmania and cytotoxic properties (Abreu et al., 1998; Abreu et al., 1999); anti-viral and antimalaria properties (Olugbuyiro et al., 2009). Detarium microcarpum was found to have the highest total phenolic, flavonoid and antioxidant values among fourteen wild edible fruits from Burkina Faso (Abdalbasit et al. 2009).In view of this, we have evaluated the hepatoprotective effect of stem bark extract of D. microcarpum in N-Diethylnitrosamine (DEN)-induced hepatocellular carcinoma in male Albino Rats. The discovery and development of chemotherapeutics that are effective for the treatment and control of liver cancer is urgently needed for present moment.
2. Materials and Methods
2.1. Plant Materials
- The plant material of D. microcarpum was collected from Girei local Government area of Adamawa State, Nigeria. The sample was identified and authenticated at the Department of Plant Sciences, Modibbo Adama University of Technology Yola, Nigeria.
2.2. Preparation of Plant Extract
- The collected plant materials were washed sliced and completely shade dry. The dried material was ground make to a fine powder and used for extraction. The powdered plant material D. microcarpum (200 g) was extracted with methanol (1 litre) in an air tight clean flat bottomed container for 48 hours at room temperature with occasional stirring and shaking (Trease and Evans, 2002). The methanol extract was filtered first through a fresh cotton plug and then through a whatman filters. The filtrate was evaporated to dryness in vacuo by a rotary evaporator at 40-50°C and the extract was kept in a well tight sterile bottle/container under refrigerated conditions until use. The fractionation (Solvent-solvent partitioning) was used as designed and described by Kupchan and Tsou (1973), as modified by Wagenen et al., (1993).
2.3. Animals
- Fifty-four (54) male albino rats (weighing between 140 ± 20 g) were obtained from National Veterinary Research Institute Vom, Jos, Plateau state, Nigeria. They were housed in polypropylene cages, and were given standard grower diet (Vital Feeds, Jos) and water ad labium for 7 days to enable them acclimatize before the commencement of the experiment. Throughout the experiment it was maintained under the laboratory conditions of 29 ± 2°C (temperature) and 12 hours light and dark cycle in the Department of Biochemistry, Modibbo Adama University of Technology, Yola, Nigeria. Guide for the Care and Use of Laboratory Animals was strictly followed.
2.4. Experimental Design
- The rats were randomly divided into nine equal groups of six rats each. Group I served as normal control, that is, no inducement and no administration of methanol extract/fractions as shown in Table 1 below. Liver carcinogenesis was induced in group II, III, IV V, VI, VII, VIII and IX by injecting diethylnitrosamine (in DMSO) intraperitonially at a dose of 50 mg/kg body weight once in a week for a period of three weeks as reported by Sumithra et al., (2013). Group II served as the negative control while group III served as the positive control group (silymarin 100 mg/kg b.w. was used as standard drug). The stem bark methanol extract of the Detarium microcarpum was administered to group IV (200 mg/kg b.w.) and group V (400 mg/kg b,w.) while group VI and VII were given fractions I (200 mg/kg bw and 400 mg/kg b.w. respectively). Group VIII and IX were given fraction II (200 mg/kg b.w. and 400 mg/kg b.w. respectively). The stem bark methanol extract, fraction I and fraction II of Detarium microcarpum were administered to the rats through oral gavages for a period of 14 days.
|
2.5. Collection of Samples
- On completion of the experimental period, animals were anaesthetized with diethyl either (2ml/kg). The blood was collected with and without EDTA as anticoagulant.
2.6. Estimation of Biochemical and Haematological Parameter
- The biochemical parameters determine are- aspartate transaminase, alanine transaminase, alkaline phosphatase (Reitman and Frankel, 1957); total bilirubin (Dangerfield and Finlayson, 1953); total protein (Lowery et al., 1959) and albumin (Doumas, 1971). As well as lipid profile parameters determine are- Serum Cholesterol, HDL-cholesterol (Trindar, 1969); triglycerides (Stein, 1987); LDL-cholesterol (Friedwald et al., 1972). Antioxidant enzymes- superoxide dismutase (Beauchamp and Fridovich, 1971), glutathione peroxidase (Mohandas et al., 1984) and glutathione reductase (Carlberg and Mannervik, 1975); total antioxidant status (Erel, 2004) and haematological profile (Sheth and Shah, 2015).
2.7. Histopathology Studies
- The liver were excised from the experimental animals of each group after collecting the blood sample and washed with normal saline. Initially, the materials were fixed in 10% neutral formalin solution. Sections were cut using microtome techniques after paraffin embedding. The sections were possessed in alcohol xylene series and were stained with hematoxylin and eosin. The different sections were examined microscopically for the evaluation of histopathological changes.
2.8. Statistical Methods
- The results of the experiment were represented as Mean value ± standard error of mean (SEM). Statistical analysis was done using Statistical Package for the Social Sciences (SPSS) version 24.0 (SPSS, Incorporation Chicago Illinois, USA). Differences between and within the group means were analyzed using One-way Analysis of Variance (ANOVA) followed by Duncan’s Multiple Range Test (MRT) for the post-hoc treatment. The results were considered statistically significant at p<0.05.
3. Results
3.1. Effect of Extract and Fractions on Liver Enzyme Markers of Rats
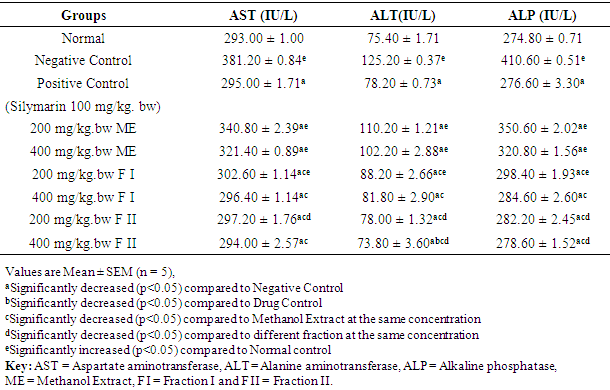
- The effects of methanol extract, fraction I and fraction II of Detarium microcarpum on liver enzyme markers (AST, ALT and ALP) of rats are as presented in Table 2. The serum levels of AST, ALT and ALP in the negative control (DEN-induced hepatocellular carcinoma) were significantly (p<0.05) elevated/high, when compared to the values of normal control group. Treatment with the standard drug, methanol extract, fraction I and fraction II of Detarium microcarpum showed statistically significant (p<0.05) decrease in the level of AST, ALT and ALP when compared to the values of negative control. The highest effect was observed in group IX, that is, rats treated with fraction II at the dose of 400 mg/kg bw.
|
3.2. Effect of Extract and Fractions on Liver Non-enzyme Markers of Rats
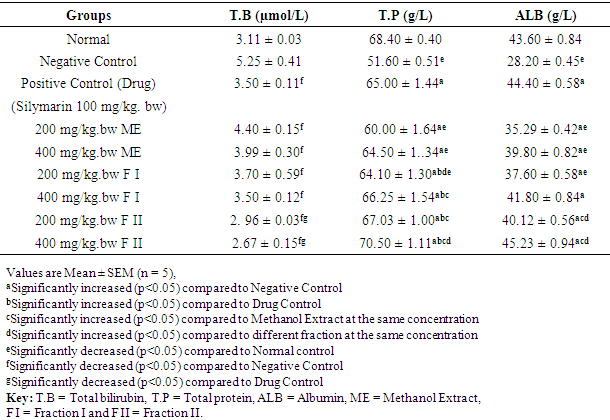
- The effect of methanol extract, fraction I and fraction II of Detarium microcarpum on liver non-enzyme markers (Total bilirubin, Total protein and Albumin) of rats are as presented in Table 3. The result shows that treatment with the standard drug, methanol extract (200 mg/kg. bw and 400 mg/kg. bw), fraction I (200 mg/kg. bw and 400 mg/kg. bw) and fraction II (200 mg/kg. bw and 400 mg/kg. bw) of Detarium microcarpum significantly (p<0.05) increased the serum level of total protein and albumin while that of total bilirubin significantly (p<0.05) decreased compared to the values of negative control. Fraction II (400 mg/kg bw) showed the highest effect.
|
3.3. Effect of Extract and Fractions on Lipid Profile of Rats
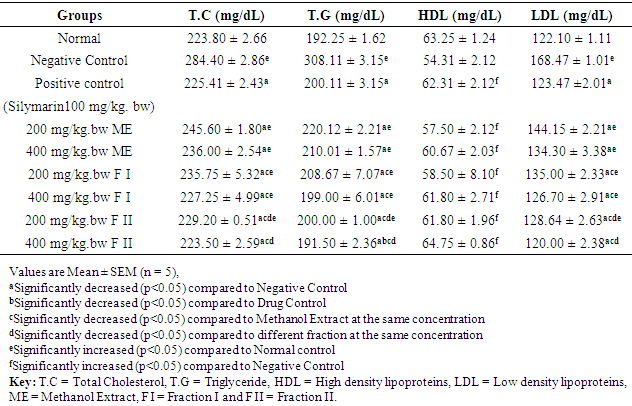
- Table 4 shows the result of the effect of methanol extract, fraction I and fraction II of Detarium microcarpum on TC, TG, HDL and LDL of rats. The serum levels of total cholesterol, triglyceride and LDL-cholesterol in the negative control group (DEN-induce hepatocellular carcinoma) significantly (p<0.05) increased compared with the normal control group. Administration of methanol extract, fraction I and fraction II of Detarium microcarpum significantly (p<0.05) decreased the serum level of total cholesterol, triglyceride, and LDL-cholesterol while the serum level of HDL-cholesterol significantly (p<0.05) increased compared to the values of negative control.
|
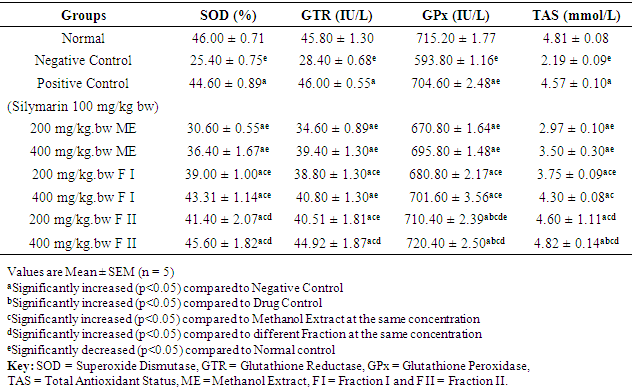
3.4. Effect of Extract and Fractions on Antioxidant Enzymes and Non-antioxidant Enzyme of Rats
- Table 5 shows the effect of methanol extract, fraction I and fraction II of Detarium microcarpum on antioxidant enzymes (SOD, GTR, GPx) and non-antioxidant enzymes (TAS) of rats. The serum levels of SOD, GTR, GPx and TAS in the negative control group (DEN-induce hepatocellular carcinoma) were significantly (p<0.05) lower compared to the values of normal control group. Treatment with the standard drug, methanol extract, fraction I and fraction II of Detarium microcarpum to the various groups showed statistically significant (p<0.05) increase in the serum level of SOD, GTR, GPx and TAS when compared to the values of negative control. The highest effect was observed in group IX, that is, rats treated with fraction II at the dose of 400 mg/kg bw.
|
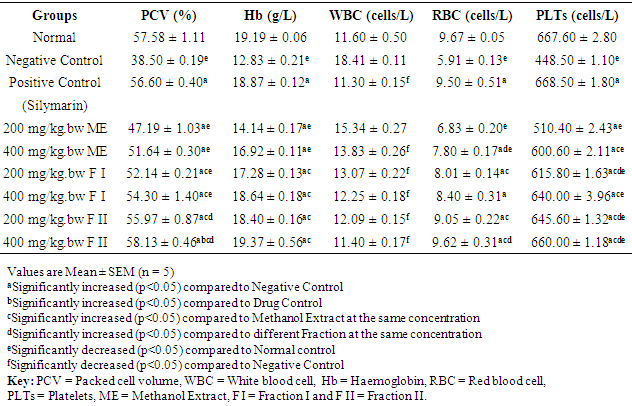
3.5. Effect of Extract and Fractions on Haematological Profile of Rats
- Table 6 shows the effect of methanol extract, fraction I and fraction II of Detarium microcarpum on haematological parameters (PCV, Hb, WBC, RBC and PLTs) of rats. The result shows that oral administration of standard drug (100 mg/kg. bw), methanol extract (200 mg/kg. bw and 400 mg/kg. bw), fraction I (200 mg/kg. bw and 400 mg/kg. bw) and fraction 2 (200 mg/kg. bw and 400 mg/kg. bw) of Detarium microcarpum significantly (p<0.05) increased the level of PCV, Hb, RBC and PLTs while the serum level of WBC significantly (p<0.05) decreased compared to the values of negative control. Fraction II (400 mg/kg bw) exerted the best effect.
|
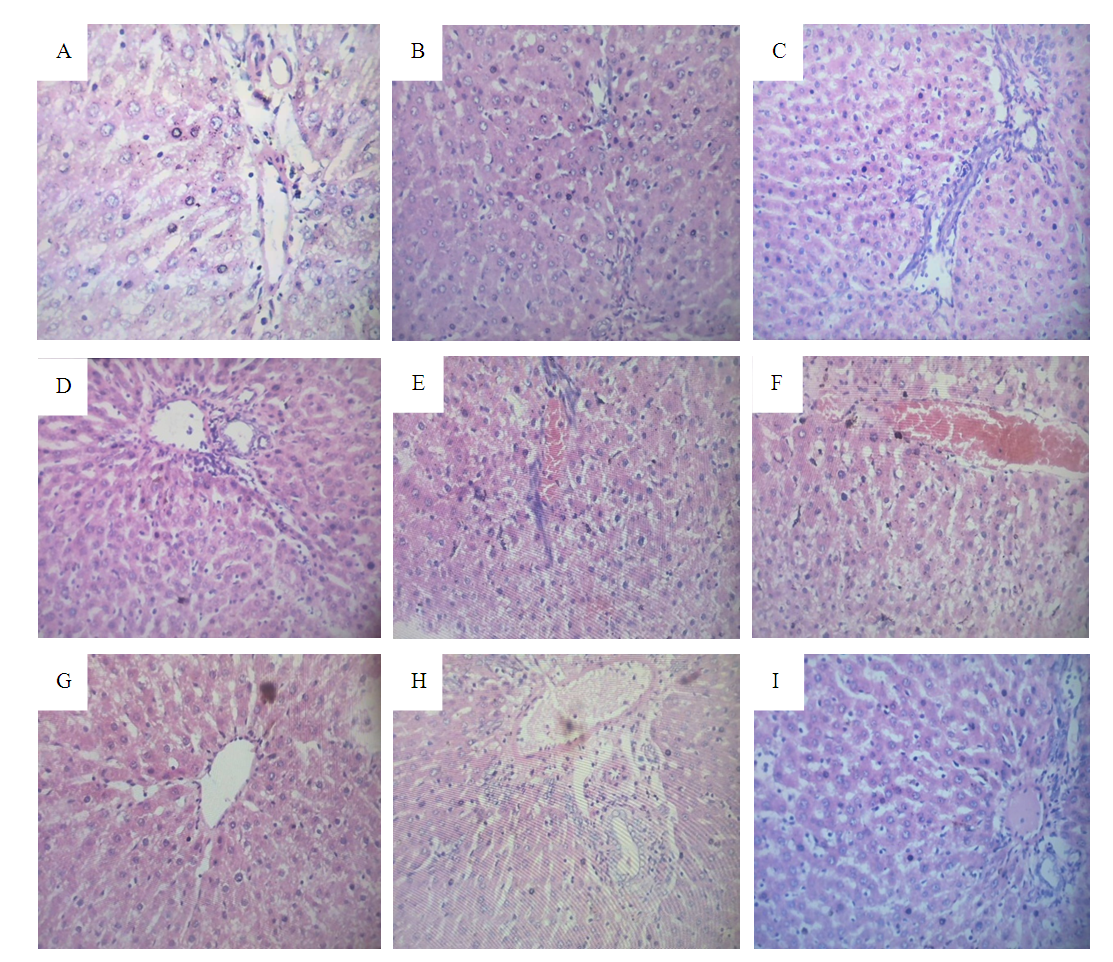
3.6. Effect of Extract and Fractions on Liver Structural Alterations in DEN-Induced Hepatocellular Carcinoma in Rats
- Histopathological evaluation of the liver tissues from rats in the normal control group (Group I) revealed absolutely normal histological features, as illustrated in figure 1 plate A below. However, there was marked of different lesions including necrosis, degeneration and proliferation of hepatic stellate cells in DEN-induced HCC rats (plate B). The result obtained shows that there was remarkable attempt at restoration of these histological features in the group of rats treated with fraction II of the stem bark extract when compare to the group treated with the methanol stem bark extract and fraction I of Detarium microcarpum respectively.
4. Discussion
- Phytochemicals are biologically active, naturally occurring chemical compound found in plants which protect plants cells from environmental hazards and photogenic attack (Nyamai et al., 2016). Antioxidants are substances that protect living cells from the damages caused by unstable molecules known as free radicals. Antioxidants interact and stabilize free radicals which may prevent damage such as the development of cancer. The main characteristic of an antioxidant is its ability to trap free radicals. The antioxidant activity has been attributed to various mechanisms such as prevention of chain initiation, the binding of transition metal ion catalysts, decomposition of peroxides, the prevention of continued hydrogen abstraction, the reductive capacity and radical scavenging and the reducing capacity of a compound may serve as a significant indicator of its potential antioxidant activity as reported by Sutharsingh et al., (2011).The present study demonstrated that activities of ALT, AST and ALP in the sera of DEN treated rats were markedly elevated compared to the normal healthy control rats. This increment is due to the production of free radicals during DEN metabolism, thus damaging the hepatocellular membrane. As a result, these cytoplasmic enzymes are released into the systemic circulation. As seen in the present study, treatment with silymarin significantly reduced (p<0.05) serum ALT, AST and ALP activities compared to DEN treated animals. This might be attributed to the ability of silymarin to scavenge the free radicals, thus preventing or reducing the hepatocellular damage caused by DEN, thereby suppressing the leakage of enzymes through plasma membranes (Pradeep and Mohan, 2007). Administration of methanol extract, fraction I and fraction II in hepatocellular damage-induced animals effectively lowers the high serum activities of AST, ALT and ALP produced by DEN especially fraction I and fraction II. This hepatoprotective effect of the both fractions could be due to a modifying influence on the biotransformation/detoxification of DEN, thus reducing its liver toxicity. This study is in agreement with that of Abarker et al., (2015); Ameesh and Murugan (2016) where they reported that, treating rats with silymarin in hepatocellular damage do decreased the level of ALT, AST and ALP when compared with negative control group.Evaluation of serum proteins such as total bilirubin (TB), total protein (TP), albumin (ALB)) and globulin is a good criterion for assessing the secretion ability/functional capacity of the liver and most commonly used biochemical markers of liver damage in addition with ALT AST and ALP. Based on the result in Table 2, it shows that inducement of hepatocellular carcinoma with DEN significantly (p<0.05) decreased the serum level of TP and ALB of the rats while the serum level of total bilirubin significantly (p<0.05) increased. This may be as a result of decreased in protein synthesis and due to over production of bilirubin, impaired uptake, conjugation or excretion of bilirubin and backward leakages from damaged hepatocytes or bile ducts (Al-Aboudi and Afifi, 2011). Sumithra et al., (2013) reported that, elevation of serum ALT, AST, ALP, LPO and bilirubin is known effect of DEN toxicity which specially affects the liver. Treatment with standard drug, methanol extract, fraction I and fraction II of Detarium microcarpum significantly increased (p<0.05) the serum level of TP and ALB of the rats when compared to the negative Control group. While that of total bilirubin significantly decreased (p<0.05) when compared to the negative control group and the effect is dose dependent as revealed by the result. The reasons behind this observation may not be unconnected with the high antioxidant capacity and the presence of flavonoids, alkaloids, phenols and other essential phytochemicals in the plant extract and its fractions as reported by Christopher et al., (2019).In the present research protocol (Table 3), it was observed that fraction I (200 mg/kg bw and 400 mg/kg bw) and fraction II (200mg/kg bw and 400 mg/kg bw) improved the levels of total cholesterol, triglycerides, HDL-cholesterol and LDL-cholesterol. These findings suggest that fraction I and fraction II can be used as a potential chemotherapeutic agent in the treatment of hepatocellular carcinoma. Studies by Bansal et al., (2015) on cancer revealed that, the levels of total cholesterol increases in the rapidly multiplying cells. Since the liver plays a major role in cholesterol metabolism in mammals, during tumour growth, the animals progressively developed marked changes in the liver and distribution of total cholesterol. Similar elevation in cholesterol levels was reported in hepatoma cells in DEN (Diethylnitrosamine) as reported by Gupta et al., (2014) and in aflatoxin induced hepatocellular carcinoma. The result further revealed that, administration of methanol extract and its fractions significantly (p<0.05) increased the serum HDL level in the rats compared to the negative control group, and the best effect was observed in fraction II (400 mg/kg body weight) this is in agreement with the reports of Bansal et al., (2015) and Gupta et al., (2014) where they independently reported that, treatment increases the serum HDL level in the rats when compared with the negative control group.In this study, DEN-induction reduced the level of antioxidant enzymes which comprises of superoxide dismutase (SOD), glutathione reductase (GTR) and glutathione peroxidase (GPx) and non-enzyme antioxidant (total antioxidant status-TAS), the finding is depicted in Table 4. Group II, which serves as the negative control group shows a low levels of SOD, GTR, GPx and TAS when compared with the normal control group (group I). The oral administration of the standard drug, methanol extract, fraction I and fraction II of D. microcarpum significantly increased (p<0.05) the serum level of SOD, GTR, GPx and TAS of the rats when compared to the negative Control group. These results obviously indicate the chemopreventive effect of the plant under investigation that elevated the level of the defence system of the animals. This result is in harmony with the work of Khan et al., (2001); Sumithra et al., (2013); Abarker et al., (2015); Ameesh and Murugan, (2016) whereby they independently reported that, there is a significant (p<0.05) increased in the level of antioxidant enzymes considered. As seen from the result, Fraction II happened to be more effective when compared with methanol extract and fraction I.The oral administration of standard drug, methanol extract of D. microcarpum stem bark and its fractions in DEN-induced rats (group III, IV, V, VI, VII, VIII and IX) significantly (p<0.05) improved the haematological parameters when compared with the negative control and normal control group. These indicated the protective effect of silymarin as a standard drug and methanol extract of D. microcarpum stem back and its fractions on the hemopoietic system. This result is in agreement with the report of Hasssan et al., (2014) and Yakubu et al., (2017) whereby they independently reported that, there is a significant (p<0.05) improvement in the level of haematological parameters when treated. Red blood cells (RBC) and haemoglobin (Hb) are important in transporting respiratory gases. Based on this research, there were significant treatment related effects (positive effect) on RBC and Hb, this implies that the extract did affect the oxygen carrying capacity of the blood and the amount of oxygen delivered to the tissues when compared with the negative control. Yakubu et al., (2017) also reported that some medicinal plants are known to cause destruction of red blood cells leading to anaemia, but this is not the case with methanol extract of D. microcarpum stem back and its fractions as observed from this study.The biochemical, lipid profile, antioxidant and non-antioxidant enzymes and haematological findings in this study were supported by the histopatological examination of the liver tissue of experimental animals as shown above figure 1 from plate A to I. Microscopic results in DEN group showed different lesions including necrosis, degeneration, karyomegaly and proliferation of hepatic stellate cell (HSCs) in agreement with other researches (Shaarawy et al., 2009). The histopatological observation of the liver section of the normal control (group 1) stained with H&E showed classical hepatic lobules. Each lobule showed anastomating plates of hepatocytes radiating from the central vein toward the periphery of the lobule. The liver sinusoids were seen in between the adjacent plates. Kupffer cells were also seen associated with the sinusoidal lining cells; normal hepatocytes were observed. In contrast, the sections of the liver from HCC control group (Group II) showed loss of lobular architecture; enlarged and darkened nuclei with variable size, dilatation of hepatic sinusoids with kupffer cell hyperplasia were also observed with a feature of necrosis and fatty change. Standard drug group also showed that there are less severe pathological changes compared to hepatocellular damage control group. This means, there is significant improvement when compared with negative control group, this is in agreement with the report of Mesallamy et al., (2011). Treatment with the methanol extract and its fractions (fraction I and fraction II) on the rats induced with DEN reduced severity of lesions in liver. Group treated with fraction I (200 mg/kg bw and 400 mg/kg bw) and fraction II (200 mg/kg bw and 400 mg/kg bw) after the induction of hepatocellular damage showed a high reduction in degeneration and distortion of hepatocytes, marked rejuvenation of hepatic architecture with restoration of bile duct histology. It also showed less fatty changes and minimal pleomorphism compared to HCC control (Group II) rats. This may be as a result of the high antioxidant status and presence of some key phytochemicals which include alkaloids, saponins, tannins, phenols and flavonoids (Christopher et al., (2019). This is in agreement with the study of Abreu et al., (1998), Abreu et al. (1999), Adamu et al., (2014), Yakubu, (2015) and Reuben et al., (2016) that reported that, Detarium microcarpum possess antimicrobial, antitumor, antileishmania and cytotoxic properties.
5. Conclusions
- It may be inferred from the present study that the hepatoprotective effect of methanol extract, fraction I and fraction II of Detarium microcarpum stem bark in DEN-induced hepatotoxicity were responsible for oxidative free radical scavenging activities by alleviating lipid peroxidation through scavenging of free radicals and enhancing the activity of antioxidants (SOD, GPx, GTR and TAS). Probably due to the antioxidant effects of alkaloids, phenols, tannins, saponins, flavonoids and free radical scavenging properties that is present in the stem bark. This plant has immense potential and have broad spectrum of activity on several ailments as earlier indicated. The global changing scenario is showing a tendency towards the use of nontoxic plant products having good traditional medicinal background. This plant can be used safely for longer duration as a cheap source of active therapeutics for alleviation of commonly occurring ailments by the poor and under privileged people of Nigeria. Also, the results from this study have confirmed the rationale for the folkoric use of the Detarium microcarpum in the treatment of hepatic disorders.
ACKNOWLEDGEMENTS
- The authors acknowledge the support received from Prof. M.S. Nadro, Head of Biochemistry Department, School of Life Science Modibbo Adama University of Technology, Yola, Nigeria and other staff for their support and encouragement in carrying out this work.
Conflicts of Interest
- The authors confirm that this article content has no conflict of interest. The authors alone are responsible for the content and writing of this article.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML