-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2019; 9(2): 35-44
doi:10.5923/j.ajb.20190902.03

Antioxidant and Antihyperlipidemic Activity of Methanol Extract of Borassus aethiopum Fruit in Triton X-100 Induced Hyperlipidemic Rats
Umaru Hauwa Aduwamai, Bala Alfa Mahmud, Dahiru Daniel
Department of Biochemistry School of Life Sciences Modibbo Adama University of Technology Yola, Adamawa State, Nigeria
Correspondence to: Umaru Hauwa Aduwamai, Department of Biochemistry School of Life Sciences Modibbo Adama University of Technology Yola, Adamawa State, Nigeria.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Hyperlipidemia is considered one of the major risk factors causing cardiovascular diseases (CVDs). The antihyperlipidemic activity of methanol extract of Borassus aethiopum fruit was tested in Triton X-100 induced hyperlipidemic rats. The hyperlipidemia was induced by a single intraperitoneal injection of Triton X-100 (100mg/kg bwt). The qualitative and quantitative phytochemical analysis of the fruit extract revealed the presence of Flavonoid, saponin, steroids, phenols, tannins, terpenoids and alkaloid with flavonoid having the highest percentage (22%). The GC-MS analysis carried out revealed the presence of alpha tocopherol, oleic acid, dibutyl phthalate, costunolide, citronellol, ascorbyl palmitate, ergosta-5, 22-dien-3, 9,19-Cyclolanost-24-en-3-ol and Ergost-22-en-3-ol. The antioxidant activity of the methanol extract was also determined using Hydrogen peroxide (H2O2), 2, 2-Diphenyl-l-Picryl Hydrazyl (DPPH) and Ferric Reducing Antioxidant Power (FRAP) assay at different concentrations (20, 40, 60, 80 and 100 mg/mL). Result obtained revealed high antioxidant activity comparable to ascorbic acid in a dose dependent manner. Treatment of hyperlipidemia with Borassus aethiopum fruit methanol extract at different concentrations (100, 200, 300 and 400 mg/kg) significantly (p≤ 0.05) reduced the hyperlipidemia via decreased levels of serum total cholesterol (TC), triglycerides (TG), low density lipoprotein cholesterol (LDL- C), very low density lipoprotein cholesterol (VLDL-C) and increase in serum high density lipoprotein cholesterol (HDL-C) when compared to control group in a dose dependent manner. Result obtained from this study indicate that the methanol fruit extract of Borassus aethiopum used in this research possess strong antihyperlipidemic activity comparable to the standard drug atorvastatin.
Keywords: Hyperlipidemia, Lipid profile, Antioxidant activity, Borassus aethiopum, Triton X-100
Cite this paper: Umaru Hauwa Aduwamai, Bala Alfa Mahmud, Dahiru Daniel, Antioxidant and Antihyperlipidemic Activity of Methanol Extract of Borassus aethiopum Fruit in Triton X-100 Induced Hyperlipidemic Rats, American Journal of Biochemistry, Vol. 9 No. 2, 2019, pp. 35-44. doi: 10.5923/j.ajb.20190902.03.
Article Outline
1. Introduction
- Hyperlipidemia has been ranked as one of the greatest risk factors contributing to the prevalence and severity of coronary heart disease. Coronary heart disease, stroke, arthrosclerosis and hyperlipidemia are the primary cause of death globally. Hyperlipidemia is characterized by elevated serum, total cholesterol, low density and very low density lipoprotein and decreased high density lipoprotein levels (Kaur and Meena 2013). Atherosclerosis is one of the leading causes of death in the world both in developed countries and as well as developing countries (Herrington et al., 2016).Cardiovascular disease (CVD) is a disease which is associated with the aberrant functioning of the heart or which affects the blood passage through its route (blood vessel) (CVD foundation, 2011). Other cardiovascular diseases include stroke, heart failure, hypertensive heart disease, rheumatic heart disease, cardiomyopathy, heart arrhythmia, congenital heart disease, valvular heart disease, carditis, aortic aneurysms, peripheral artery disease and thromboembolic disease, (WHO, 2011). Cardiovascular disease is a worldwide health problem currently, growing in developing countries as significant parameter of non-communicable disease burden. CVD causes more than 4 million deaths each year across Europe, accounting for 45% of all deaths (Nick et al., 2016). CVDs account for one third of total deaths around the world, it is believed that CVDs will turn out to be the main cause of death and disability worldwide by the year 2020 (Ginghina et al., 2011; Jorgensen et al., 2013).In the last ten years in Nigeria according to Iloh et al., (2013), cardiovascular diseases have become the leading and major clinical and public health problem. It is associated with high rates of disability, case fatality, unnecessary and sudden death. The national survey in Nigeria and previous studies reports revealed that the prevalence of cardiovascular diseases is escalating in all parts of Nigeria (Iloh et al., 2013).The available drugs have been associated with number of side effects coupled with high cost. In regard to this, there is need for an alternative therapy in the field of hyperlipidemia management using available and easily accessible effective medicinal plant with scientific approval and validation and also culturally acceptable.In spite of tremendous development in the field of allopathic medicines during the 20th century, plants still remain one of the major sources of drugs in modern as well as in traditional system of medicine (Saikia and Sristisri 2011). Borassus aethiopum is an example of such plants. Borassus aethiopum is a tropical plant and its various parts including roots, leaves, flowers and fruits have been used for different and various purpose such as nutritional agents, treatment for sexually transmitted diseases, cutaneous fungal infections and viral infections particularly measles ( Sakande et al., 2012). The antipyretic activities of this plant have been reported (Sakande et al., 2014).
2. Materials and Methods
2.1. Materials
2.1.1. Plant Materials Collection
- The fresh fruits of Borassus aethiopum were collected in April 2018 from Garkida, a village under Gombi Local Government Area of Adamawa state, Nigeria. The sample was identified and authenticated at the Department of Plant Sciences, Modibbo Adama University of Technology Yola, Nigeria. The edible parts of the fruits were peeled and air dried at room temperature under shade and reduced to appropriate size by grinding to fine powder.
2.1.2. Experimental Animals
- Forty two (42) male albino rats (weighing about 90-100g) were obtained from the National Veterinary Research Institute (NVRI) Vom-Jos, Plateau State. The animals were housed in a ventilated plastic cage and acclimatized for seven (7) days prior to the commencement of the experiment. The animals were given standard dry pellet diet (finished vital feeds, Jos) and water ad libtium.
2.2. Method
2.2.1. Plant Extraction
- The Method described by Das et al., (2010) was adopted. Fifty grams (50g) of fine fruit powder were dissolved in 500ml methanol. The solution was allowed to stand for 24hours. The extracts were then filtered using a whatmann filter and concentrated using a rotary evaporator, the water bath was set at 40 degree Celsius.
2.2.2. Qualitative Phytochemical Screening of Methanol Extract of Borassus aethiopum Fruit
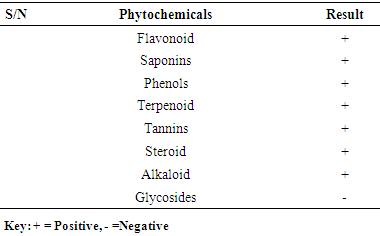
- Flavonoid, alkaloids, tannins, terpenoid, phenols, saponins, steroids and glycosides were determined using the method of Trease and Evans (1989).
2.2.3. Quantification of Phytochemicals
- The presence of alkaloids, saponins, flavonoids, total phenols and tannins were determined using the methods of Trease and Evans (1989), Harbone (1973) and Sofowora (1993).
2.2.4. GC-MS Analysis
- Method: The GC-MS analysis was carried according to the method of Vengaiah et al., (2015). Principle: A combination of two different analytical techniques, Gas Chromatography (GC) and Mass Spectrometry (MS), is used to analyze complex organic and biochemical mixtures. The GC-MS instrument consists of two main components. The gas chromatography portion separates different compounds in the sample into pulses of pure chemicals based on their volatility by 23 flowing through an inert gas (mobile phase), which carries the sample, through a stationary phase fixed in the column. Spectra of compounds are collected as they exit a chromatographic column by the mass spectrometer, which identifies and quantifies the chemicals according to their mass-to-charge ratio (m/z). (Syed and Khushnuma, 2014). Procedure: The analysis was performed using Agilent Gas Chromatography mass spectrometer (GC-MS): GC Model 7890A, MS 5975C (Inert MSD) equipped with 7638B Auto sampler. Helium was used as the carrier gas at a constant flow rate of 1 ml/min and an injection volume of 2 μl was employed (split ratio of 10:1). Injector temperature was 250°C; Ion- source temperature 280°C. The oven temperature was programmed from 110°C (isothermal for 2 min.), with an increase of 10°C/min, to 200°C, then 5°C/min to 280°C, ending with a 9 min. isothermal at 280°C. Mass spectra of compounds in sample obtained by electron ionization (EI) at 70 eV; a scan interval of 0.5 seconds and fragments from 45 to 450 Da. Total GC running time was 36 min. The relative % amount of each component was calculated by comparing its average peak area to the total areas (Vengaiah et al., 2015).
2.2.5. Antioxidant Activity
2.2.5.1. Hydrogen Peroxide-Scavenging Activity
- Method: Hydrogen peroxide scavenging activity of the fruit extract was determined using the method of Jayaprakasha (2004). Principle: Hydrogen peroxide is a weak oxidizing agent and can inactivate a few enzymes directly, usually by oxidation of essential thiol (-SH) groups. It can cross cell membranes rapidly and inside the cell. H2O2 reacts with Fe2+ and possibly Cu2+ ions to form hydroxyl radical which may be the origin of many of its toxic effects. Procedure: A solution of hydrogen peroxide (20 mM) was prepared in phosphate buffer saline (PBS at pH 7.4). Various concentrations of the extract and standards in methanol (1 ml) were added to 2 ml of hydrogen peroxide solutions in PBS. After 10 min, the absorbance was measured at 230nm against a blank solution that contained extracts in PBS without hydrogen peroxide. The percentage scavenging activity of hydrogen peroxide and standard compounds was calculated using the following formula:% scavenged [hydrogen peroxide] =
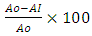 Where; Ao = absorbance control AI = absorbance of sample
Where; Ao = absorbance control AI = absorbance of sample 2.2.5.2. DPPH Radical Scavenging Activity
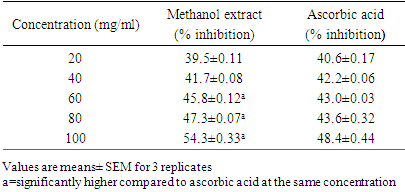
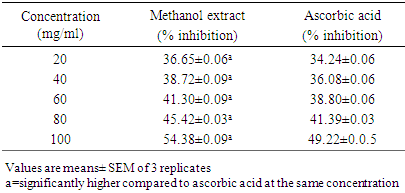
- Method: The DPPH (2, 2-diphenyl-l-picryl hydrazyl) radical scavenging scavenging activity was carried out according to the method described by Patil et al., (2009). Principle: The stable 2, 2-diphenyl-2-picryl hydrazyl (DPPH) is a free radical and accepts an electron or hydrogen radical to become a stable diamagnetic molecule which is widely used to investigate radical scavenging activity. In DPPH radical scavenging assay, antioxidants react with DPPH (deep violet color) and convert it to yellow colored α,α-diphenyl-β-picrylhydrazine. The degree of discoloration indicates the radical-scavenging potential of the sample.Procedure: About 1ml of various concentrations (20, 40, 60, 80 and 100 mg/mL) of the extracts in methanol were added to 4 ml of (0.004% w/v) methanol solution of DPPH. After 30 minutes the absorbance of the preparations was taken at 517 nm by a UV spectrophotometer which was compared with the corresponding % inhibition of standard ascorbic acid. The free radical scavenging activity (FRSA) was calculated using:
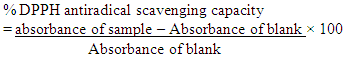
2.2.5.3. Ferric Reducing Antioxidant Power (FRAP Assay)
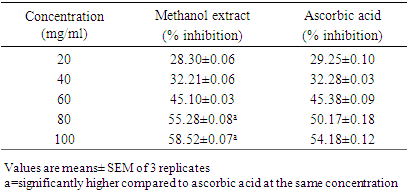
- Method: The Ferric reducing antioxidant power (FRAP Assay) was carried according to the method described by Benzie and Strain (1996). Principle: Ferric reducing antioxidant power (FRAP Assay) assay is a widely used method that uses antioxidants as reductants in a redox-linked colorimetric reaction, where Fe3+ is reduced to Fe2+. Ferric (Fe3+) to ferrous (Fe2+) ion reduction at low pH causes formation of colored ferrous-probe complex from a colorless ferric-probe complex. Procedure: In ferric reducing antioxidant power assay, 1 ml of test sample of methanol extract in different concentration (20, 40, 60, 80 and 100 mg/100 mL) was mixed with 1 ml of 0.2 M sodium phosphate buffer (pH 6.6) and 1 ml of 1% potassium ferricyanide in separate test tubes. The reaction mixtures were incubated in a temperature-controlled water bath at 500°C for 20 min, followed by addition of 1 ml of 10% trichloroacetic acid. The mixtures were then centrifuged for 10 min at room temperature. The supernatant obtained (1 ml) was added with 1 ml of deionised water and 200 μl of 0.1% FeCl3. The blank was prepared in the same manner as the samples except that 1% potassium ferricyanide was replaced by distilled water. The absorbance of the reaction mixture was measured at 700 nm. The reducing power was expressed as an increase in A700 nm after blank subtraction
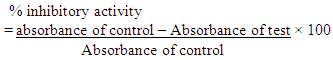
2.3. Experimental Design
2.3.1. Induction of Hyperlipidemia
- Hyperlipidemia was induced in wistar albino rats by single intraperitoneal injection of freshly prepared solution of triton X-100 (100mg/kg) in physiological saline solution after overnight fasting.
2.3.2. Grouping of Experimental Animal
- Forty two (42) rats were grouped into seven (7) groups of 6 rats per each group. I. Group (1) received normal feed + water (normal control) II. Group (2) received Triton X-100(100mg/kg body weight), experimental control-no treatment III. Group (3) received Triton X-100(100mg/kg body weight)+ Atovarstatin 10mg (standard control) IV. Group 4, 5 6, and 7 received Triton X-100 (100mg/kg body weight) and were treated with 100, 200, 300 and 400 mg/kg body weight of the Borassus aethiopum fruit extract respectively.Experimental Design
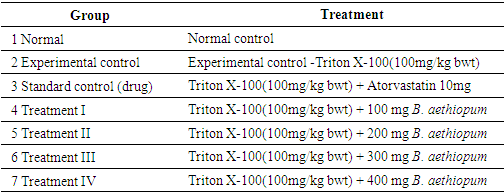
2.3.3. Biochemical Analysis
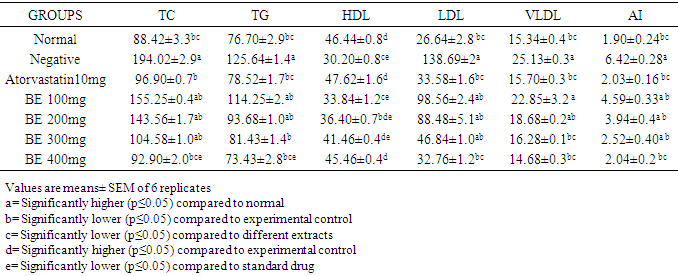
- After seven days of treatment, rats were fastened for 18 hours. The rats were then anesthetized using chloroform, the blood samples were collected by cardiac puncture. The serum was separated by centrifugation of blood at 3000 rpm/10mins for estimation of biochemical parameters (lipid profile) which includes TC, TG, HDL-C, LDL-C, VLDL-C (Vijayaraj et al., 2011).
2.3.3.1. Estimation of Serum Total Cholesterol (TC)
- Method: The method of CHOD- PAP for estimation of serum cholesterol was used as described by Safiullah et al., (2015). Principle: Total cholesterol is measured enzymatically in serum or plasma in a series of coupled reactions that hydrolyze cholesteryl esters and oxidize the 3-OH group of cholesterol. One of the reaction products is measured quantitatively in a peroxidase catalyzed reaction that produces a color. Absorbance is measured at 500nm. The color intensity is proportional to cholesterol concentration.Procedure: The samples were pipetted into the reaction vessel using a micro pipette. Test samples (T): 0.02 ml serum, 2.00 ml reaction solution; the standard sample (S): 0.02ml standard and 2.00 ml reaction solution, while for the blank sample (B): 0.02 ml DW and 2.00ml reaction solution. The mixture were mixed well and incubated for 10 minutes at +20 to 25°C or 5 minutes at 37°C. The absorbance was read at 505/670 nm against the reagent blank.
2.3.3.2. Estimation of Serum Triglycerides (TG)
- Method: GPO-PAP method was used to estimate the serum triglycerides as described by Safiullah et al., (2015).Principle: Sample triglycerides incubated with lipoprotein lipase (LPL) liberate glycerol and free fatty acids. Glycerol is converted to glycerol-3-phosphate (G3P) and adenosine -5-diphosphate (ADP) by glycerol kinase and ATP. Glycerol-3-phosphate (G3P) is then converted by Glycerol phosphate dehydrogenase (GPO) to dihydroxyacetone phosphate (DAP) and hydrogen peroxide (H2O2). In the last reaction, hydrogen peroxide (H2O2) reacts with 4-aminophenazone (4-AP) and p-chlorophenol in the presence of peroxidase (POD) to give a red colored dye. The intensity of the color formed is proportional to the triglycerides concentration in the sample.Procedure: For this analysis 0.01 ml of serum was taken in a test tube (T) in which 1ml reaction solution was added. In another test tube (S) 0.01ml standard and 1ml reaction solution was added. The solution was mixed well and incubated at 25°C for 10 min. The absorbance of standard and test against reagent blank was read at 540 nm.
2.3.3.3. Estimation of HDL-cholesterol
- Method: CHOD-PAP method was used to estimate the serum HDL cholesterol level as described by Safiullah et al., (2015).Principle: During the first phase, LDL, VLDL particles and Chylomicrons generate free cholesterol, which through an enzymatic reaction, produce hydrogen peroxide. The generated peroxide is consumed by a peroxidase reaction with DSBmT yielding a colorless product. During the second phase, specific detergent solubilizes HDL-Cholesterol. In conjunction with CO and CE action, POD + 4-AAP develop a colored reaction which is proportional to HDL-cholesterol concentration.Procedure: For this analysis 2ml of serum was taken in a test tube and 0.5 ml of precipitation reagent was added. The mixture was shaken thoroughly and left to stand for 10min at 25°C and then centrifuged for 15min at 4000rpm. Within 2hr after centrifugation, the clear supernatant was used for the determination of HDL-C. One ml of the supernatant was taken in a test tube (T) and 1 ml of reaction solution was added to it. In another test tube 0.1 ml DW was taken and 1ml reaction solution (B) was added. The mixtures were mixed thoroughly, incubated for 5min at 37°C and measured the absorbance of the sample against reagent blank at 546 nm.
2.3.3.4. Estimation of LDL Cholesterol
- Method: The Estimation method described by Friedwald et al., (1972) was used in determining LDL-C.Principle: This LDL-C method requires the measurement of triglycerides (TG), high density lipoprotein (HDL-C) and total Cholesterol (TC). Procedure After getting the parameters required, the LDL-C was calculated by using Friedwald formula:
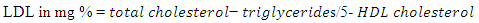
2.3.3.5. Estimation of VLDL Cholesterol
- Method: The Estimation method of Friedwald et al., (1972) was used in determining VLDL-C.Principle This VLDL-C method requires the measurement of triglycerides. VLDL-C by Friedwald estimation is then calculated by dividing the triglyceride by five (5). Triglycerides level above 400mg/dl preclude estimation by Friedwald estimation (Friedwald et al., 1972). ProcedureAfter getting the parameters required, the VLDL-C by Friedwald estimation was then calculated by using Friedwald formula:
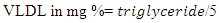 Atherogenic index (AI): was calculated using the formula:AI=TC/HDL-C.
Atherogenic index (AI): was calculated using the formula:AI=TC/HDL-C.2.4. Statistical Analysis
- One way Analysis of Variance (ANOVA) was used. Result was expressed as mean ± SEM. LSD multiple range test was used to determine significant differences between means. The difference between the means was regarded as significant at p<0.05 and the differences of the mean was expressed using SPSS software version 23.
3. Result
- 3.1. The phytochemicals that were found to be present in the methanol fruit extract of Borassus aethiopum is as revealed in table 1. Flavonoid, tannins, phenols, saponins, terpenoid, steroid, and alkaloids were found to be present while glycoside was absent.
|
|
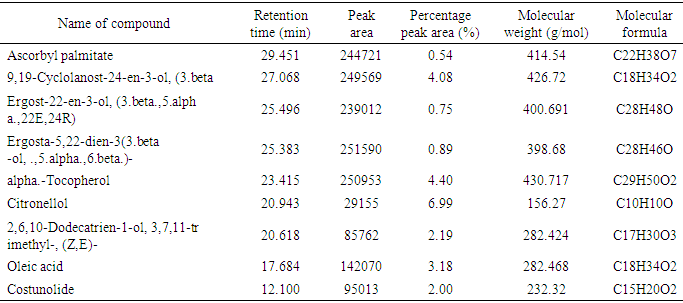
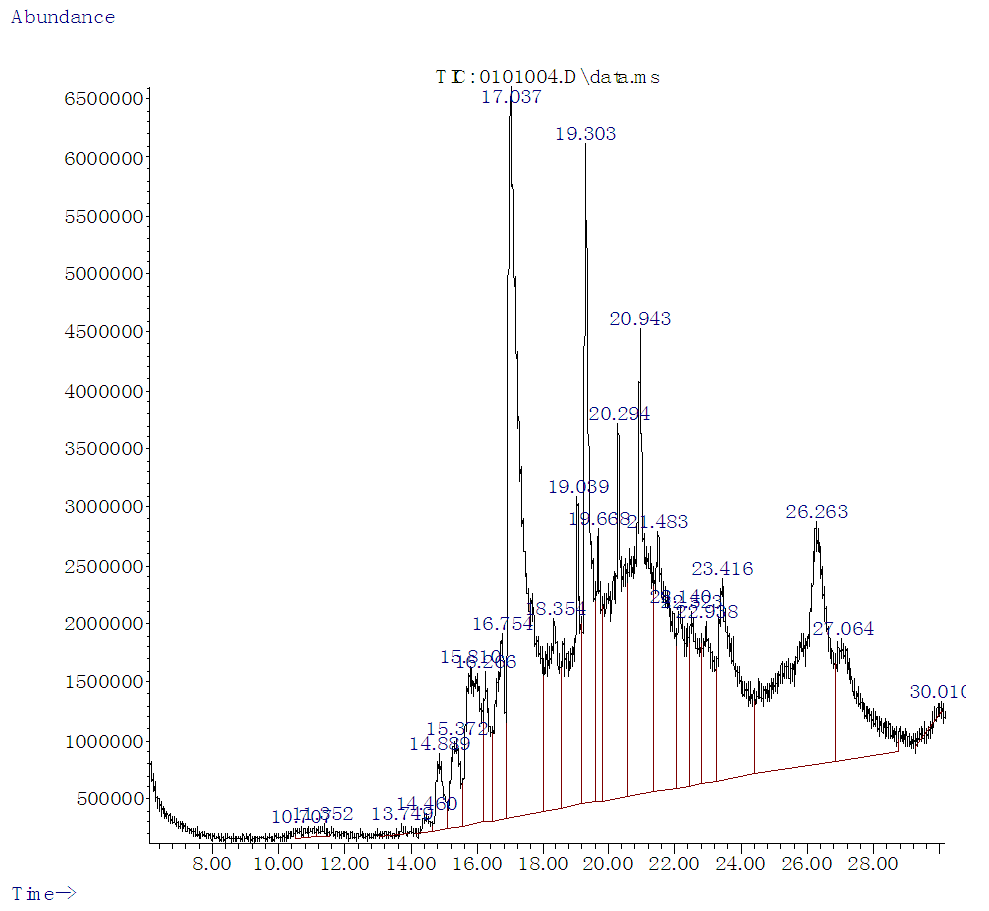 | Figure 1. GC –MS Chromatogram of Methanol Extract of Borassus aethiopum Fruit |
|
|
|
|
|
4. Discussion
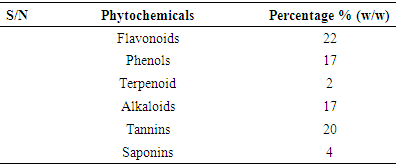
- The phytochemical analysis of the methanol fruit extract of Borassus aethiopum revealed the presence of flavonoids, phenols, terpenoid, alkaloids, saponins and tannins. The quantification analysis in terms of percentage revealed that the fruit extract possess flavonoid in high amount followed by tannins, phenols, alkaloid, saponin and terpenoid arranged in descending order. Flavonoids as compounds originating from plants, are part of the human diet and have many positive impacts on the human organism. They act as natural antioxidants and have an effect on many diseases. Flavonoids have a variety of biological effects in numerous mammalian cell systems, in vitro as well as in vivo. They have been shown to exert antimicrobial, antiviral, antiulcerogenic, cytotoxic, antineoplastic, mutagenic, anti-inflammatory, antioxidant, antihepatotoxic, antihipertensive, hypolipidemic and antiplatelet activities (Mona et al., 2014). Flavonoids are known to improve cardiac function, decrease anginas and lowers cholesterol levels. These compounds act by regulation of inflammation mediators (Jiang et al., 2010). The flavonoid contained in the fruit extract of Borassus aethiopum may have contributed to the anti hyperlipidemic activity of the extract. Phenolics, phenols or polyphenolics (or polyphenol extracts) are chemical components that occur ubiquitously as natural colour pigments responsible for the colour of fruits of plants. Phenolics in plants are mostly synthesized from phenylalanine via the action of phenylalanine ammonia lyase (PAL). They are very important to plants and have multiple functions. The most important role acts in plant defence against pathogens and herbivore predators, (Builders et al., 2010).Plant sterols have been investigated as an alternative for lowering plasma cholesterol levels, and several studies have shown that they significantly reduce plasma total and LDL cholesterol. Anti-atherosclerotic effects of plant sterols are well documented. The anti-atherogenic effects may be due, not only to their cholesterol-lowering activities, but also to other properties, such as effects on the coagulation system, an antioxidant system, and hepatic and lipoprotein lipase activities (Mona et al., 2014). Saponins are known to have hypocholesterolaemic, immunostimulant, hypoglycemic effect and anticarcinogenic properties. Saponins are also believed to have antifungal and hypocholesterolemic effects. These effects are believed to be due to combination with bile acids to form micellar aggregates. Saponins prevent hyperlipemia and liver injury induced by lipid peroxidation (Nyamai et al., 2016). The mechanism of action of these compounds in this case is through inhibition of lipid peroxidation and inhibition of lipid peroxide production. This process lowers the amount of cholesterol assimilated in the system. Clinical studies have shown that phytosterol intake leads to up to 15% reduction of LDL-cholesterol (Katan 2003). Tannins are also associated with a number of biological activities, such as anti-inflammatory, anti-asthmatic, anticancer, antimicrobial, anti-allergy, antihypertensive and cardioprotective. The beneficial effects of tannins on human health have been attributed mainly to their strong free radical-scavenging and antioxidant activities (Mona et al., 2014). The work of (Jain and Surana 2015) also reported that some plants with hypolipidemic activity possessed significant amount of phytochemicals. The GC MS shows several active component present in the fruit extract. Ascorbyl palmitate acted as an antioxidant and as an anti-inflammatory agent (Hira et al., 2016). Therefore, it plays a vital role in hyperlipidemia management since it confers antioxidant activity. Vitamin E is a naturally occurring fat-soluble antioxidant which has been proposed as a treatment for both primary and secondary protection against cardiovascular (CV) events. Vitamin E has a potential effects as antioxidant as reported by (Raluca et al., 2015) and (Maciej et al., 2012). α- tocopherol (one of vitamin E) decreased serum total cholesterol and increasing HDL-C, factors, plays an important role in atherogenesis implies that the development and progression of atherosclerosis can be inhibited by antioxidants (Marysabel et al., 2003). In addition it increased the liver glutathione and reduced serum lipid peroxide (Alqayim et al., 2015).Vitamin E is classified as an antioxidant due to its ability to scavenge lipid radicals and terminate oxidative chain reactions. It can terminate radical chain reactions by interacting with the lipid peroxyl radical, preventing it from generating a new radical and perpetuating the chain reaction by oxidizing other lipids. Following its oxidation, vitamin E can be recycled back to its native unoxidized form by various soluble antioxidants such as vitamin C and ubiquinol. This process prevents the accumulation of vitamin E radicals and their subsequent peroxidation of lipids, and is considered by some to be critical for the antioxidant activity of vitamin E. It has been suggested that all of the other biological functions of vitamin E are actually a result of its antioxidant activity (Moshe et al., 2013). Vitamin E has been also shown to benefit rabbits that are fed an atherogenicdiet by inhibiting the development of atherosclerotic lesions (Marysabel, 2003). Costunolide, a sesquiterpene lactone, present in most of the medicinal plants has been identified as an active principle responsible for the medicinal property of the plants and the compound has been examined to possess several pharmacological activities (Rajalakshmi and Anita 2014). Costunolide has many medicinal importance including conferring antioxidant activity and the antioxidant efficacy of costunolide has been reported by (Eliza et al., 2010).The radical scavenging activity of the fruit revealed that both DPPH, H2O2 and FRAP activity of the methanol extract of B. aethiopum exhibited significantly higher (p< 0.05) antioxidant activity compared with ascorbic acid. The work of (Vijay and Vimukta 2014) also revealed that antioxidants play a vital role in treating cardiovascular diseases. Oxidants and free radicals are harmful for the body health when their overload cannot steadily be destroyed and consequently generate an occurrence called oxidative stress (Sima et al., 2018). Antioxidants have been shown to prevent LDL oxidation in vitro and retard the progression of atherosclerosis in animal model (Keaney and Frei, 2012). Therefore, the antioxidant activity conferred by the methanol extract of Borassus aethiopum fruit may have also contributed to its antihyperlipidemic activity. Antioxidants plays a key role in the formation and development of chronic diseases such as cancer, rheumatoid arthritis, cardiovascular and autoimmune disorders or even aging (Sima et al., 2018). Antioxidants have been shown to prevent LDL oxidation in vitro and retard the progression of atherosclerosis in animal model (Keaney and Frei, 2012). Therefore, the antioxidant activity conferred by the methanol extract of Borassus aethiopum fruit may have also contributed to its antihyperlipidemic activity. The result of the present study clearly indicated that the methanol fruit extract of Borassus aethiopum were all active against triton X-100 induced hyperlipidemic rats. Treatment with the extract at different concentrations significantly decreased the lipid profile parameters when compared with the negative group at (P<0.05). Treatment with the extract shows a decrease in LDL cholesterol level which is a bad cholesterol because high plasma concentration of cholesterol, in particular those of low-density lipoprotein (LDL) cholesterol is one of the principal risk factors and the process of atherogenesis and has been considered by many to consist largely of the accumulation of lipids within the artery wall and even much more (Peter et al., 2017). Hence, high LDL is associated with hyperlipidemia, while the extract shows a considerable increment in HDL-cholesterol which has been known to be a good cholesterol.The elevation of triglyceride could lead to increased secretion of VLDL. Increasing VLDL secretion has been reported in triton X-100 induced hyperlipidemic rats. Increase in TG level induces imbalance in lipid metabolism leading to hyperlipidemia (Pragda et al., 2012). The elevated levels of low-density lipoprotein and very low density lipoproteins associated with cholesterol and triglycerides is one of the primary risk factors for atherosclerosis (Herrington et al., 2016). An increased rate of low density-lipoprotein cholesterol oxidation may increase the risk of premature atherosclerosis. HDL-cholesterol exerts its cardio protective effects at many levels by prevention of low density lipoprotein cholesterol oxidation, vascular wall inflammation, and thrombosis, by the preservation of endothelial vasomotor, proliferative and survival function, by possible prevention of macrophage apoptosis and by increasing the number of endothelial progenitor cells. HDL has a well characterized role in reverse cholesterol transport (RCT) that removes macrophage cholesterol from the plaque. In addition, HDL-C has several anti-inflammatory effects (Biswajit and trinath 2012). The present study agrees with the work reported by (Mallikarjuna 2018).
5. Conclusions
- The study has revealed that Borassus aethiopum fruit extract contains terpenoids, flavonoids, steroids, alkaloids, phenols, saponins and tannins with flavonoid having the highest percentage. Borassus aethiopum also possess high antioxidant activity comparable to vitamin C. The methanol fruit extract of Borassus aethiopum used for this research showed significant (p<0.05) decrease in the lipid profile parameters compared to the negative group in a dose dependent manner. The activity was found to be comparable to the standard drug atorvastatin.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML