-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2019; 9(1): 7-16
doi:10.5923/j.ajb.20190901.03

Mitigation of Aluminium Phosphide-induced Hematotoxicity and Ovarian Oxidative Damage in Wistar Rats by Hesperidin
Olusegun Kayode Afolabi1, Emmanuel Bukoye Oyewo1, Gbadebo Emmanuel Adeleke1, Jelili Abiodun Badmus1, Adedoja Dorcas Wusu2
1Biochemistry Department, Faculty of Basic Medical Sciences, Ladoke Akintola University of Technology, Ogbomoso, Nigeria
2Department of Biochemistry, Faculty of Science, Lagos State University, Lagos, Nigeria
Correspondence to: Olusegun Kayode Afolabi, Biochemistry Department, Faculty of Basic Medical Sciences, Ladoke Akintola University of Technology, Ogbomoso, Nigeria.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Aluminium phosphide is an oral rodenticide and insecticide that is toxic to multiple organs in non-target organisms with no known antidote. This study investigated aluminium phosphide-induced hematotoxicity and ovarian oxidative damage in rat and evaluated the effectiveness of hesperidin as a therapeutic agent against the toxic effects. Rats receiving a sub-lethal dose of aluminium phosphide (1.15 mg kg-1 body weight) for 30 days, exhibited significant impaired hematological parameters with distorted leucocyte and thrombocytic indices. Aluminium phosphide exposure produced macrocytic, hyperchromic anemia. The toxicity also included a reduction in the white blood cell, lymphocyte, and granulocyte counts. Ovarian oxidative stress indicators comprising malonaldehyde and lipid hydroperoxides levels were markedly increased, while the antioxidant enzymatic system was inhibited. Co-treatment with hesperidin ameliorated the aluminum phosphide-induced anemia, thrombocytopenia and leukopenia by improving erythrocyte indices, and boosting white blood cell count. Besides, hesperidin markedly enhanced the ovarian anti-oxidative status through the activation of catalase and superoxide dismutase, while also reducing malonaldehyde and lipid hydroperoxides concentrations in the ovary. The results demonstrated the ovarian damaging capability and hematotoxicity of aluminium phosphide and established the ameliorative potential of hesperidin.
Keywords: Aluminium phosphide, Hesperidin, Hematotoxicity, Ovarian damage, Oxidative stress, Antioxidant
Cite this paper: Olusegun Kayode Afolabi, Emmanuel Bukoye Oyewo, Gbadebo Emmanuel Adeleke, Jelili Abiodun Badmus, Adedoja Dorcas Wusu, Mitigation of Aluminium Phosphide-induced Hematotoxicity and Ovarian Oxidative Damage in Wistar Rats by Hesperidin, American Journal of Biochemistry, Vol. 9 No. 1, 2019, pp. 7-16. doi: 10.5923/j.ajb.20190901.03.
Article Outline
1. Introduction
- In recent years, the use of pesticides such as aluminium phosphide (AlP) has increased, leading to an improvement in the quality and quantity of agricultural products in many developing countries [1]. Owing to some of its properties, such as its toxicity to insects at all stages of life cycle, short half-life and a low decomposition residue, AlP has been considered an ideal pesticide for use in many agricultural processes [2,3]. Its ready availability has caused an increased incidence of exposure and toxicity to non-target organisms, especially humans and animals [4]. The incidences which may be deliberate, accidental or occupational have resulted in high mortality rates in many countries [5-8].AlP exposure results in toxicity that affects multiple organs in the body system. AlP in contact with moisture or gastric juice becomes hydrolyzed and releases highly toxic gaseous phosphine (PH3) which is responsible for the toxic effects of AlP [8]. PH3 interferes with the mitochondrial electron transfer process. The inhibition of oxidative phosphorylation leads to impairment of cellular respiration and activation of peroxide radicals [1]. The peroxides in-turn facilitate the production of oxygen free radicals that can cause cellular injury through oxidative damage, a major contributory process to AlP-induced toxicity [8,9]. Also, AlP through the action of PH3 interferes with the function of cellular enzymes and proteins. PH3, while increasing superoxide dismutase (SOD) activity produces an inhibitory effect on the antioxidant enzymes, catalase (CAT) and peroxidase, thereby depleting the scavenging ability of the cell [10]. AlP thus can induce multiple organ damage and these toxic effects are manifested in systems such as the cardiovascular, hepatic, renal, hematological and nervous systems [5,11-13]. There is no known antidote for AlP or phosphine poisoning and treatment has largely been supportive [10]. But since oxidative imbalance is a major feature of the toxic effect, supplementation of antioxidants to prevent the effects of AP exposure in populations of risk, is of interest. Investigations have shown phytochemicals like flavonoids to be useful in the treatment of oxidative stress-related injury. Flavonoids are natural substances containing variable phenolic structures and studies have shown that they have potent antioxidant and free radical-scavenging properties that offer protective effects [14]. In a previous study, we reported hesperidin (HSD), a phytoflavanone with antioxidant property, as a potential therapeutic agent against AlP-induced testicular damage [15]. Hesperidin is a flavonone glycoside known to produce a wide range of pharmacological effects, such as anti-inflammatory [16-18] anticarcinogenic [18,19] and antioxidant [20]. HSD has been reported to augment hematological parameters [21,22]. Consequent to previous findings showing the protective effects of hesperidin against chemical toxicity [15,23,24], the present study aimed at evaluating hesperidin’s protective effects against aluminium phosphide induced hematotoxicity and ovarian oxidative stress.
2. Materials and Methods
2.1. Chemicals
- Hesperidin, triphenylphosphine (TPP), xylenol orange, 5,5’ -dithiobis(2-nitrobenzoic acid) were obtained from Sigma-Aldrich (Munich, Germany), trichloroacetic acid and thiobarbituric acid were from Qualigens Fine Chemicals (Mumbai, Maharashtra, India). All other chemicals were of analytical grade.
2.2. Animals and Treatment
- Twenty-eight healthy adult female Wistar rats of between 8 and 10 weeks old and weighing 120–140 g were procured from The Animal House, Faculty of Basic Medical Sciences, Ladoke Akintola University of Technology. Rats were housed in plastic cages in a ventilated room under controlled laboratory conditions of a normal light-dark cycle (12 h light/dark) and temperature (25 ± 2°C). The animals were provided with laboratory standard rat feed and water ad libitum. In this study, the National Institute of Health guidelines for laboratory animal care and use were followed [25]. Experimental design and animal handling were executed according to the guidelines approved by the Research Ethical Committee of the Faculty of Basic Medical Sciences, Ladoke Akintola University of Technology, Nigeria that is in agreement with the Guide for the Care and Use of Laboratory Animals published by the National Institute of Health.
2.3. Experimental Design
- Rats were randomly divided into four groups of seven animals each. Animals in Control group were orally administered 1 mg kg-1 body weight (bwt) of corn oil, 2 h after administration of the same volume of saline. AlP-treated group was orally administered AlP at a dose of 1.15 mg kg-1 bwt (one-tenth LD50) in corn oil [26]. Animals in HSD group orally received a dose of 2oo mg kg-1 bwt of HSD dissolved in saline, while AlP plus HSD-treated group received the same dose of 200 mg kg-1 bwt HSD 2 h prior to administration of AlP at 1.15 mg kg-1 bwt in corn oil. All treatments were given for 30 days. One-tenth LD50 for AlP was used to produce a sub-lethal toxic effect in the animal, while the HSD dose was based on our earlier study which produced a therapeutic effect against AlP intoxication [15].
2.4. Sample Preparation
- At the end of the treatment, rats were fasted overnight, anesthetized with diethyl ether and killed by cervical dislocation. Blood sample was drawn by cardiac puncture into sample bottles containing ethylenediaminetetraacetic acid (EDTA). The ovaries were excised, rinsed in cold 1.15% potassium chloride and weighed. Right ovaries were kept in 10% neutral formalin for hematoxylin and eosin (H&E) staining. Left ovaries were homogenized in 0.1 mol L-1 phosphate buffer at pH 8.0. Homogenates were centrifuged at 3000 g for 5 min at 4°C and supernatants collected for biochemical analysis.
2.5. Hematological Parameters
- Hematological parameters including, red blood cell count (RBC), hemoglobin (Hb), hematocrit (HCT), red blood cell distribution width (RDW), mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), mean corpuscular hemoglobin (MCH), white blood cell count (WBC) and differential leucocytic count, thrombocytic indices [total platelet count (PLT), mean platelet volume (MPV), total platelet crit (PCT) and platelet distribution width (PDW)] were analyzed. The analyses were conducted with the use of an automated hematological assay analyzer (Medonic CA 620, Sweden).
2.6. Biochemical Analysis
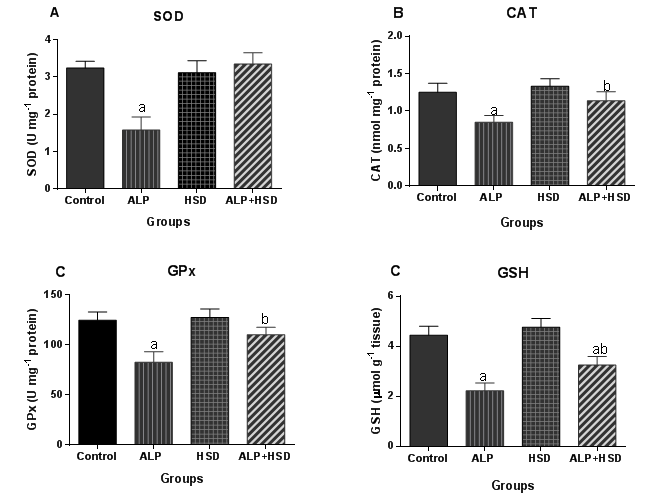
- Protein concentration in ovary homogenate was determined according to the method described by Lowry et al. [27] using bovine serum albumin as a standard. Lipid peroxidation was quantified by measuring MDA content in the ovary. Ovarian MDA was determined using the thiobarbituric acid reactive substance assay, as described by Buege and Aust with slight modifications [28]. The MDA concentration was expressed as nmol g-1 of wet tissue. Lipid hydroperoxides (LOOH) concentrations in ovarian homogenate were estimated using the method of Nourooz-Zadeh et al. [29]. The method of Moron, Depierre [30] was used in determining reduced GSH in the ovary. The activity of CAT was measured by the method of Aebi [31]. The method of Misra and Fridovich [32] was used in determining SOD in the rat ovaries. GPx activity was determined using H2O2 as a substrate in the presence of reduced glutathione [33].
2.7. Histological Examination
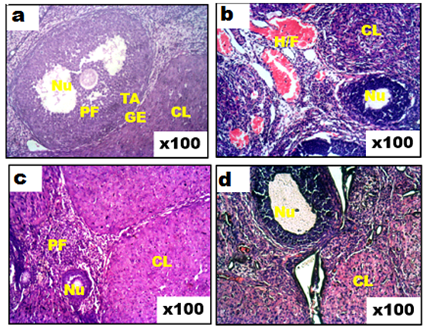
- Ovarian tissues were fixed in 10% neutral-buffered formalin and embedded in paraffin wax for histological examination. Sections (4 µm) were prepared and stained with hematoxylin and eosin for light microscope examination by a histopathologist.
2.8. Statistical Analysis
- One-way analysis of variance was used for the statistical analysis after which multiple comparisons were carried out by Tukey’s test. All data were expressed as the mean ± standard deviation (SD) and the differences were considered statistically significant at p < 0.05. Data were analyzed using GraphPad Prism version 6.
3. Results
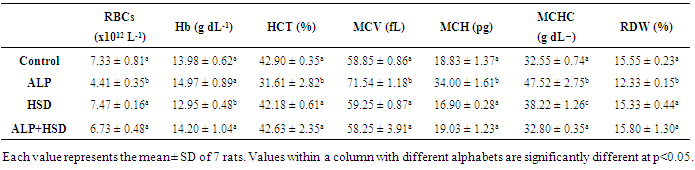
- The changes in hematological parameters in control and treated rats are presented in Table 1. After 30 days, RBC, HCT and RDW were significantly lower (p < 0.05) in rats exposed to AlP compared to the control (40%, 26% and 21% respectively). Treatment with HSD significantly raised these parameters in the AlP exposed rats by 53%, 36 %, and 28% respectively. Hb however, was unaffected by the exposure. Mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and the mean corpuscular hemoglobin concentration (MCHC) were also determined in this study (Table 1). These red cell indices were altered by exposure to AlP. In AlP-treated group, MCV, MCH, and MCHC were elevated by as much as, 22%, 81%, and 46% respectively but the administration of HSD reduced these values by 19%, 44%, and 31% respectively.
|
|
|
4. Discussion
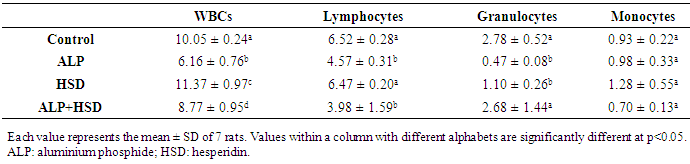
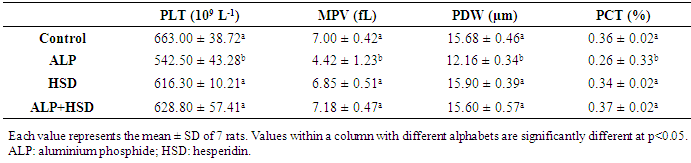
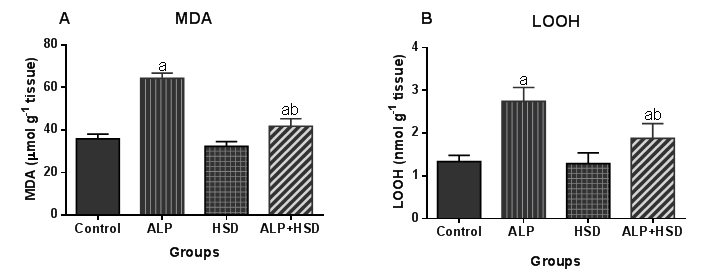
- Hematological parameters are often evaluated to assess the functional health status and the internal environment of an organism [34,35]. Previous studies have shown that exposure to chemicals like pesticides can alter hematological parameters in animals [36] and humans [37,38]. In the present study, the main hematological alterations induced by AlP exposure include anemia, thrombocytopenia, and leukopenia. Our study associated AlP exposure with significant decreases in RBC, HCT, and RDW values, while MCV, MCH, and MCHC values were significantly increased. The reduction in both RBC and HCT values is indicative of an AlP-induced anemia in the rats, which is in agreement with earlier reports on the hematoxic effect of pesticides, including AlP [13,36,39]. After AlP ingestion, phosphine concentration is reported to increase in the blood and liver [40,41]. Studies have also shown that the absorbed AlP, as well as, its gaseous product phosphine can react with free hemoglobin and hemoglobin in normal red blood cells to produce hemichrome, a derivative of methemoglobin and Heinz bodies [5,42] with the concomitant induction of free radicals [8,42]. Oxidation of lipids and proteins by these free radicals can cause an increase in the production of lipid peroxides leading to hemolysis of RBC [43]. The major pathological consequences of free radical-induced membrane lipid peroxidation include increased membrane rigidity, decreased cellular deformability, reduced erythrocyte survival, and lipid fluidity, all of which will eventually result in the lysis of the erythrocyte [44]. The decreased RBC observed in our study may, therefore, result from increased erythrocyte destruction predicated by oxidative stress. Besides, pesticide residues are known to induce anemia by interfering in several steps in heme biosynthesis, causing a shortening of circulating erythrocytes life span [45,46]. The anemia observed in this study might thus, be due to the effect of AlP on erythropoietic tissues and heme biosynthesis in rats. RBC parameters such as Hb, MCHC, MCH, PCV, and MCV were studied to ascertain the type of anemia induced by AlP. Indices such as MCH and MCHC depict the level of hemoglobin content while MCV reflects the average size of the red blood cell, all of which aid in the diagnosis of the type of anemia. The low RBC and HCT along with the high MCV, MCH, and MCHC in the current study suggested that AlP exposure provoked macrocytic, hyperchromic anemia in the rats. The increased hemoglobin content in the red cell as reflected by the high MCHC and MCH values may thus, be a compensatory mechanism to improve the oxygen-carrying capacity of the blood already compromised by the anemia induced by AlP and may also explain the normal Hb level observed. Although erythrocyte lipid peroxides and antioxidant levels were not measured in this study, the observed distorted hematological parameters may be connected with oxidative stress induced by AlP. This is because studies have implicated oxidative stress in AlP-induced hematotoxicity [47]. The present results demonstrated that hesperidin treatment normalized the otherwise depressed RBC, HCT, and RDW levels while stabilizing the MCV, MCHC and MCH levels in AlP intoxicated rats. HSD attenuated the anemic response promoted by exposure to AlP in the rats. This may partly be due to its ability to improve the erythrocyte membrane integrity by the alleviation of oxidative damage to the erythrocyte membranes through its scavenging effect [48,49]. Besides, HSD is reported to ameliorate depleted levels of endogenous antioxidant enzymes like SOD, CAT, and glutathione-S-transferase which might have contributed to the improvement seen of the blood indices [50]. Amelioration of oxidative stress in blood by hesperidin has also been associated with improvement in hematological profile [49]. HSD has been suggested to possess osmoregulatory and biomembrane stabilizing properties and thus may have improved the erythrocyte indices in the AlP intoxicated rats through these mechanisms [51]. Following hesperidin administration, the level of RBCs and its related indices were appreciably improved. This indicates that the flavonoid can stimulate the formation or secretion of erythropoietin, which stimulates stem cells in the bone marrow to produce red blood cells [52]. The stimulation of this hormone enhances the rapid synthesis of RBC which is supported by the improved level of MCH and MCHC [53]. These parameters are used to define the concentration of hemoglobin and to suggest the restoration of the oxygen-carrying capacity of the blood. Mahmoud et al. [52] have previously reported that the action mechanism of hesperidin may be attributed to its ability to lower lipid peroxidation level that causes hemolysis of erythrocytes. A decreased expression of adipose tissue adiponectin has been associated with anemia, with the administration of hesperidin in the same study, reportedly ameliorating the depressed expression of the protein, leading to an improved hematological status in experimental diabetic rats [52]. This ability of hesperidin to improve adiponectin expression may, therefore, partially explain the improved status of the hematological parameters in AlP intoxicated rats. ROS are involved in redox-sensitive signaling pathways through down-regulation of transcription factors and it has been shown that erythropoietin production and erythroid differentiation are regulated by this [54-56]. Suppression of erythropoietin production by ROS generation can, therefore, be reversed by antioxidants. Several studies have investigated the effects of antioxidants on erythropoiesis and hematological parameters [57-59]. Distortion in leucocyte differentials has been recognized as an indication of environmental stress, which provides a summation of an organism's immune status [60]. Leucocytes are immunological cells that defend against infectious diseases and xenobiotics. An increase of these immune cells is linked to an amelioration of the body immune system while a reduction is associated with immunological suppression [61]. Our data revealed the deleterious effect of AlP on the total and differential leucocyte counts in the animals with AlP exposure depleting WBC, lymphocyte and granulocyte counts. These are incongruent with several studies which described the proliferation of the WBC by insecticides and some pharmaceuticals [62,63]. There is however inconsistency in the effect of AlP on leukocyte as AlP exposure is reported to cause leukocytosis in the African Cat fish [47] while Ntelios et al. [64] associated it with leukopenia; the latter being in agreement with our study. The observed leucopenia in the animals may be attributed to depression of leucopoiesis, alteration in the cell membrane or disintegration of WBC [36]. Leukopenia usually occurs when WBC is reduced by infection or by treatment such as chemotherapy or radiation therapy, or when a hematopoietic stem cell abnormality does not allow normal growth/maturation within the bone marrow. The WBC count is a biomarker of systemic inflammation as the cells are involved in the regulation of immunological function in most organisms [34]. The leukopenia observed is primarily due to a decrease in the numbers of the mononuclear cells, lymphocytes and granulocytes. The observed lymphopenia might be due to the direct toxic action of AlP on leucopoiesis in lymphoid organs [65]. As suggested by Ambali et al., 2011 in their study involving chlorpyrifos, AlP could have evoked lymphopenia either by decreasing lymphocyte production and/or increasing its rate of removal through rapid destruction. Additionally, AlP ROS-induced deregulation of monocytes programmed cellular death may play a part in the observed leukopenia in our study, as ROS is reported to play a role in programmed cell death [66,67]. The observed decrease in these defense cells in AlP-exposed rats is indicative of an immune response suppression and immunotoxic effect of AlP in the rats. In contrast to lymphocyte and granulocyte, monocyte numbers were not altered by AlP. Hesperidin in this study, significantly improved WBC count in AlP exposed rats while restoring the granulocyte and monocyte to level comparable to the control. Thrombocytic indices in the present study indicated that AlP induced thrombocytopenia in the rats. this is consistent with the report of Fayyaz that reported thrombocytopenia in patients exposed to AlP [68]. The level of platelets in this study was significantly lowered by AlP intoxication when compared to the normal group. Platelets play an important role in hemostasis where they form the primary plug sealing vascular defects and provide the requisite phospholipid surface for the recruitment and activation of clotting factors. Thrombocytopenia occurred in parallel with anemia in the AlP-intoxicated rats with decreased platelet count. Thrombocytopenia is known to develop in response to either antibody production against attached viral antigens on platelet surfaces or nonspecific binding of antigen-antibody complexes to platelet surfaces [69]. Several mechanisms have been adduced to xenobiotic-induced thrombocytopenia, including marrow suppression of megakaryocytes or generalized marrow stem cell suppression [70] and increased platelet destruction and consumption resulting in impaired platelet function [45]. Pesticides, like many other environmental toxins, are known to generate oxidative stress and data have suggested that reactive oxygen species (ROS) production plays a significant role in the toxicity of these compounds [71,72]. Oxidative stress results when increased intracellular production of ROS overwhelms the cellular antioxidant defense systems. This is usually followed by increased cytotoxicity and oxidative degradation of tissues. Normal levels of ROS are known to play important roles in ovarian physiology whereas; abnormally high levels have detrimental effects on the organ [73]. Oxidative stress resulting from the increased generation of free radicals or decreased antioxidant system activity is a major factor responsible for reproductive impairment [74]. Besides generating lipid peroxidation in ovarian follicles, oxidative stress also promotes granulosa cell apoptosis which can cause follicular atresia and reduction in oocytes number, while also damaging their quality [75,76].Pesticide-induced female infertility has been ascribed to some factors among which is oxidative stress, due to the serious threat posed to the reproductive system by free radicals [74]. Pesticides perturb oxidative balance by generating ROS leading to oxidative stress. In the present study, AlP disrupted the ovarian oxidative balance by significantly increasing MDA and LOOH levels, both markers of oxidative stress. The elevated levels of ovarian MDA and LOOH imply that AlP exposure induces oxidative stress primarily due to enhanced lipid peroxidation production, with consequential disruption of the lipid bilayer of the cell membrane leading to cellular dysfunction [77]. Although the information on the effect of AlP on ovary is scanty, the data from this study are in agreement with previous studies on the ability of environmental chemical agents like pesticides, to induce increased generation of ROS in the ovary [78-80]. Production of lipid peroxides through the interaction of ROS with the unsaturated lipids of the ovarian cells produces degradation products with toxic aldehyde moieties such as MDA which could interfere with the ovarian reproductive system. The increased generation of lipid peroxidation products as observed with the high levels of LOOH in AlP exposed rats may also be due to the indirect effect of the phosphide on free radical scavenging enzymes and glutathione status. This is plausible as phosphine, a product of phosphide degradation in the presence of moisture, has been reported to have a high affinity for sulfhydryl groups in these enzymes and molecules [81]. In addition, phosphine ability to impair mitochondrial functions by the disruption of the electron transport chain may also be a factor [13]. Induction of ROS in the ovary by AlP is of great import as environmental factors have been linked with the initiation of antral follicle apoptosis which has a far-reaching effect on the reproductive function of the ovary [73]. Other studies have also shown that exposure to agents that can cause oxidative stress can rapidly reduce the number of follicles and oocytes in animals with consequent reproductive dysfunction [76,82]. Along with the increase in lipid peroxidation, our study also showed a significant decrease in the activities of ovarian antioxidant enzymes (SOD, CAT,) and the lowering of the cellular redox potential as depicted by a decrease in GSH levels. This is consistent with the findings of Kariman et al. [8] that AlP exerts its toxicity by not only increasing the rate of lipid peroxidation but by also inhibiting the endogenous antioxidant system. The antioxidant enzymes are the first line of defense against free radical attack in the cell and the inhibition of their activities might have further contributed to the oxidative insult experienced by the tissue. SOD scavenges ROS by catalyzing the dismutation of superoxide radicals to the less toxic hydrogen peroxide [83,84]. The inhibition of its activity may increase superoxide radicals, contributing in part to the observed increased lipid peroxidation in the ovary of AlP-exposed rats. The lowered SOD activity resulting from AlP exposure could cause an imbalance in ROS levels in the follicular fluid which is physiologically required for normal ovarian functions. A threshold value for ovulation might be impeded following reduced SOD activity [85]. The ovarian CAT activity was also inhibited following AlP exposure. CAT helps in the detoxification of hydrogen peroxide to non-toxic end-products, but its inhibition by AlP in this study indicated that its peroxide radicals scavenging ability has been compromised which could result in the formation of highly reactive hydroxyl radicals [13]. The accumulation of H2O2, which is a potent oxidant, could result in the disruption of steroidogenesis in the ovarian cells [71]. Chromosomal defects and DNA damage could also be induced in the oocyte nucleus following CAT inhibition [86]. The data in the present study indicated that AlP exposure decreased ovarian GPx activity. GPx along with CAT metabolizes H2O2 to water and molecular oxygen and has been reported to play significant roles in gametogenesis and in vitro fertilization [85,87]. The reduced activity of this enzyme could, therefore, impair these important processes. The reduction may not be unconnected with the depletion of the ovarian GSH content, a cysteine-containing antioxidant, through the utilization in combating the oxidative stress induced by AlP exposure. GSH, with a reducing activity in its thiol group, scavenges free radicals either through direct chemical reactions or through reduction of peroxides as a cofactor for GPx [88,89]. Our results thus, further confirm earlier reports from several investigations indicating that AlP alters the activity of cellular antioxidants [15]. However, treatment with HSD prevented the AlP-induced inhibition of SOD and CAT activities, depletion of GSH, and the increase in MDA and LOOH concentrations in the ovary. HSD has been reported to provide strong cellular antioxidant protection against chemical-induced oxidative damage [50,90]. Histopathology of rat ovaries revealed atrophied and atretic ovarian cells, along with wasted, disorganized and atretic follicles. There were also observed severe degenerative changes in the ovary in addition to necrotic ovarian cells and appearance of hemorrhoid. HSD treatment was able to ameliorate much of the AlP-induced damage by preserving the histological architecture of the ovary, majorly through its ability to neutralize ROS in this tissue. These results are consistent with previous studies that reported the ability of HSD in protecting against ovarian toxicity induced by chemical agents [91-93].
5. Conclusions
- The findings of the present study indicate that AlP exposure provoked hematotoxicity while also inducing oxidative stress in the ovaries of Wistar rats. However, co-treatment with HSD restored the hematological indices while protecting the rat ovary from the AlP-induced toxicity. It is posited that HSD mitigated the hematotoxicity and ovarian oxidative stress through its ability to scavenge and trap free radicals generated by AlP exposure. HSD thus, represents a potential therapeutic agent in protecting against AlP-induced toxicity.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML