-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2018; 8(5): 93-99
doi:10.5923/j.ajb.20180805.02

Role of Serum Trypsin Level in Diagnosis and Prognosis of Pancreatitis and Compared with Healthy Subjects of Rajasthan
Hemlata Sharma, RK Vyas, Shalini Vyas
Department of Biochemistry, Sardar Patel Medical College, Bikaner, Rajasthan, India
Correspondence to: Hemlata Sharma, Department of Biochemistry, Sardar Patel Medical College, Bikaner, Rajasthan, India.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Inflammation of pancreas is called pancreatitis. Cell death is induced directly by intracellular trypsin which is very harmful. Aim of this study was to find out the role of serum trypsin level in diagnosis and prognosis of pancreatitis. Material and methods: 200 subjects were selected out of them 100 were selected as study group and 100 were selected as control group and distributed according to their age and sex. Serum trypsin was estimated by sandwich elisa kit method. Result: meantrypsin level was found to be 587.94 ng /ml in male patients group as well as in all male control group. Value of trypsin was 186.28 ng/ml in female patients group and mean serum trypsin level was 696.34 and mean value of serum trypsin in female control group was 206.20. The P value was Highly significant in this group. (P<0.0001). Serum trypsin level was calculated in many other groups and compared with healthy subjects and each and every time the level of serum trypsin was found to be increased in all patients. Conclusion: We came to the conclusion that serum trypsin activity looks specific and can be used for diagnosis of Alcoholic and gall stone pancreatitis.
Keywords: Inflammation, Pancreatitis, Trypsin, Sandwich elisa, Alcoholic pancreatitis, Gallstone pancreatitis
Cite this paper: Hemlata Sharma, RK Vyas, Shalini Vyas, Role of Serum Trypsin Level in Diagnosis and Prognosis of Pancreatitis and Compared with Healthy Subjects of Rajasthan, American Journal of Biochemistry, Vol. 8 No. 5, 2018, pp. 93-99. doi: 10.5923/j.ajb.20180805.02.
Article Outline
1. Introduction
- Inflammation of pancreas is called pancreatitis. [1] Pancreatic acinar cell distruption is considered as premost initiating event in the pathogenesis of acute pancreatitis. [2] acute pancreatitis is a disease with multiple etiologies requiring various approaches regarding its diagnosis as well as treatment was not appreciated till the mid of 20th century. Recently gallstone and alcohol account for 35%-40% and 30% respectively as etiologic factor of acute attacks of pancreatitis. [3] cell death is induced directly by intracellular trypsin which is very harmful. Trypsin is formed in small intestine where trypsinogen is activated by pancreas. Three forms of this disease; mild, moderate and severe is classified by revised Atlanta classification. [4, 5]REVISED DEFINITION AND CLASSIFICATION OF ACUTE PANCREATITIS – in clinical and research communications the following definition and classification are used.DIAGNOSIS OF ACUTE PANCREATITIS-DEFINITION- Two of the following three features are used in the diagnosis of acute pancreatitis. We can say that the diagnosis is dependent upon these 3 features:1. Pain of abdomen with pancreatitis (severe epigastric pain radiating to the back)2. Lipase activity of serum/ amylase activity of serum at least more than three times of upper limit of normal range.3. Characteristic findings of acute pancreatitis like CECT or MRI or ultrasonography of abdomen. [6-10]
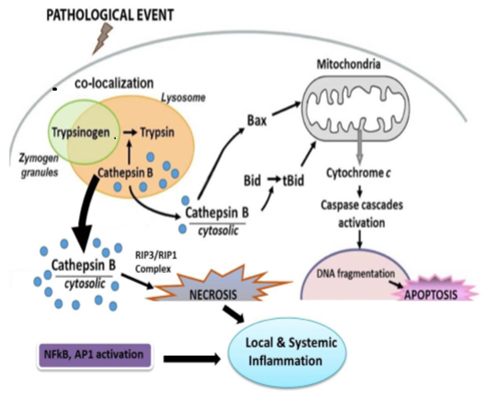 | Figure 1. Schematic representation of major pathophysiologic events in pancreatitis |
2. Materials and Methods
- The present study was conducted on 200 subjects (age between 15-75 years) in the Biochemistry Department of Sardar Patel Medical College and Surgery and Gastroenterology Department of PBM hospital. 100 subjects were pancreatitis suspected or clinically confirmed pancreatitis patients (by radiological findings) suffering from abdominal pain. Severity of disease was calculated by glassgow criteria and 100 normal persons were selected as control group. Blood was collected in a clean and dry test tube and samples were allowed to clot for 2 hours at room temperature before centrifugation. The supernatant was collected and the assay was carried out immediately. Trypsin was estimated by kit based on sandwich enzyme linked immunosorbent assay technology.
2.1. Design of Study a Case-control Analytical Study
2.1.1. Setting
- This study was carried out in the Department of Biochemistry in collaboration with the Department of General surgery and Gastroenterology at S.P. Medical College and Associated group of P.B.M. Hospitals, Bikaner. All participants completed a medical history form and provided informed consent.
2.1.2. Study Population
- Study included 200 subjects. 100 were study group and 100 were control group subjects. These patients were suffering from abdominal pain and were pancreatitis suspected and clinically diagnosed (with radiological findings) pancreatitis patients. In group 2nd patients with gallstone with abdominal pain and pancreatitis suspected or clinically diagnosed (with radiological findings) were selected. In this study 75% patients were males and 25% were females. Patients were distributed age wise in which 42% patients were between 15-55 years and 58% patients were between 56-75 years.
2.1.3. Study Period & Study Approval
- Present study was done from June 2016 to June 2018. The Institutional Ethical Committee at the Sardar Patel Medical College and Associated Group of P.B.M. Hospitals, Bikaner, Rajasthan, India approved the study. The Developmental Research Committee at the Rajasthan University of Health Sciences, Jaipur, India also approved the study.
2.1.4. Study Protocol
- The following criteria are undertaken for the selection of the patients in the study:- Inclusion Criteria: depends according to Atlanta symposium 1992. Patients with abdominal pain and having gallstone or alcoholic age between 15-75 and having clear radiological findings of abdomen were included. Exclusion Criteria: Serious ill/moribund patient, Severe hepatic or renal disease, Cancer, Severe psychiatric disorder (e.g. schizophrenia), Stroke, Pregnant women, Autoimmune diseases, Familial hyperlipidemia Patients with a history of any other complicated disease were excluded. Patients with history of any sugery case which could have an impact on pancreatic function were excluded. Chronic smokers were excluded.Principle of assay- This kit was based on sandwich enzyme linked immunosorbent assay technology. Anti-TRY antibody was precoated onto 96-well plates and the biotin conjugated anti-TRY antibody was used as detection antibodies. The standard, test sample and biotin conjugated detection antibody were added to the wells subsequently and washed with wash buffer. HRP-striptividin was added and unbound conjugates were washed away with wash buffer. TMB substrates were used to visualize HRP enzymatic reaction. TMB was catalyzed by HRP to produce a blue coloured product that changed into yellow after adding acidic stop solution. The density of yellow is proportional to the TRY amount of sample captured in plate. Read the O.D absorbance at 450 nm in a microplate reader and then the concentration of TRY can be calculated.
3. Summary of Procedure
- 1. Wash plate 2 times before adding standard, sample and control (zero) wells2. Add 100μL standard or sample to each well for 90 minutes at 37°C 3. Add 100μL Biotin- detection antibody working solution to each well for 60 minutes at 37°C4. Aspirate and wash 3 times 5. Add 100μL SABC working solution to each well. Incubate for 30 minutes at 37°C 6. Aspirate and wash 5 times 7. Add 90μL TMB substrate. Incubate 15-30 minutes at 37°C8. Add 50μL Stop Solution. Read at 450nm immediately9. Calculation of results
4. Observation Tables
|
|
|
|
|
|
|
|
|
5. Result
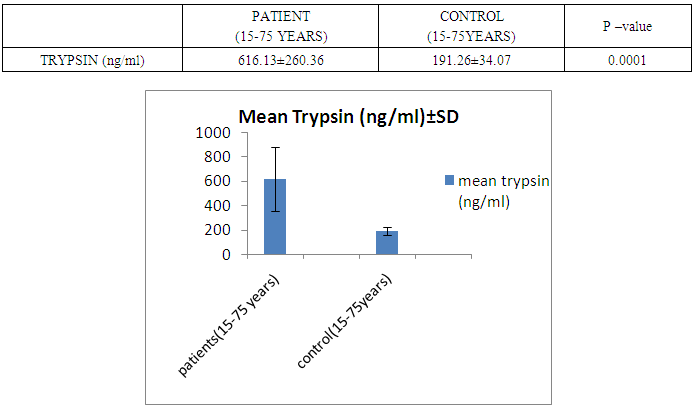
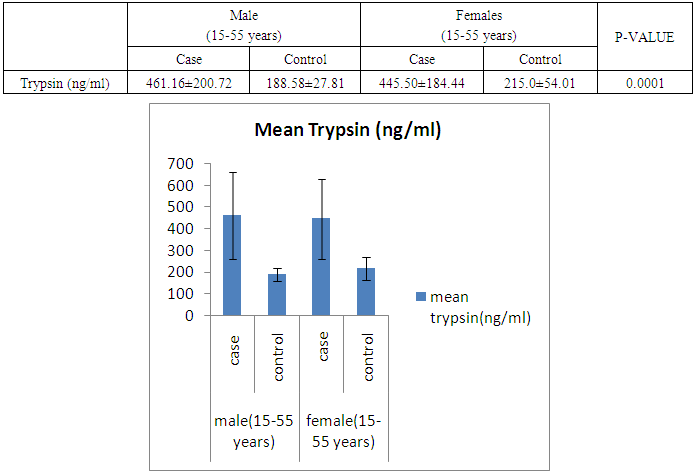
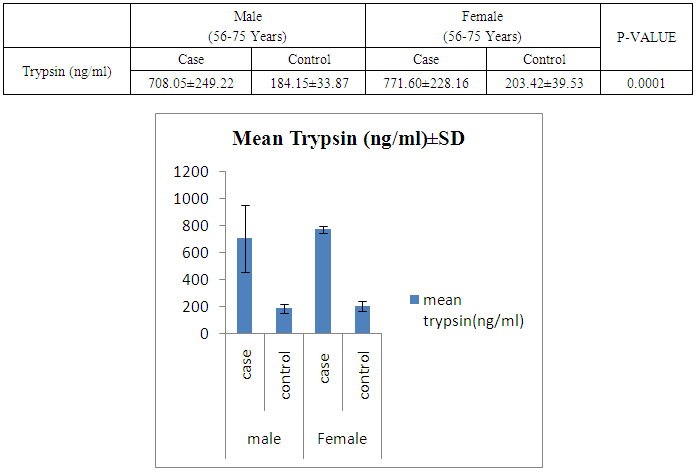
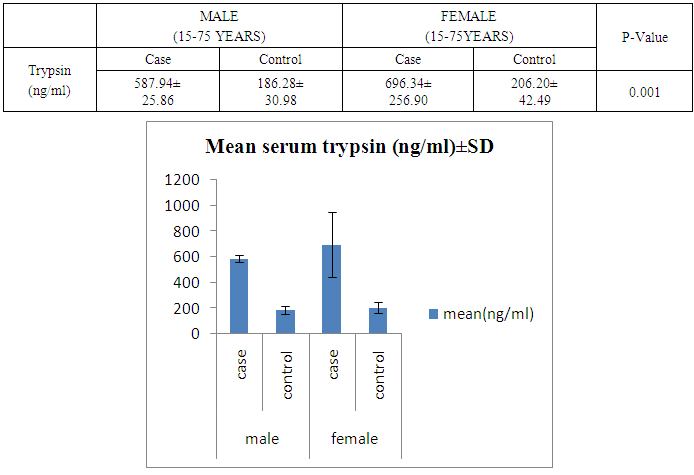
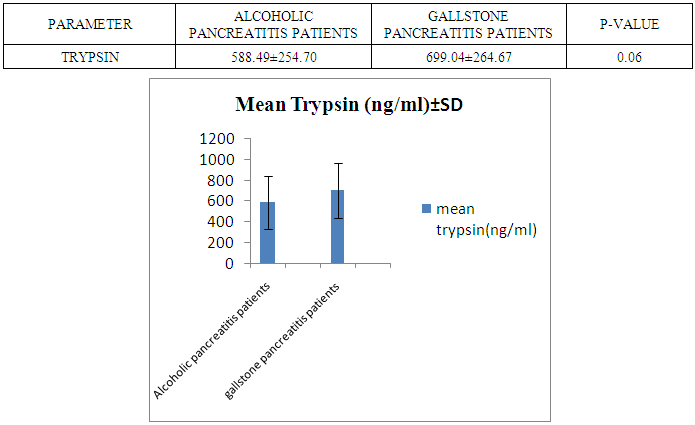
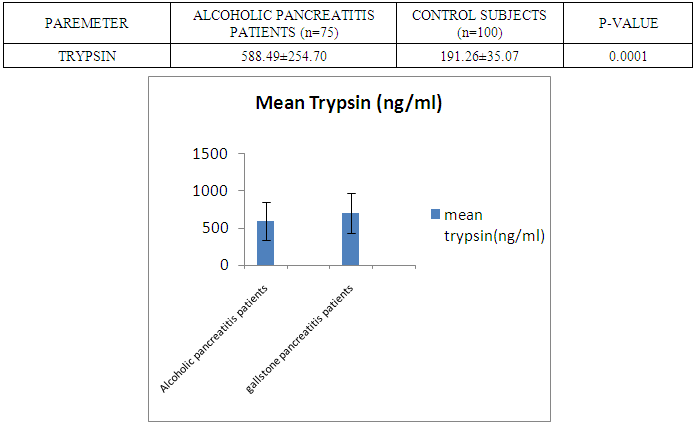
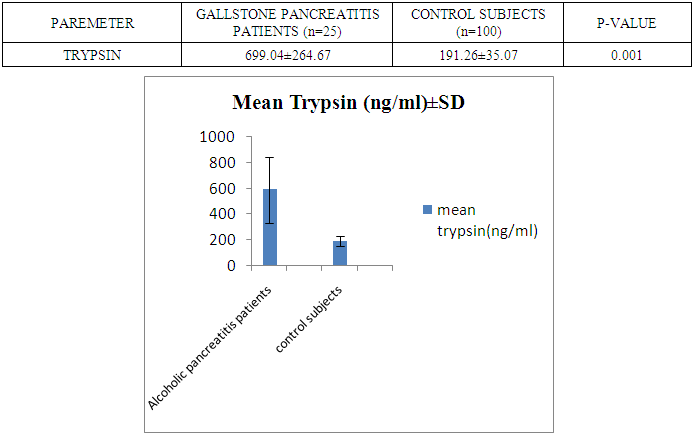
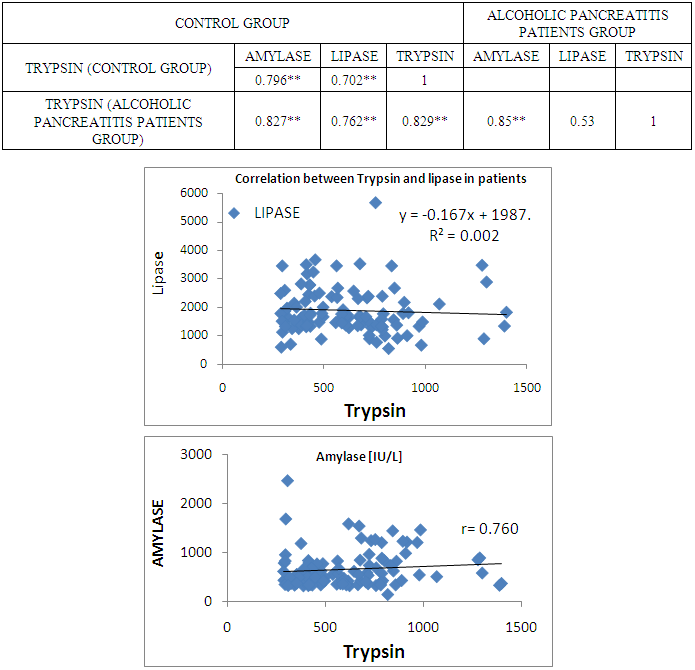
- The present study was conducted on 200 subjects aged between 15-75 years male and female groups in the Department of Biochemistry of SPMC, Bikaner and Department of Surgery and Department of Gastroenterology of PBM Hospital. 100 subjects with abdominal pain and gallstone or severe alcoholic with radiological findings of pancreatitis was selected in this study and 100 normal persons were selected as control group. Severity was measured by glassgow criteria by available clinical history of patients. Table 1 depicts comparison of mean serum trypsin level in patients (n=100) and control group (n=100). Mean ± sd value of serum trypsin was 616.13 ± 260.36(ng/ml) in patients and in control group this value was 191.26 ± 34.07 (ng/ml). P- value was found to be highly significant when compared with control group of same age group. Serum trypsin level was found very high than control group.In table 2 the comparison was done according to their age and sex in both subjects (patients and control). mean serum trypsin level was 461 ± 200.72(ng/ml) in male patients (age group 15-55 years) and in control of same age group the mean value of trypsin was 188.58 ± 27.81(ng/ml), as well as in female patients (age 15-55 years) the mean serum level was 445.50 ± 184.44 (ng/ml) and in control females of same age group it was 215.0 ± 54.01(ng/ml). The p- value was found to be highly significant. (P≤0.0001) when compared with healthy subjects.In table 3 in male patients of age group 56-75 years, mean value of serum trypsin was 708.05 ± 249.2 (ng/ml) and in control male subjects of same age group it was 184.18 ± 33.87 (ng/ml), which was highly significant, as well as in female patients of same age group serum trypsin concentration was 771.60 ± 228.16 (ng/ml) and in female control group of same age group it was 203.42 ± 39.53 (ng/ml). The p-value was found to be highly significant (P≤0.0001) when compared with healthy subjects of same age group.In table 4 mean trypsin level in all male patients was found 587.94 ± 25.86 (ng/ml) and in whole male control group the mean value of serum trypsin was 186.28 ± 30.98 (ng/ml) as well as in all female patients group, it was 696.34 ± 256.90 (ng/ml) and in female control group the mean value of serum trypsin was 206.20 ± 42.49 (ng/ml) the P-value was found significantly high in this group (P≤ 0.001) serum trypsin was found increased in study group. As table 5 shows patients who were suffering from alcoholic pancreatitis, mean value of serum trypsin was found 588.49 ± 254.70 (ng/ml) and in patients who were suffering from gallstone pancreatitis mean value of trypsin was 699.04 ± 264.67 (ng/ml). Comparison was not so significant. In this P –value was found 0.06 in this group.As represented in table 6, the mean serum trypsin level of patients (alcoholic pancreatitis, (n=75) was 588.49 ± 254.70 (ng/ml) and in control subjects (n=100), it was 191.25 ± 35.07 (ng/ml). The comparison was done in both the groups. The P-value was statistically highly significant in both groups. In table 7 serum level of trypsin in gallstone pancreatitis patients and control subject was compared. Table 8 depicts the correlation coefficient analysis between mean serum trypsin level and other biochemical parameters. Serum trypsin was positively correlated with amylase (r=0.760), lipase (r=0.629), total bilirubin (r=0.772), AST (r=0.712), ALT (r=0.789), ALP (r=0.767). The correlation of trypsin with other parameters was highly significant.Table 9 depicts the correlation between serum trypsin and other biochemical parameters level in control and alcoholic pancreatitis patients. The correlation between serum trypsin and amylase in control group (r=0.796) and correlation of serum trypsin and lipase in control group (r=0.702) was statistically significant. In alcoholic pancreatitis patients group, there was significant correlation of trypsin with amylase (control group) (r=0.827), lipase (control group) (r=0.762), trypsin (control group) (r=0.829) which was positively correlated as well as mean trypsin level of alcoholic pancreatitis patients group was correlated with amylase and lipase of same group. The r value of serum trypsin and amylase was 0.85 which was found to be significant in this group and correlation of serum trypsin with lipase was done, the r value was 0.53.
6. Discussion
- In our study, trypsin level was found to be increased in all patients when inflammation of pancreas occurs. Serum trypsin is formed in small intestine when its pro enzyme form the f level of serum trypsin level is found in pancratitis. In our study, we observed that in elder patients (male or female) serum trypsin level is found increased than younger age group and control group. The increase in serum level of trypsin is the conversion of trypsinogen to active trypsin is mediated by the relaease of its activation peptide. Trypsin then activates other pancreatic enzymes which leads to tissue damage and eventually autodigetion of pancreatic cells or pancreas. [12] Correlation of trypsin with other pancreatic enzyme was found to be highly significant. The r-value between trypsin and amylase or lipase was positively correlated. Ventrucci M et al (1983) described that in acute pancreatitis serum amylase, pancreatic amylase and salivary isoamylase, lipase and trypsin –like immunoreactivity had same diagnostic sensitivity. [13] Acute pancreatitis occurs due to reduced trypsin activity or intracellular protective mechanism to prevent trypsinogen activity are overwhelmed. [14] In gallstone pancreatitis pathogenesis, pancreatic duct obstruction plays a major role which increases pancreatic duct pressure, bile reflux, trypsin activation and pancreatic autodigestion. [15] Occurence of repeated episodes of acute pancreatitis with cellular necrosis according to necrosis –fibrosis hypothesis eventually leads to the development of chronic pancreatitis as the necrotic tissue fibrosis is replaced by healing process. A major protective mechanism in preventing inappropriate activation of pancreatic enzyme cascade by inihibiting up to 20% of trypsin activity by SPINK-1 which is a potent protease Inhibitor. [16] The radioimmunoassay of trypsin measures its immunological concentration and not its enzymatic biological activity (Elias et al., 1977; Temler and Felber, 1976; Borgstrom and Ohlsson, 1978; Lake Bakaar et al., 1980a). [17-20] our study was based on sandwich enzyme linked immune sorbent assay technology. TMB substrate was used to visualize HRP enzymatic reaction. This assay has high sensitivity and excellent specificity for detection of trypsin. Talukdar et al evaluated the mechanism by which trypsin induces cell death in acinar cells and observed that trypsin makes co-localized vesicles fragile which causes cathepsin B to escape from co-localized organelles into the cytosol, which in turn cause cell death during pancreatitis. [21] Evaluating the role of trypsin activation, Gaiser et al [22] showed that expression of active trypsin in pancreas was sufficient for induction of acute pancreatitis. This study was closely related with our study. Another aim of study was to evaluate relationship between trypsin and other parameters in pancreatitis. Trypsin level was positively correlated with amylase and lipase.
7. Conclusions
- We conclude that serum trypsin activity looks specific to acute alcoholic pancreatitis and gall stone pancreatitis. It can be used for the diagnosis of pancreatitis. It is suggested that trypsin is a specific test, so it should be prospectively measured in different types of pancreatitis. Further research is encouraged to determine the efficacy of applying this biomarker for diagnosis and treatment in a clinical setting.
ACKNOWLEDGEMENTS
- Dr. R.K. Vyas, Sr. Professor & Head, is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.This work was supported by Dr. R.P. Agarwal, Principal, Dr. bhupendra Sharma, Dr. sushil phalodia Sardar Patel Medical College, Bikaner.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML