-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2018; 8(5): 87-92
doi:10.5923/j.ajb.20180805.01

A Biochemical Study of the Effect of Quercetin on Cisplatin Induced Rat Tissues Toxicity
Mahdi Ali Abdullah1, Ali Ashgar Abd2, Semaa Ahmed Baker2
1Deparment of Pathology and Microbiology, Duhok University, Duhok, Iraq
2Deparment of Biology, College of Education for Pure Science, Mosul University, Mosul, Iraq
Correspondence to: Mahdi Ali Abdullah, Deparment of Pathology and Microbiology, Duhok University, Duhok, Iraq.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The present study was aimed to analyze the protective effectof quercetin againstcisplatin-induced hepatotoxicity, nephrotoxicity and cardiotoxicity in male albino rats. The animals were divided into six groups of 6 animals in each group and treated as follow: a control group, quercetin (50mg/kg), cisplatin (4 and 8 mg/kg, I.P), and a groups that received both quercetin and cisplatin. Cisplatin induced a significant increase in ALT, AST, LDH, ALP, Cholesterol,Triglycerides, Urea and Creatinine with both serum and homogenize tissues. However, both Glucose and Albumin levels were significantly decreases. Conversely, quercetin significantly modulated hepatotoxicity, nephrotoxicity and cardiotoxicity by the effect with oxidative markers. The study showed that the Quercetin could be promising antioxidants for reducing the risk of cisplatin induced nephrotoxicity, cardiotoxicity and hepatotoxicity.
Keywords: Cisplatin, Chemical analysis, Iraq, Quercetin, Toxicity
Cite this paper: Mahdi Ali Abdullah, Ali Ashgar Abd, Semaa Ahmed Baker, A Biochemical Study of the Effect of Quercetin on Cisplatin Induced Rat Tissues Toxicity, American Journal of Biochemistry, Vol. 8 No. 5, 2018, pp. 87-92. doi: 10.5923/j.ajb.20180805.01.
1. Introduction
- Cisplatin (Cis-dichloro diammine platinum) CDDP, is a commonly used as chemotherapeutic drugs for treatment of several solid tumor [1]. However, cisplatin has serious side effects on normal a cell which is causes toxicity of many of organs [2, 3]. This toxicity is closely associated with an increase in lipid peroxidation and reactive oxygen species (ROS) in the tissues as a result of increase and decrease of endogenous enzymes in the body systems [4]. Excess ROS causes significant oxidative damage by attacking bimolecular such as membrane lipids, DNA, and proteins in cells [5]. The oxidative stress is associated with many disease states including neurological diseases such as Alzheimer’s brains and Parkinson’s disease, chronic heart disease, and kidney and liver diseases [6]. The most endogenous antioxidants and tissues enzymes are related to oxidative tissue as glutathione (GSH), glutathione peroxidase (GSH-PX), superoxide dismutase (SOD), catalase (CAT), alanine aminotransferase (ALT), blood urea nitrogen (BUN), albumin, creatinine and aspartate aminotransferase (AST) are compounds that act as free radical scavengers. Recently many of natural products, such as lycopen, grape seed extract, green tea caffeic acid phenethyl ester and royal jelly are demonstrated to have a protective role against cisplatin-induced kidney and liver oxidative damages [7, 8, 6].As well as Quercetin is a safe and common dietary flavonoid found in onions, apples and tea [4]. It is able to scavenge oxygen radicals, inhibit lipid peroxidation and exert as anti-carcinogenic activities [9]. Few studies were found dealing with the role of Quercetin on the biochemical alteration of renal and hepatic toxicity of cisplatin (19). Therefore, this work was designed to assess the productive effect of orally administered of Quercetin against cispaltin induced nephrotoxicity, cardiotoxicity and hepatotoxicity.
2. Materials and Methods
- 1- Animals and Experimental protocolThirty six Male Albino Wister rats, weighting 200- 300 gm were used in this study; they were obtained from and maintained in the college of veterinary medicine university of Duhok, they were housed at temperature 20-25°C and fed commercial pellets with tap water. The Rats were randomly divided into 6 groups each groups (6 rats) and treated as follow:Group I: received single dose I.P (intraperitoneal) of distilled water 2 ml as a control.Group II: received single dose I.P of cisplatin (4mg/kg).Group III: received single dose I.P of cisplatin (8mg/kg)Group IV: received single dose I.P of cisplatin (4mg/kg) and Quercetin 50mg/kg once daily for 7 days before and 7 days after a single dose of cisplatin 4 mg/kg I.P. Group V: received single dose I.P of cisplatin (8mg/kg) and Quercetin 50mg/kg once daily for 7 days before and 7 days after a single dose of cisplatin 4 mg/kg I.P. Group VII: received oral dose of Quercetin 50mg/kg daily respectively for 7 successive. Animals were anesthetized by ether, the blood was collected directly from the retro-ocular vein [10]. After that rats were slathered and blood poured into plain tubes, the clot was dispersed with glass rod and then centrifuged at 3000 rpm for 15 minute and placed in -20°C. The serum was used for the estimation of biochemical test. While internal organs liver and kidney keep in -20°C for homogenizing. 2- Biochemical studyThe Biochemical test used in this study included these enzymes alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), alkaline Phosphatase (ALP), Glucose, Albumin, Cholesterol, Triglycerides, Urea and Creatinine.At the end of experiment blood samples were obtained from retro-ocular vein the blood was allowed to clots for 20 min and centrifuged at 3000 rpm for 10 min then the Serum samples were used from all groups for determination of different biochemical analysis, the colorimetric methods used with UV Visible Spectrophotometer width length 505 nanometer (Jenway Ltd, U.K) according to the different standardized commercially available kits each of one from different company. Tissue homogenate from each organs ( kidney and liver) was prepared and storage at -20°C then taken one gram from each organs after removed, cleared from adhering connective tissue; and homogenize using Ultrasomic Homogenize ( Sonic Rupture 400, U.S.A) with 10ml of 0.1M Tris- HCL buffer PH 7.4 according to [11]. The homogenates were Centrifuge -4°C (D3024R with AS24-2 aluminum alloy rotor Kit, China) at 3000 rpm for 15 min and the supernatants obtained were transferred into eppendorf tubes, and preserved at -20°C in a deep freezer until used for biochemical analysis.The study was analysis different of biochemical biomarkers by using specific kits for each marker and according to its manuscripts.Statistical AnalysisThe data were analyzed using one-way analysis of variance (ANOVA) followed by post- hoc Duncan multiple range test (36). Comparison test for comparing means from different groups. The data were expressed as mean ±SEM and a value of P<0.05 was considered statistically significant [12].
3. Results
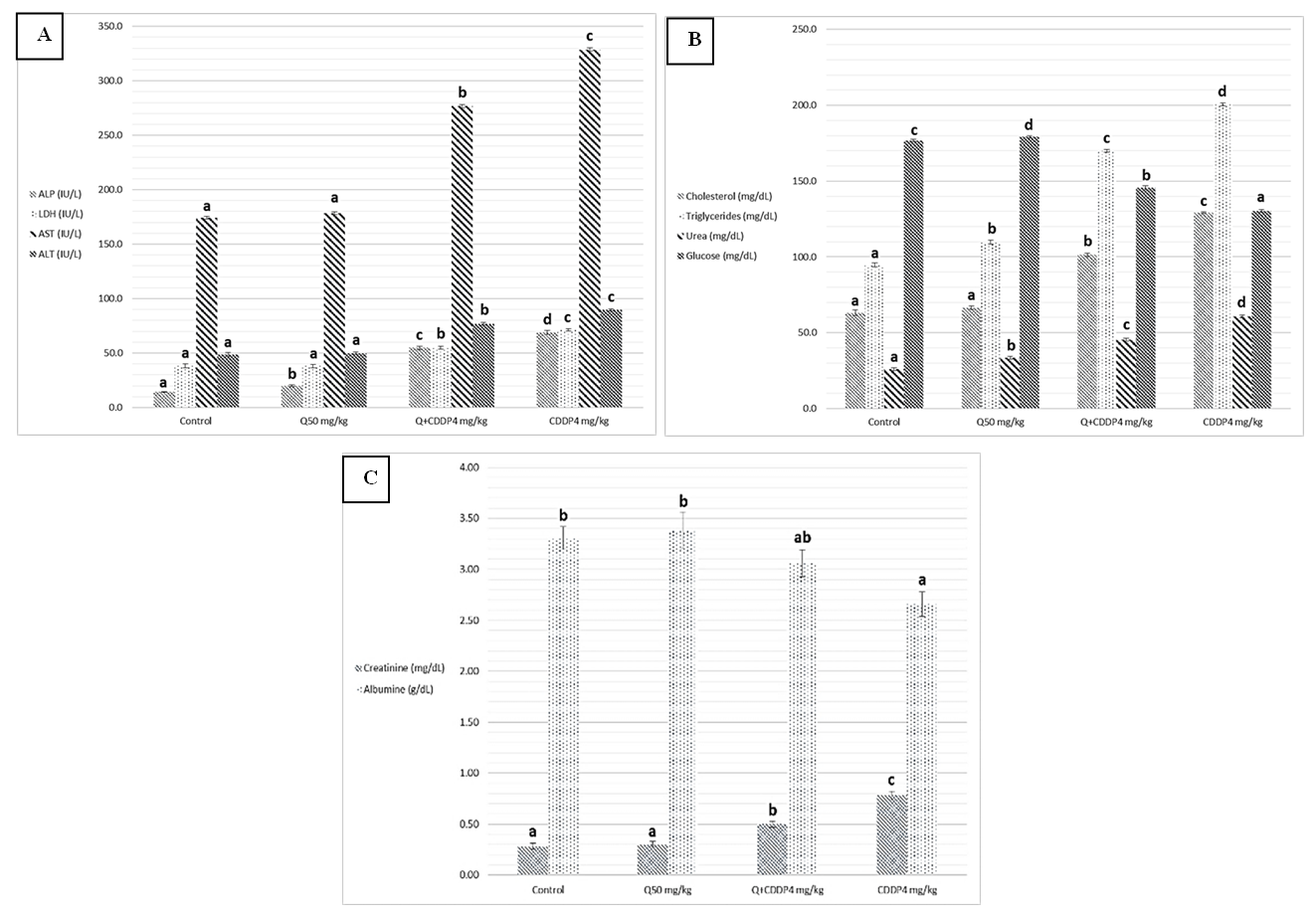
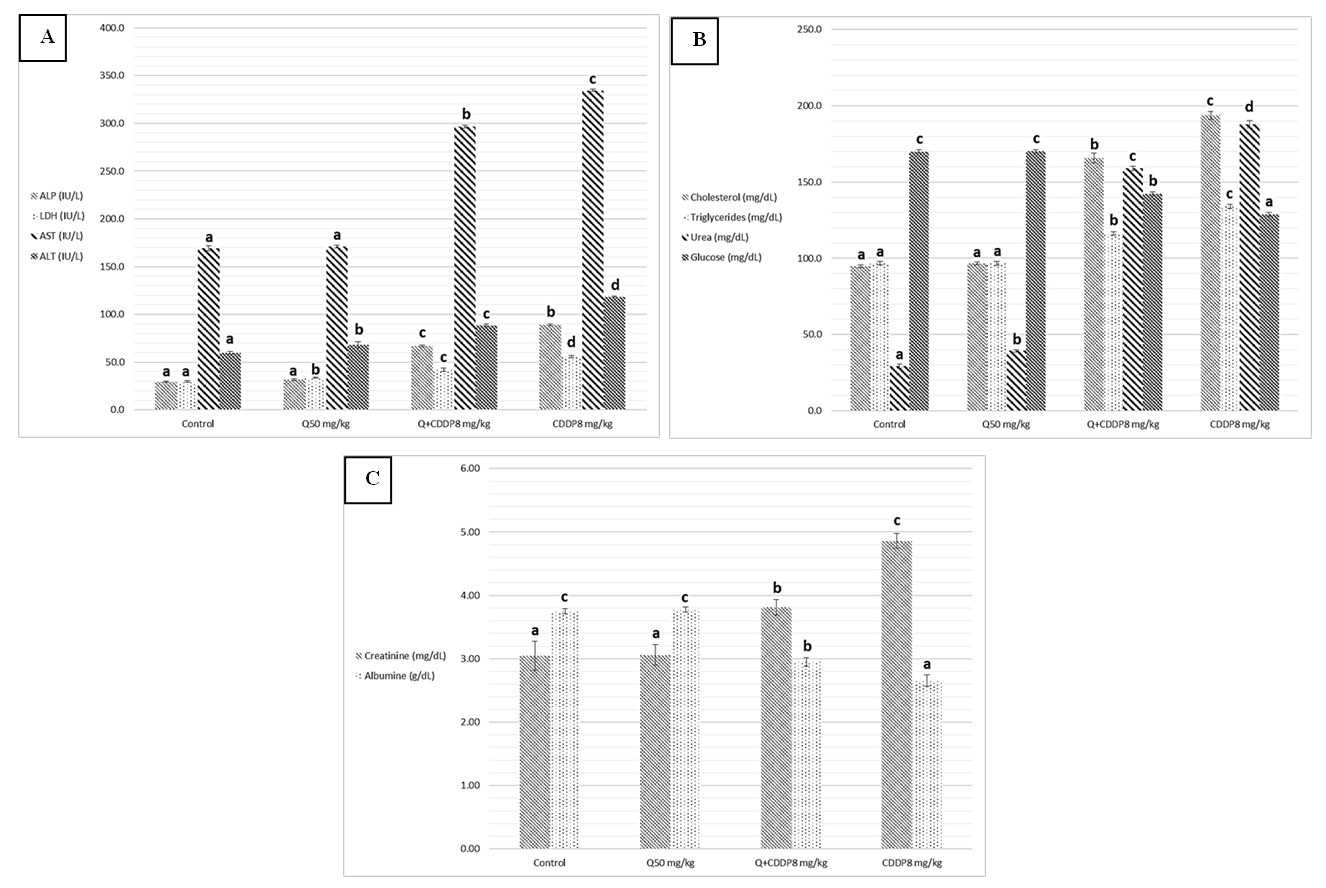
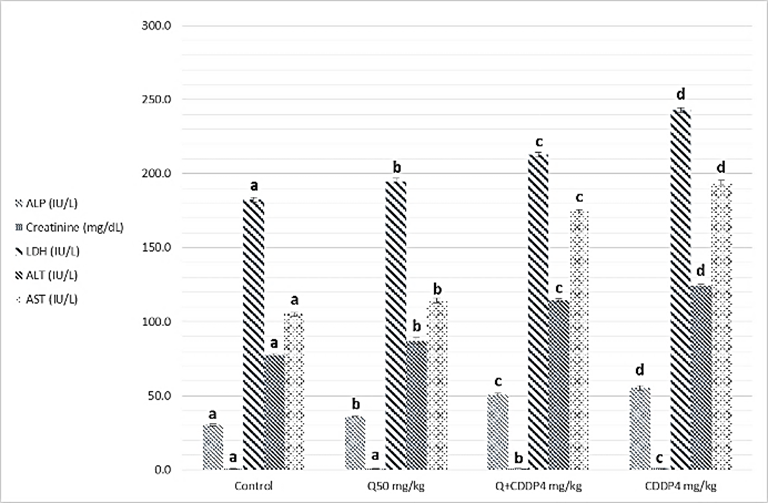
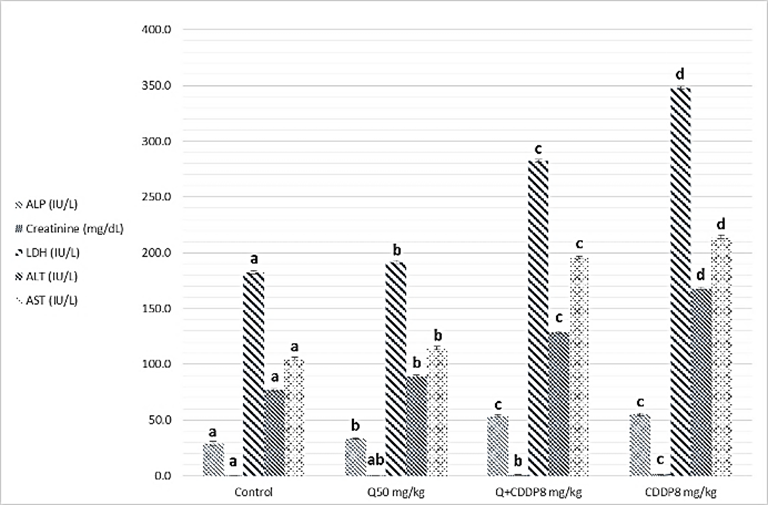
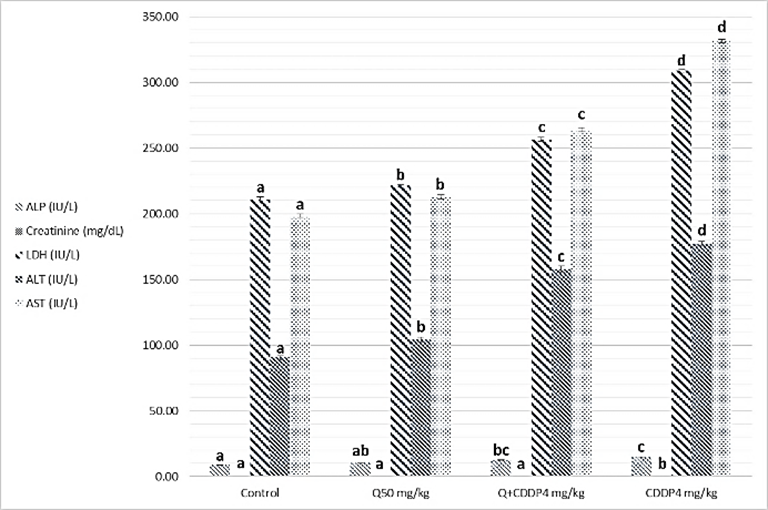
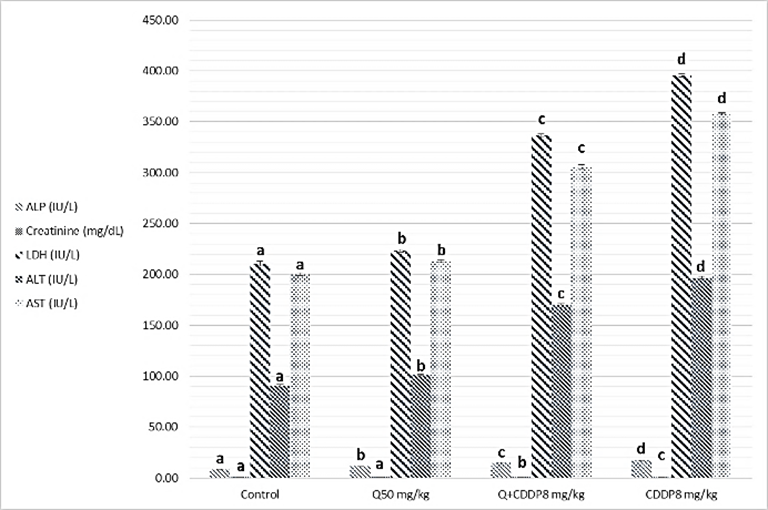
- Results of biochemical analysis showed a significant increase levels of ALT, AST, ALP, LDH, urea, cholesterol, Triglycerides and creatinine in serum with cisplatin group at both doses four and eight mg/kg, compared to the control groups, Quercetin and Quercetin with cisplatin groups as showing in figure 1 and 2 (A, B and C), while analysis of biochemical parameters of homogenize tissue showed a significant increase levels of ALT, AST, ALP, LDH and creatinine concentrations only (p <0.005) as showing in figure (3, 4, 5 and 6). In other hand the level of both Glucose and albumin serum was significantly decreased (p <0.005) as showing in figures 1 and 2 (B and C).
4. Discussion
- Among the biochemical aspects, the increase level of many enzymes as AST, ALT, ALP, creatinine, urea, cholesterol, Triglycerides and LDH in serum and homogenized tissue of cisplatin treated rats group was found to be related with damage of the liver and kidney which are conversely associated to the functions and structure of organs especially with high dose compared to low dose of cisplatin as well as compared to groups of cispaltin treated with Querecitin, Querecitin alone and control groups. The elevation of such enzymes levels in both serum and homogenize tissue has been attributed to the damaged and revealed dysfunction of renal and hepatic cells because these enzymes are normally found in the cytoplasm of these cells and are released into the circulation when damage of cells as well as renal failures caused by the administration of cisplatin as a result of reduced activities of several enzymes that play a role in renal function, such as adenosine triphosphate production which serve as uncoupling of the mitochondrial oxidative phosphorylation [13-15]. In the same time the results were showed a decrease of level albumin and Glucose in serum, lower enzyme levels may be due to either lower release from the tissues to the serum, or decreased production of active enzymes and consequently lower activity in the serum [14, 16]. It has been postulated that the nephrotoxic mode of action of the drug cisplatin is similar to that of other heavy metals, and is related to the decrease in the intracellular concentrations of glutathione and protein-bound SH groups, which are required for normal cellular function as well as Mitochondrial damage might be attributed to inhibition of DNA synthesis and gluthatione depletion induced oxidative stress and those finding are similar to our results [17-19]. The increase levels of urea and creatinine its anther indication of the damage of the renal glomeruli and these data similar to other previous studies [20], have mentioned that cisplatin causes elevation the levels of the kidney function biochemical markers such as serum urea and creatinine. In opposite side the concentrations of creatinine and urea were decreased in rats that were administered cisplatin with Quercetin. These findings may be in concord with many other studies, which found Quercetine overcame the elevation of creatinine and urea levels caused by cisplatin [21]. The elevated of serum urea and creatinine concentrations in cisplatin injected rats could be attributed to the elevated of ROS [22]. In other hand the decrease levels of these biomarkers with administration of Querectein was thought to be their antioxidant properties and improve of both the hepatic and renal dysfunctions as well as damage that induced by hepatotoxicity and nephrotoxicity which is confirmed by many of other reports which showed that antioxidant reduced damage of cells and had protective role [19, 23, 8]. In the present study showed that administration of Quercetin before or after cisplatin produced a significant decrease of levels biomarkers both in serum and homogenize tissue in comparison with rats treated with cisplatin and control group. These results suggested the possible role of Quercetin which mediated a protection against cisplatin induced depletion of renal and hepatic glutathione could be ascribed to its antioxidant properties [24]. Furthermore, it has been reported that the main metabolites of Quercetin which are glucoronic acid conjugates and glutathione conjugates have antioxidant activity [20].In the present work was found that cisplatin induces structural and functional changes in both kidney and liver as proved in result of this study, the significant increases of enzymes level in serum and homogenize tissue as creatinine and urea, in addition to the reduction of albumin and glucose level is a good marker that is a Cisplatin induced kidney damage, while significant increase in serum and homogenize tissue of AST, ALT, ALP and LDH levels indicated the liver and heart damage beside the elevation the level of both cholesterol and triglycerides. Our conclusion showed that the Quercetin could be promising as antioxidants for reducing the risk of cisplatin induced nephrotoxicity, hepatotoxicity and cardiotoxicity So, the supplementation of this antioxidant could maintain the integrity of lipid in kidney and liver tissue under oxidative stress during chemotherapy.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML