-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2018; 8(4): 75-84
doi:10.5923/j.ajb.20180804.02

Effect of Chemical and Thermal Treatments on Browning Inhibition of Senescent Plantain (Musa paradisiaca) Puree for Semolinas Preparation
Arlette A. N’Guessan, Olivier K. Kouadio, Jean T. Gonnety
Unité de Formation et de Recherche en Sciences et Technologie des Aliments, Université Nangui Abrogoua, Abidjan, Côte d’Ivoire
Correspondence to: Jean T. Gonnety, Unité de Formation et de Recherche en Sciences et Technologie des Aliments, Université Nangui Abrogoua, Abidjan, Côte d’Ivoire.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Polyphenol oxidases (PPOs) from several fruits, including plantain, have been involved in the undesirable brown discoloration of food products that resulted in negative effects on colour, taste, and nutritional value. The present work was carried out to evaluate the effect of chemical and thermal treatments on browning inhibition of senescent plantain (Musa paradisiaca) puree for the preparation of a local semolina called “M’Bahou”. A screening of PPOs activities from senescent plantain was carried out. The physicochemical characteristics and thermal stability of main PPOs activities were determined in order to develop methods of anti-browning. The anti-browning methods focused on chemical treatments alone or combined with heat treatment. The effectiveness of these methods was determined by measuring colour parameters of purees. Sensory analyses were carried out in order to evaluate the effect of the anti-browning treatments on organoleptic quality of “M’Bahou” semolina. Dopamine oxidase, pyrocatechol oxidase and pyrogallol oxidase activities were the main PPO activities from senescent plantain that were optimally active at pH range (5.6 to 7.0). At 60°C, their D-values ranged from 31 to 47.27 min. Hence, heat treatment at 60°C for 35 min reduced browning of senescent plantain puree. Sodium metabisulfite was found to be the most potent inhibitor of PPOs from senescent plantain followed by ascorbic and citric acids. Pre-treatment of plantain puree with a combination of metabisulfite and ascorbic acid (5:50 mg and 7:30 mg) per 100 g of puree or heating combined with the addition ascorbic and citric acids (50:50 mg) indicated low browning. A comparison between sensorial characteristics of semolinas obtained from these pre-treatment methods revealed no significant difference in terms of odour, texture, and overall acceptability of semolinas. But for health reasons related to metabisulfite in Asthmatics, we would recommend the use of the lowest concentration (5:50 mg; metabisulfite/ascorbic acid) or the method which combined using of heat treatment and ascorbic acid/ citric acid for semolina preparation.
Keywords: Browning index, Food additives, Polyphenol oxidase, Senescent plantain, Semolina
Cite this paper: Arlette A. N’Guessan, Olivier K. Kouadio, Jean T. Gonnety, Effect of Chemical and Thermal Treatments on Browning Inhibition of Senescent Plantain (Musa paradisiaca) Puree for Semolinas Preparation, American Journal of Biochemistry, Vol. 8 No. 4, 2018, pp. 75-84. doi: 10.5923/j.ajb.20180804.02.
Article Outline
1. Introduction
- Plantain (Musa paradisiaca) is an important starchy, staple and commercial crop in West and Central Africa where fifty per cent of the world’s plantain crop is produced [1]. It constitutes a major source of carbohydrate for millions of African people. Plantain contains low protein and fat but rich in starch and mineral elements, especially potassium [2]. Food uses and consumption patterns of plantain are quite diverse [3, 4]. The plantain flour is used to make donuts, biscuits, bread [5, 6] and also food for children and pregnant women [7]. In Côte d'Ivoire, unripe and ripe plantain pulps are generally used to make popular foods such as foutou and foufou [8]. The production of plantain is estimated at 1,624,354 million tons [9] but, about 35 to 60% of this production is lost after harvested due to the lack of storage conditions and the inappropriate processing technologies for food [10]. After harvest, plantain exhibits a respiratory peak during natural ripening. The ethylene gas triggers the enzymatic reactions in plantain fruits causing ripening [11] and over-ripening of pulps for the most of time. After maturity, the plantain fruit evolves in 9 different stages of ripening [12]. From stage 1 to stage 7, plantain still retains its market value. Beyond stage 7, there is senescence, where there is rapid deterioration coupled with the growth of microorganisms as a result of drastic softening of the fruit tissue [13, 14]. The over-ripeness and senescence of pulp make plantain unused for common diet but some alternative products such as cakes [15], beer [16] and juice [17] were generated. In the eastern part of Côte d’Ivoire, a local semolina product from senescent plantain called “M’Bahou” is also produced to reduce post-harvest losses. The “M’Bahou” semolina supplements the dietary needs of rural population and is well appreciated by urban consumers. The process of “M’Bahou” semolina requires senescent plantain pulp that is crushed and blended with cassava flour. A long-time of treatment of the plantain pulp leads to more oxidation and production of coloured products [18]. Several users of plantain have noted the presence of a polyphenol oxidase activity [19] and the occurrence of enzymatic browning during the preparation of plantain [20]. The enzymatic browning occurring in the crushed pulp remains a critical point that impacts negatively the “M’Bahou” semolina. This browning makes the product dark, limits the storage and contributes to reduce its organoleptic quality [21]. It leads also to a change in flavour and a reduction in the nutritional quality [22, 23]. Several methods such as the use of sodium metabisulfite as a chemical browning inhibitor, as well as thermal processing have been used to inhibit enzymatic browning and limit its impact on the quality of plantain products [24, 25]. Ruthra and al. [26] evaluated the effect of the combination of citric acid, ascorbic acid and potassium metabisulfite and their combinations on minimally processed banana. They observed that potassium metabisulfite (0.2%) and combination of potassium metabisulfite and ascorbic acid were most effective in control of browning. Metabisulfite (0.1 and 0.2%) was also found to be the most effective pre-treatment for preventing browning in ripe and over-ripe banana pulp, as these concentrations were likely to maintain the original colour of banana pulp [27]. According to Koffi and al. [28], heating whole bananas or the puree was effective in inhibiting PPO, but the addition of 100 mg/L potassium metabisulfite was the most effective treatment in inhibiting browning. Bazaz and al., [29] showed that banana pulp (stage 4 of ripening) subjected to heat treatment (65°C, 30 min) and chemical treatment can be stored for long term without deterioration. All these works were generally carried out on unripe, ripe or over-ripe plantain. Investigations on the browning inhibition of senescent plantain for its valorisation are scarce.The present study was carried out to assess the effect of chemicals and thermal treatments on browning inhibition of senescent plantain (Musa paradisiaca) puree for semolina preparation in Côte d’Ivoire. It should also provide helpful information about the physicochemical characteristics of PPO activities as well as the impact of these treatments on the organoleptic quality of “M’Bahou” semolina.
2. Materials and Methods
2.1. Materials
- Plantain sample preparationMature plantains (Musa paradisiaca, variety Corn 1) were harvested in the experimental plot of Azaguié (located 50 km north of Abidjan 5° 38'N, 4° 05' W) located in Côte d’Ivoire. Plantains were allowed to ripen naturally at room temperature to senescence (stage 9).
2.2. Methods
- Extraction of Polyphenol Oxidase (PPO)A sample of senescent plantain (150 g) was crushed in a blender (Moulinex, France) and homogenized for 10 min in 300 ml of NaCl 0.9% (w/v). The resulting homogenate was centrifuged at 8000 g for 10 min at 4°C (Refrigerated centrifuge TGL-16M, China). The collected supernatant was the crude enzymatic extract used for PPO activity assays [30].Enzyme assayThe PPO activities were determined using various phenolic compounds consisting of dopamine, pyrocatechol, pyrogallol, guaïacol, L-Tyrosine and L-Phenylalanine as substrates. The reaction mixture adjusted to 2 mL with extraction buffer at appropriate pH, contained 0.1 mL of crude enzymatic extract and 10 mM of substrate. This reaction mixture was incubated at 25°C for 10 min. O-quinones content starting from the dopamine and guaïacol was estimated at 480 nm and those resulting from the others phenolic compounds at 420 nm [31]. Experiments were performed in triplicate, and the results expressed as units of enzymatic activity per mg of protein. One unit of enzymatic activity (U) was defined as an increase in absorbance of 0.001 per min (standard test conditions).Optimal pH of PPO activitiesThe effect of pH on the crude PPO activities was determined by measuring the oxidation of phenolic substrates in different buffers at various pH values ranging from pH 2.6 to 10.6. The buffers (100 mM concentration) used were sodium acetate from pH 3.6 to 5.6, sodium phosphate from 5.6 to 8.0 and sodium citrate from pH 2.6 to 7.0 and Tris-HCl from pH 8.6 to 10.6. The PPO activities were determined at 25°C under the standard test conditions.Optimal temperature of PPO activities The effect of temperature on PPO activities was performed in 100 mM sodium phosphate buffer pH 7.6 (for dopamine oxidase and pyrogallol oxidase), in sodium acetate buffer pH 5.6 (for pyrocatechol oxidase) after 10 min incubation at temperatures ranging from 5 to 80°C under standard test conditions [30, 32].Effect of some chemical agents on PPO activitiesTo determine the effect of some food additives as effective inhibitors of PPO activities, the crude enzymatic extract was preincubated at 25°C for 30 min with the chemical agents. Citric acid, ascorbic acid, and sodium metabisulfite were tested at different concentrations and the activity of PPO was assayed under the standard test conditions. Residual activities were expressed as percentage refers to a control without chemical agents.Thermal inactivation and kinetic parameters (t1/2 and D-values) determination The thermal inactivation of PPO activities was determined at temperatures ranging from 45 to 85°C. The crude enzymatic extract in appropriate buffers [100 mM sodium phosphate pH 7.6 (for dopamine oxidase and pyrogallol oxidase) and sodium acetate pH 5.6 (for pyrocatechol oxidase)] was preincubated at different temperatures. Aliquots were withdrawn at intervals and cooled at room temperature for 10 min. The enzymatic activities of the aliquots were measured under standard conditions. The Kinetic data analysis of thermal inactivation of the PPO activities was done from the equation 1 (1). This equation is derived from the equation of first-order reactions [33].
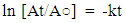 | (1) |
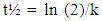 | (2) |
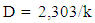 | (3) |
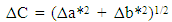 | (4) |
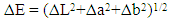 | (5) |
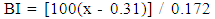 | (6) |
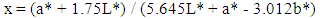 | (7) |
3. Results and Discussion
- Physicochemical characteristics and thermal stability of PPOs from senescent plantainThe results of the screening of PPO activities as presented in Figure 1 showed that diphenolic compounds were suitable substrates for PPOs from the pulp of senescent plantain. Our results were in agreement with those of Ngalani and al. [20], who reported that PPOs of plantain pulp (variety '' French '', ripening stage 4 and 5) were able to oxidize diphenolic substrates more than monophenolic substrates. The fact that dopamine, pyrocatechol and pyrogallol were the most suitable diphenolic substrates suggested that dopamine oxidase, pyrocatechol oxidase and pyrogallol oxidase activities were the main enzymatic activities involved in the enzymatic browning of senescent plantain pulp (variety Corn 1). To this end, browning prevention strategies during the processing of plantain pulp should focus on these enzymatic activities. Hence, their physicochemical characterization has been considered.
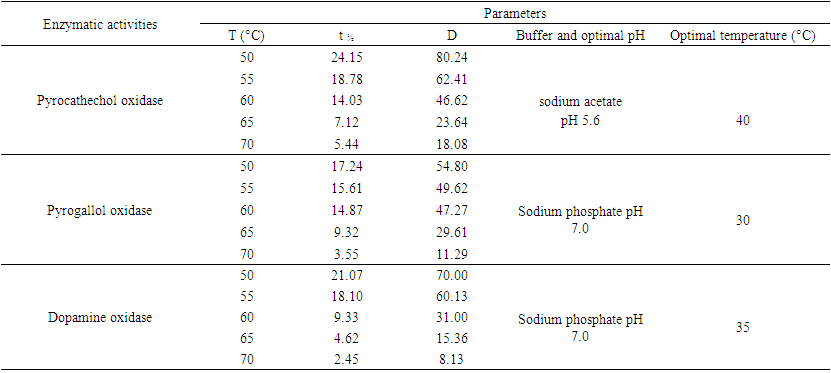 | Table 1. Some physicochemical characteristics and kinetic parameters of polyphenol oxidase (PPO) activities of the pulp from senescent plantain (Musa paradisiaca; variety Corn1) |
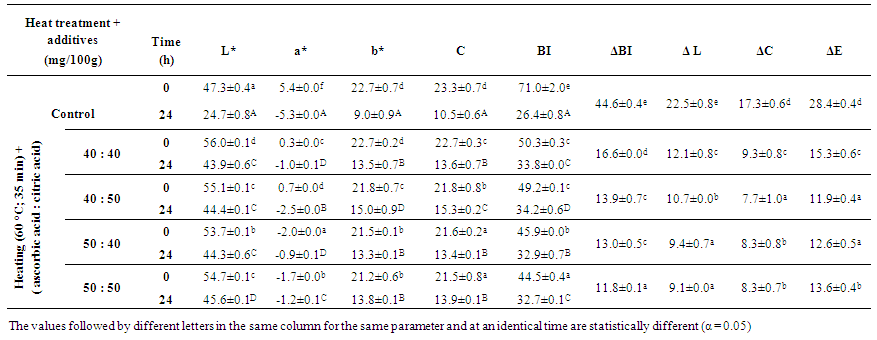
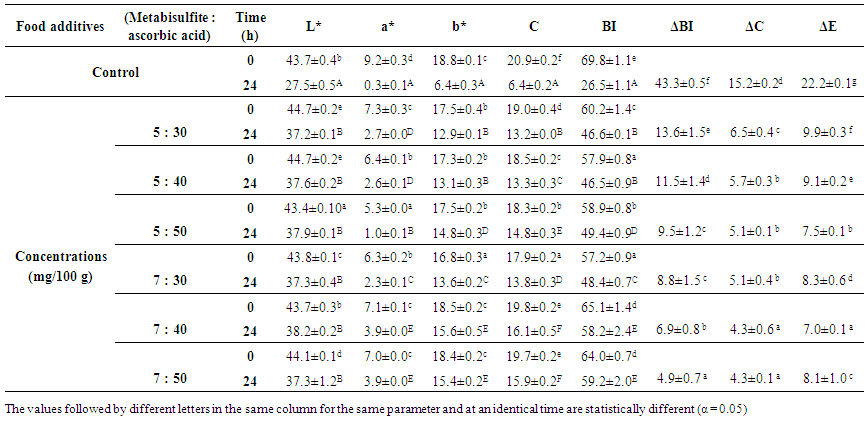 | Table 2. Initial and final values of colour parameters of senescent plantain (Musa paradisiaca, variety Corn 1) puree treated with the combination of metabisulfite and ascorbic acid |
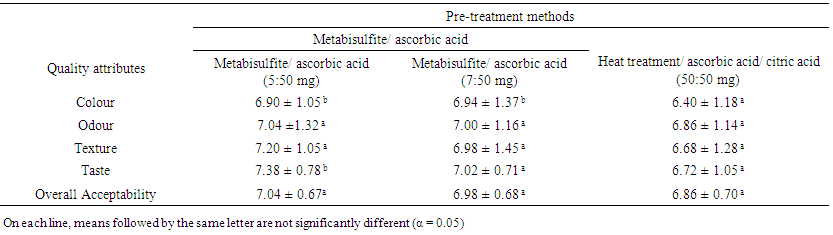 | Table 4. Mean acceptability of the quality attributes of “M’Bahou” semolinas obtained from three pre-treating methods of senescent plantain (Musa paradisiaca, variety Corn 1) pulps or purees |
4. Conclusions
- The screening of PPO activities has shown that dopamine oxidase, pyrocatechol oxidase and pyrogallol oxidase were enzymatic activities mainly involved in the browning of senescent plantain. Based on the physicochemical properties and thermal stability of these PPOs activities, treatments carried out on the senescent plantain pulp and puree led to reduce browning of plantain puree. The combined addition of metabisulfite and ascorbic acid to the puree was the most effective method of anti-browning. The thermal treatment of pulps combined with addition of ascorbic and citric acids had good effects on the organoleptic quality of the generated “M’Bahou” semolina, but a slightly preference was observed for semolina obtained from the puree to which the combination of metabisulfite and ascorbic acid (5:50 mg) per 100 g of puree was added. Colour, odour, texture and taste of semolina were found to be acceptable with fairly good grades. For health reasons related to metabisulfite in Asthmatics, we would advise users to use the combination of metabisulfite and ascorbic acid (5:50 mg) or the method using heat pretreatment combined with the use of ascorbic and citric acids to produce semolina of good quality. We believe that this study will enable the valorisation of senescent plantain and thus contribute to food security.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML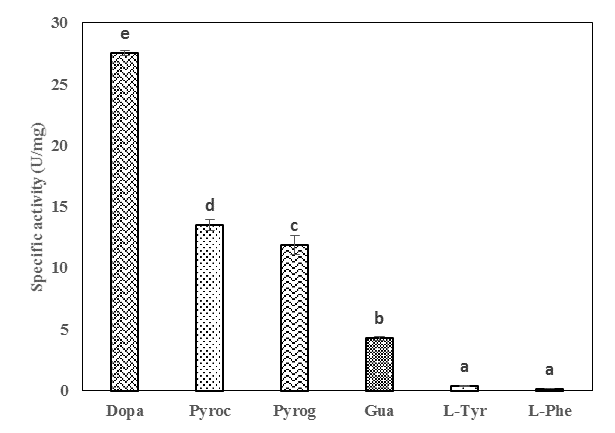
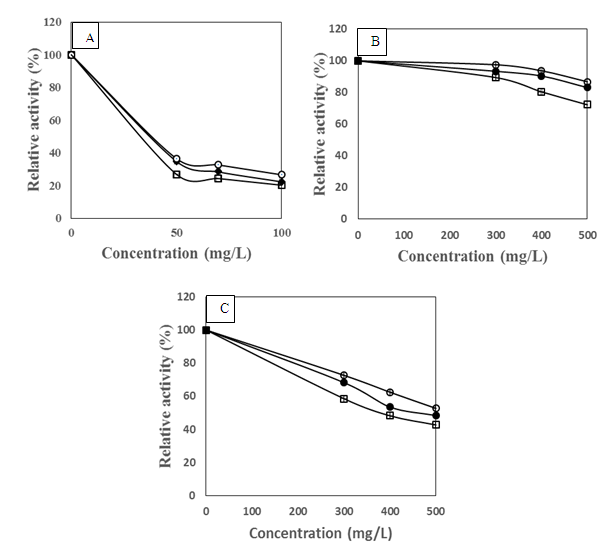
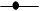 Pyrocathecol oxidase activity,
Pyrocathecol oxidase activity, 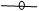 Pyrogallol oxidase activity,
Pyrogallol oxidase activity,  Dopamine oxidase activity
Dopamine oxidase activity