-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2018; 8(4): 69-74
doi:10.5923/j.ajb.20180804.01

Anti-diabetic Effects of Aqueous Extract and Oil of Moringa oleifera Seed on Liver and Kidney Functions in Streptozotocin-induced Diabetes in Rats
M. S. Nadro, A. S. Audu, E. Glen
Department of Biochemistry, Modibbo Adama University of Technology, Yola, Adamawa State, Nigeria
Correspondence to: A. S. Audu, Department of Biochemistry, Modibbo Adama University of Technology, Yola, Adamawa State, Nigeria.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

There is no satisfactory treatment for diabetes in the modern allopathic system of medicine. Therefore, there is a need to develop newer treatment strategies of plant origin which might have fewer side effects and cost effective. The present study investigated the possible therapeutic effects of aqueous extract and oil of Moringa oleifera seed on certain biochemical markers in streptozotocin-induced diabetes mellitus in albino rats. Thirty five rats were used in the study, five for each group. Diabetes was induced by a single intraperitoneal injection of streptozotocin (65 mg/kg body weight). The effects of aqueous extract and oil of Moringa oleifera seed on blood glucose, body weight, albumin, urea, creatinine, electrolytes (Na+, K+ and Cl-)-, the activities of enzyme markers of liver damage (aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were examined in the serum of control and treated groups. Oral administration of 100 mg/kg and 200 mg/kg body weight of aqueous extract and 1 ml/kg and 1.5 ml/kg body weight oil to diabetic rats for four weeks were found to significantly (P≤0.05) reduced serum glucose. Also, urea and creatinine level were significantly decreased in the treated groups as compared to the diabetic untreated group. The treatment also improved hepatic function as observed in the decreased activities of enzymes markers of liver damage in the treated groups as compared with that of the diabetic control group. The present results showed that Moringa oleifera seed has anti-hyperglycaemic effect and consequently may alleviate liver and renal damages associated with streptozotocin-induced diabetes mellitus in rats.
Keywords: Diabetes, Anti-diabetic, Moringa oleifera, Streptozotocin-induced, Aqueous extract
Cite this paper: M. S. Nadro, A. S. Audu, E. Glen, Anti-diabetic Effects of Aqueous Extract and Oil of Moringa oleifera Seed on Liver and Kidney Functions in Streptozotocin-induced Diabetes in Rats, American Journal of Biochemistry, Vol. 8 No. 4, 2018, pp. 69-74. doi: 10.5923/j.ajb.20180804.01.
Article Outline
1. Introduction
- Diabetes is a disorder that affects the amount of sugar in the blood. Insulin, a hormone released from the pancreas, controls the amount of glucose in the blood. Glucose in the bloodstream stimulates the pancreas to produce insulin. Insulin allows glucose to move from the blood into the cells. Once inside the cells, glucose is converted to energy, which is used immediately, or it is stored as fat or glycogen until it is needed (Malviya et al., 2010). Symptoms of high blood sugar include frequent urination, increased thirst, and increased hunger. If left untreated, diabetes can lead to numerous alterations in cell membrane properties such as enhanced rigidity, permeability for cations, and transmembrane potential in its absolute magnitude (Jaiswal, 2013, WHO, 2013). Acute complications include diabetic ketoacidosis and non-ketotic hyperosmolar coma. (Kitabchi et al., 2009). Serious long-term complications include cardiovascular disease, stroke, kidney failure, foot ulcers and damage to the eyes (WHO, 2013). There is no satisfactory treatment for diabetes. Once a person develops diabetes, he will suffer from the condition for the rest of his or her life (Ezugwu, 2001). The World Health Organization (WHO) has recommended the development of oral hypoglycaemic agents from medicinal plants as herbal natural remedies to treat diabetes mellitus due to their being cost effective and safe (Santosh et al., 2008). Moringa oleifera originated from India and is now grown across the world. So, people in many developing countries (particularly in Africa) have been using Moringa oleifera to treat and manage the symptoms of diabetes for years. This is primarily because of its natural benefits. Moringa oleifera has been shown to naturally boost the immune system, which usually becomes compromised in those who suffer from type I and type II diabetes. Moringa oleifera has also been shown to possess many key anti-inflamatory benefits. There are no negative side effects associated with the use of Moringa oleifera, meaning that it is a safe, natural way for people to manage their blood sugar and other complications associated with diabetes (Bey, 2013).Therefore, the aim of this study is to evaluate the anti-diabetic activities of aqueous extract and oil of Moringa oleifera seed in streptozotocin induced diabetic rats. More so, information from this study will provide basis for supporting the claim of the use of Moringa oleifera as an anti-diabetic agent.
2. Materials and Methods
2.1. Chemical and Reagents
- The reagents included streptozotocin (STZ) (Tocris Bioscience, London), olive oil (Roberts Laboratories Limited, Belton, England), Accuchek active glucose test strip (Roche National Diagnostic Products, Australia), Glucose reagent (Randox, United Kingdom), and glycohemoglobin Reagent (Pointe Scientific Inc., USA).
2.2. Plant Materials
- The Moringa oleifera seeds were harvested in the month of February, 2015 from different trees in Takum, Takum Local Government Area of Taraba State, Nigeria where they were totally dried up. The plant specimens were authenticated at the Department of Plant Science, Modibbo Adama University of Technology, Yola, Adamawa State. Moringa oleifera seed oil was extracted using the solvent extraction method described by Olson (2010).
2.3. Experimental Animals/Ethical Considerations
- Male adult albino rats weighing 85-95g and numbering 35 were procured from Veterinary Research Institute, Vom, Plateau State, Nigeria. The animals were housed in plastic cages with a 12 h each of light and dark cycle. They were allowed free access to pelletised grower diet of Vital Feeds, (a product of United Africa Company of Nigeria (UACN) based in Plateau state, Nigeria) and water ad libitum. The international principles of Laboratory Animals’ Care (issued by Council for International Organization of Medical Sciences and The International Council for Laboratory Animal Science) were followed throughout the experiment.
2.4. Preparation of the Extracts
- Dry Moringa oleifera seeds were pounded, using a pestle and mortar and passed through a mesh sieve (30-40). The powdered seed was used for the subsequent extractions. Aqueous extract was prepared by soaking exactly 100 g of the powdered Moringa oleifera in 250 ml distilled water at room temperature for 72 hours and filtered using the Whatman filter paper. The bulk filtrate obtained was reduced in vacuo at 40°C and the solid residue stored at 4°C until needed.For the seed oil, 10 g of the seed powder was weighed and placed on a filter paper which was folded carefully. The filter paper containing the sample was then inserted into the Soxhlet apparatus. The weight of the filter paper and sample was recorded. Then 200ml of the solvent (hexane) was measured using a measuring cylinder and then poured into a 500ml round bottom flask with the sample and heated at 60°C for 5 h after which the sample (filter paper and seed) was removed and transferred into the air oven to dry at 100°C for 15 minutes. This sample was then weighed and the difference calculated as: weight of sample before extraction – weight of sample after extraction, divided by the initial weight of sample, and multiplied by 100 to give the percentage oil yield. The oil was recovered by solvent evaporation. It was heated at a low temperature until the solvent finally evaporated leaving behind the oil extracted (Olson, 2010).
2.5. Induction of Diabetes
- Diabetes was experimentally induced by slow intraperitoneal injection of diabetogenic dose of streptozotocin (65mg/kg body weight) dissolved in 5 ml distilled water, and administered within a few minutes of its preparation (Burcelin et al., 1995). The diabetic state was confirmed in the rats on the seventh day and animals with fasting glucose levels greater than 300mg/dl were selected for this study.
2.6. Experimental Design
- Group 1: (Normal control) - Normal + no treatmentGroup 2: (Negative control) - Diabetic + no treatmentGroup 3: (Positive control) - Metformin (5 mg/kg b.w.)Group 4: (Treatment 1) - Aqueous extract (100 mg/kg b.w.)Group 5: (Treatment 2) - Aqueous extract (200 mg/kg b.w.)Group 6: (Treatment 3) -Oil (1 ml/kg b.w.)Group 7: (Treatment 4) - Oil (1.5 ml/kg b.w.).All the animals were allowed free access to food and water ad libitum. At the end of the experiment, the rats were anaesthetized by placing them in a sealed jar with cotton wool soaked in chloroform. Blood was collected through cardiac puncture from each animal in a centrifuge tube and allowed to stand for 10-15 minutes after which the blood was centrifuged at 3000 revolution per minute (rpm) for five minutes. The serum were carefully collected and transferred to new sample bottles and subsequently used for biochemical analysis.
2.7. Determination of Biochemical Parameters
2.7.1. Determination of Glucose
- Blood samples were collected by cutting the tail tip of the rats for glucose determination. Fasting blood glucose levels of each group was determined every week for a period of four weeks. Determination of blood glucose was done by glucose- oxidase principle (Beach and Turner, 1958) using one touch basic instrument and results were expressed in mg/dl.Principle Glucose oxidase is an enzyme extracted from the growth medium of Aspergillus niger. Glucose oxidase catalyse the oxidation of Beta D- glucose present in the plasma to D glucono -1, 5 - lactone with the formation of hydrogen peroxide; the lactone is then slowly hydrolysed to D-gluconic acid. The hydrogen peroxide produced is then broken down to oxygen and water by a peroxidase enzyme. Oxygen then react with an oxygen acceptor such as ortho toluidine which itself converted to a coloured compound, the amount of which can be measured colorimetrically.
2.7.2. Determination of Alanine Aminotransferase (ALT)
- Alanine aminotransferase (ALT) was determined by the colorimetric method as describe by Reitman and Frankel (1957).
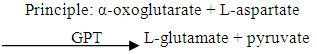 The pyruvate formed reacts with 2, 4- dinitriphenylhydrazine to form pyruvate hydrozone which was measured photometrically.
The pyruvate formed reacts with 2, 4- dinitriphenylhydrazine to form pyruvate hydrozone which was measured photometrically.2.7.3. Determination of Aspartate Aminotransferase (AST)
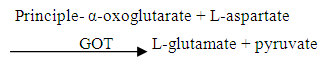 The principle for the determination of AST is based on the rate of formation of glutamic acid. In AST, the amount of oxaloacetic acid formed is proportional to the transaminase activity. The amber colour which developed in an alkaline medium is the measure of the amount of glutamic acid formed (Reitman and Frankel, 1957).
The principle for the determination of AST is based on the rate of formation of glutamic acid. In AST, the amount of oxaloacetic acid formed is proportional to the transaminase activity. The amber colour which developed in an alkaline medium is the measure of the amount of glutamic acid formed (Reitman and Frankel, 1957).2.7.4. Determination of Creatinine
- Serum creatinine was determined by using Jaffe method (without deproteinanization) as described by Henry et al., (1974). The principle was based on the color formation when creatinine in alkaline medium reacts with picrate to form a coloured complex. The rate of formation of the coloured complex is measured photometrically.
2.7.5. Determination of Urea
- Serum urea was determined by Diacetylmonoxime (DAM) method as described by Wybenga et al., (1971). The principle was based on the colour formation that occurred when urea reacts with hot acidic DAM in the presence of Thiosemicarbazide acid to give a rose purple coloured complex which was measured photometrically.
2.8. Statistical Analysis
- Values obtained were expressed as mean ± SEM and data were analysed using analysis of variance (ANOVA) with Bonferroni Post hoc test multiple comparison versus control groups using the Statistical Package for the Social Sciences (SPSS) software version 21. The values p< 0.05 were considered significant (Duncan et al., 1977).
3. Results
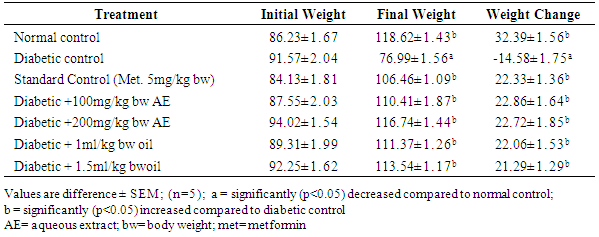
3.1. Effect of Treatments with Aqueous Extract and Oil of Moringa oleifera Seed on Body Weight Change (g) in Streptozotocin-Induced Diabetic Rats for 28 Days
- The result obtained in this regard (Table 1) showed a consistent and progressive decrease in body weight (-14.58±1.75) in the untreated diabetic group whereas, the normal and treated diabetic rats showed significant (P<0.05) increase in body weight at the end of the experiment.
|
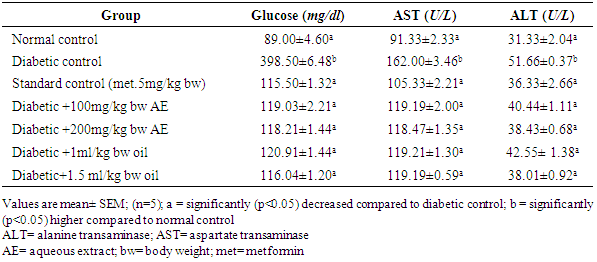
3.2. Effect of Aqueous Extract and oil of Moringa oleifera Seed on Blood Glucose, Serum ALT and AST Activities in Streptozotocin-Induced Diabetic Rats
- Prolonged treatment with aqueous extract and oil of Moringa oleifera seed has shown a significant decrease (p<0.05) in blood glucose level in all the treatment groups when compared against the diabetic control. Similarly, serum activities of Aspartate aminotransferases (AST) and Alanine aminotransferases (ALT) were found (Table 2) to be significantly (p<0.05) lower in the treatment groups when compared against the untreated group.
|
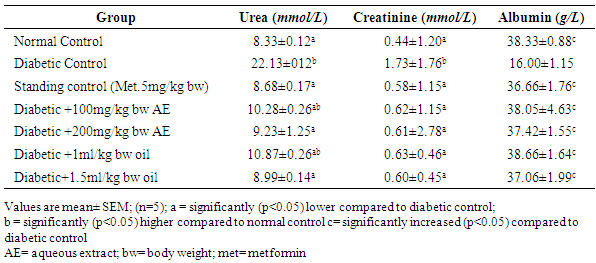
3.3. Effect of Aqueous Extract and Oil of Moringa oleifera Seed on the Serum Urea, Creatinine and Albumin Levels in Streptozotocin Induced Diabetic Rats
- The diabetic control group showed (Table 3) high levels of serum urea and creatinine (22.13±012) and (1.73±1.76), respectively, when compared against all the treatments groups which are significantly lower (p<0.05). Also, there is significant decrease in the level of serum albumin in the diabetic untreated group when compared against all the treatment groups.
|
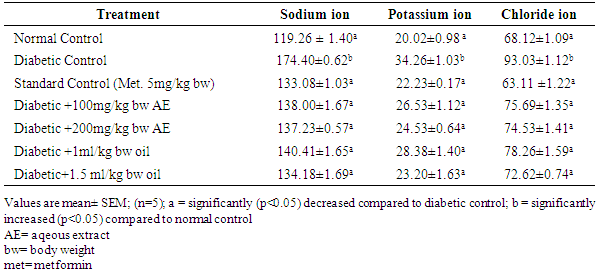
3.4. Effect of Aqueous Extract and Oil of Moringa oleifera Seed on the Electrolytes (mmol/L) Levels in Streptozotocin-Induced Diabetic Rats
- The results of electrolytes levels in streptozotocin-induced diabetic rats are shown in Table 4. There was a significant increase (p<0.05) in levels of sodium ion, potassium ion and chloride ion in the diabetic control group as compared to the groups treated with the standard, aqueous extract and oil of Moringa oleifera seed.
|
4. Discussion
- The streptozotocin-induced diabetes is characterized by severe loss in body weight of untreated rats, which is due to increased muscle wasting in diabetes (Reyes et al., 2006). The result from this study supports this claim, because weight loss was registered in the case of diabetic untreated group, whereas, treatment with aqueous extract and oil of Moringa oleifera seeds for a period of 28 days had significantly increased weight. The weight gain following treatments may be due to protein sparing effect by the extract (Bello et al., 2011).ALT and AST are enzyme markers of liver damage. The level elevates in case of hepatic injuries or malfunctioning (Qureshi et al., 2009). The diabetic control group showed high activities of AST and ALT, which might be due to leakages from damaged hepatocytes or bile ducts (Yoshinari and Igarashi, 2010). The decreased levels of ALT and AST observed in all the experimental groups treated with the extracts of Moringa oleifera seed when compared with the diabetic control, is an indication of improvement in the hepatic function by the extract.A significant elevation in serum creatinine and urea levels indicates an impaired renal function of diabetic animals (Singh, 2011). An increase in creatinine and urea levels is seen when there is damage to the kidney or when the kidney is not functioning properly. Increment of blood creatinine and urea levels with the increment of blood sugar level clearly indicates that the increase blood sugar level causes damage to the kidney (Shrestha et al., 2008). Research conducted by Anjaneyulu and Chopra (2004) - found that increases in serum creatinine and urea levels in diabetic rats indicated a progressive renal damage. Diabetes nephropathy is the kidney disease that occurs as a result of diabetes, therefore the decreased levels of creatinine and urea in the treated groups shows that treatment of the disease with extracts of Moringa oleifera seedcan guardagainst diabetes nephropathy. The reduction in albumin level was observed in diabetic rats, and this is consistent with the results obtained by (Bakirel et al., 2008). The decrease in albumin may be due to albuminuria, which is an important clinical marker of diabetic nephropathy. The results of the present study demonstrated that the treatment of diabetic rats with Moringa oleifera seed extracts caused noticeable elevation in the albumin levels as compared with their normal levels (Sy et al., 2005).Increases in plasma glucose concentration can lead to changes in plasma sodium concentration through several mechanisms. Elevations in glucose concentration increase plasma tonicity, creating an osmotic driving force that favours the movement of water from the intracellular space to the extracellular space, thereby diluting the extracellular concentration of sodium (Liamis et al., 2013). The plasma sodium concentration is usually low as a result of this osmotic flux of water. Increased or normal plasma sodium concentrations in the presence of hyperglycemia indicate a clinically significant deficit in total body water (Liamis et al., 2008). Treatment with aqueous extract and oil of Moringa oleifera seed significantly increased sodium concentration in all treated groups compared with the diabetic control group.This study also showed a significant increase in the serum level of potassium in the diabetic control group compared with the treated groups. The disease is characterized by disturbances in nephron function, which lead to impaired renal excretion of hydrogen and potassium and result in hyperkalemia and a hyperchloremic normal-gap acidosis (Elisaf et al., 2006). Treatment with aqueous extract and oil of Moringa oleifera seed significantly decreased the level of potassium ion in all treated groups compared with the diabetic control group.Hyperchloremia indicated in the diabetic control group can occur when the kidney tubules (either proximal or distal) do not reabsorb adequate quantities of the bicarbonate filtered by the glomerulus (Kokko and Jacobson, 2005). Disorders causing intrinsic damage to the tubules (e.g., interstitial nephritis). Treatment with aqueous extract and oil of Moringa oleifera seed significantly decreased the serum chloride ion concentration in all treated groups compared with the diabetic control group.
5. Conclusions
- The results of the present study have confirmed the use of aqueous extract and oil of Moringa oleifera seed in traditional medicine for the management of diabetes mellitus. The results suggested that the extracts play a significant role as potent hypoglycemic agents which are capable of improving other clinical conditions associated with diabetes mellitus, such as the kidney function and liver function. The aqueous extract and oil of Moringa oleifera seed therefore, have not only hypoglycemic activity, but also nephron-protective activity.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML