-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2018; 8(3): 60-68
doi:10.5923/j.ajb.20180803.03

Cell Free DNA and CD38 Expression on T helper Cells as Biomarkers of Disease Activity in Rheumatoid Arthritis
Mona Abd EL Rahman Eldosoky1, Reham H. M. Hammad1, Hend G. Kotb2, Noha Abdel-Rahman Eldesoky3, Hemmat A. Elabd4
1Clinical Pathology Department, Faculty of Medicine for Girls, Al-Azhar University, Egypt
2Internal Medicine Department, Faculty of Medicine for Girls, Al-Azhar University, Egypt
3Biochemistry Department, Faculty of Pharmacy for Girls, Al-Azhar University, Egypt
4Physical Medicine, Rheumatology and Rehabilitation, Faculty of Medicine for Girls, Al-Azhar University, Egypt
Correspondence to: Mona Abd EL Rahman Eldosoky, Clinical Pathology Department, Faculty of Medicine for Girls, Al-Azhar University, Egypt.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

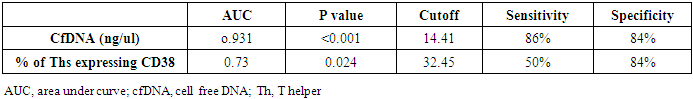
Background: Rheumatoid arthritis (RA) is an inflammatory autoimmune disease. Disease activation is associated with irreversible disability impairing patient quality of life. Circulating cell-free DNA (cfDNA) is a cell damage marker, while CD38 molecule expressed on immune cells is involved in activation, and survival signaling. Objectives: The study aimed to assess if levels of plasma cfDNA and T helpers (Ths) expressed CD38 in RA could serve as biomarkers for RA activity. Materials. Patients and methods: Study was conducted on 57 subjects, divided into three groups; Active RA group (n=22), RA in remission (n=13), and Control group (n=22). Plasma cfDNA concentration was determined by quantitative PCR. Expression of CD38 on Ths was assessed by flow cytometry. Results: Concentration of cfDNA was significantly higher in active group compared to remission group (p<0.001), while no significant difference was detected between remission and control group (p=1). Ths expressing CD38% were significantly lower in active group compared to remission group (p=0.03), while no significant difference was detected between remission and control group (p=0.3). Concentration of cfDNA was directly correlated to (DAS28-ESR) score for RA activity (r=0.857, p<0.001), while Ths expressing CD38 showed negative correlation with (DAS28-ESR) (r= -0.524, p=0.001). Regarding the output data of ROC curve for cfDNA and Th cells expressing CD38%, at cut off =14.41 ng/ul and 32.45% respectively, the specificity for both was 84% while sensitivity was (86% and 50%) for cfDNA and Ths expressing CD38% respectively, with Area Under Curve (AUC) of (0.93 and 0.73) for cfDNA and Th cells expressing CD38% respectively. Conclusion: Up-regulation of plasma cfDNA, and down-regulation of Ths expressing CD38 may serve as indicators for RA activity. Using up regulation of cfDNA as a biomarker of RA activity showed higher discriminative ability than down regulation of Ths expressing CD38 in differentiating RA activity from remission. Down regulation of CD38 expression on Ths during RA activity would drag the attention to its protective role and suggest a potential role in therapy.
Keywords: Rheumatoid arthritis, cfDNA, CD38
Cite this paper: Mona Abd EL Rahman Eldosoky, Reham H. M. Hammad, Hend G. Kotb, Noha Abdel-Rahman Eldesoky, Hemmat A. Elabd, Cell Free DNA and CD38 Expression on T helper Cells as Biomarkers of Disease Activity in Rheumatoid Arthritis, American Journal of Biochemistry, Vol. 8 No. 3, 2018, pp. 60-68. doi: 10.5923/j.ajb.20180803.03.
Article Outline
1. Introduction
- Rheumatoid arthritis (RA) is an inflammatory autoimmune disease, usually presented with constant pain, progressive irreversible joint destruction with significant disability, and impairment of the quality of life (QoL). Reduced health related QoL (HRQoL) in RA patients is associated with increased use of healthcare resources, therefore, RA has a considerable financial impact on patients’ family, health care payers, and society [1]. An over 2 decades study performed on Egyptian RA patients revealed female predominance, younger age at disease onset and more frequent polyarticular pattern at disease onset [2].The underlying pathology of most cases of RA starts years before the clinical diagnosis is made [3]. Abnormal immune activation is the most likely etiology for the development of RA [4]. Monitoring of activity of disease is very crucial to choose or modify treatment for the goal of achieving clinical remission [5].The RA status of activity and remission can be determined through disease activity indices, such as the Disease Activity Score in 28 joints-erythrocyte sedimentation rate (DAS28-ESR), and Clinical Disease Activity Index (CDAI). Subjective measurements, such as patient reported outcomes (PRO) are incorporated into these indices. Also, different indices weight similar criteria to different extents. Therefore, the classifications based on each evaluation may not be the same [6]. Hence, there is a growing need for development of novel objective biomarkers to aid in assessment of RA activity and hence severity of the disease independent on the patients reports.Circulating cell-free DNA (cfDNA) is an extracellular DNA found in plasma or serum. Accumulation of cfDNA in serum/plasma may be the result of excess release from massive cell destruction, insufficient elimination of dead cells, extracellular DNA traps formed during inflammation, or combination of all previous causes. Levels of cfDNA may indicate the extent of cell damage and inflammation, thus, determination of cfDNA levels will facilitate monitoring the disease activity and assessment of the effectiveness of a selected therapeutic strategy [7].The RA involves accumulation and activation of many cell subsets that play a crucial role in initiating and maintaining the disease process [8]. In accordance, Studying T cells are of great importance to elucidate the pathomechanism of RA; also, the comparison of the activation states of T cells in different stages of the disease may be important to monitor the course of the disease [9].CD38 is a cell surface molecule. The ligation of CD38 in immune cells delivers activation signals and induces cytokine production and secretion by T and B lymphocytes [10, 11]. It was noted that high CD38 expression on CD4 T cell subset was associated with a protective anti apoptotic role as CD38 induce proliferation, and survival of human lymphocytes [12]. One of the hypotheses explaining pathogenesis of RA; is that autoantigens are presented to auto reactive T helper (Th) cells, which then orchestrate the inflammatory processes detected in the disease [13]. Studies stressing on the definite function of CD38 expressed on T cells in autoimmune disorders are only few without enough reports to clear the role of this molecule in RA.The aim of our study was to find out if levels of plasma cfDNA and frequency of Th cells expressing CD38 in RA may serve as indicators for activity progression in RA. Also, we aimed to assess if there is a relation between cfDNA as apoptotic marker and CD38 as a signalling molecule involved in activation, proliferation and survival.
2. Materials and Methods
2.1. Study Participants
- This prospective case-control study was conducted on 57 participants divided into 3 groups; RA patients with active disease (n=22), RA patients in remission (n=13), Control group of age and sex matched healthy participants (n=22). All patients were recruited from outpatient Rheumatology clinic and inpatient Rheumatology Unit, AL-Zahraa Hospital, Al-Azhar University Cairo, Egypt during the period from (February 2018 to June 2018). RA was diagnosed using the American College of Rheumatology/European League Against Rheumatism 2010 revised criteria for RA [14]. DAS28-ESR Score was calculated for all patients to assess activity of disease. Active group, included RA patients with both high and low activity states, enrolled in single active group (n=22) assessed by DAS28-ESR score. DAS28-ESR score of greater than 5.1 indicates highly active disease, score less than 3.2 indicate low disease activity; Remission group, included patients with DAS28-ESR score less than 2.6; Control group, included sex and age matched subjects with no history of autoimmunity. Exclusion criteria: included ongoing rheumatic or inflammatory joint diseases other than RA, evidence of malignancy or infection and autoimmune diseases. All procedures performed in the study were in accordance with the ethical standards of the Faculty of medicine, Al-Azhar University Research Committee and with the 1964 Helsinki declaration and its later amendments ethical standards.
2.2. Sample Collection
- Approximately 10 ml of venous blood were drawn from each subject and divided into four aliquots; 1st aliquot was 4 ml blood transferred into EDTA tube, centrifuged for 10 minutes at 3500 rpm then plasma was removed carefully and re-centrifuged at 13000 rpm for 10 minutes. The supernatant is then transferred to microcentrifuge tubes and stored at -80°C for extraction and measurement of cfDNA by real-time quantitative PCR (qPCR). 2nd aliquot was 2 ml transferred to heparin tube for flow cytometry to be analysed within 24 hours. 3rd aliquot was 2 ml blood transferred into EDTA tube for complete blood count and differential count. 4th aliquot was 1.6 ml transferred into citrate tube for ESR measurement.
2.3. Flow Cytometry Assay
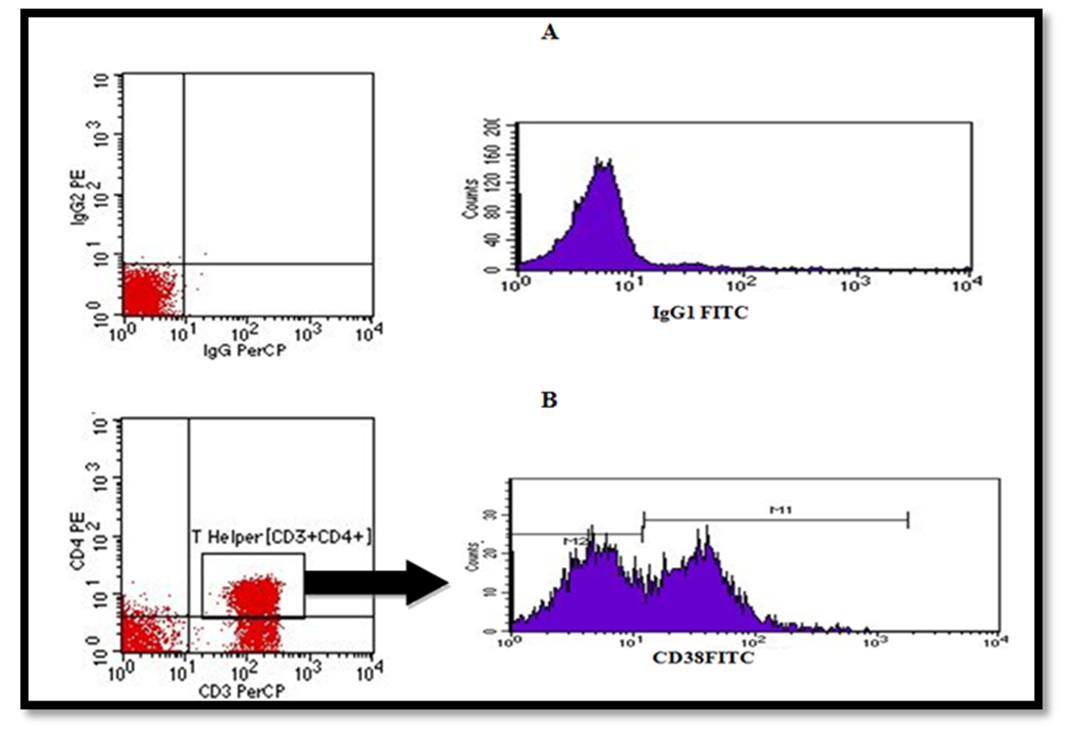
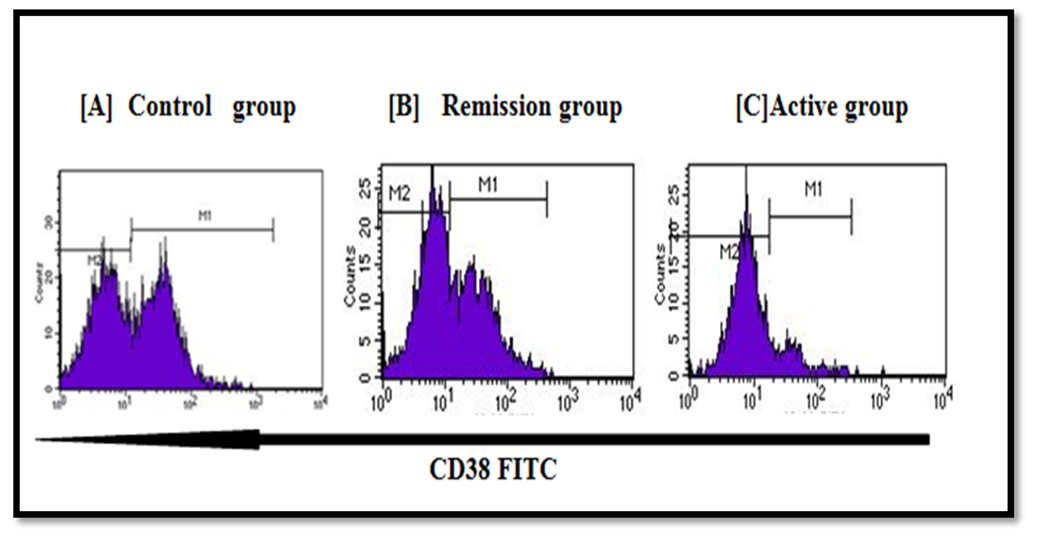
- Flow cytometry was conducted at the Clinical Pathology Department, Al-Zahraa Hospital, AL-Azhar University using four colors FACS Calibur (BD, Biosciences, San Jose, USA). CellQuest Pro software (BD Biosciences, San Jose, USA) was used for data analysis. Compensation setting was established before acquiring the samples using colour calibrite beads (BD, Biosciences, San Jose, USA and lot no. 5093879). After adjusting the sample count for acquisition, unstained samples were acquired to detect the sample auto-florescence. Isotype controls, mouse IgG1 FITC /IgG2a PE control (cat. no. 342409) and mouse IgG PerCP control (cat. no. 349044) were obtained from (BD, Biosciences, San Jose) for detection of nonspecific binding. Samples were analysed for expression of FITC conjugated anti-human CD38 (BD Biosciences, San jose. Cat. no. 560982, lot no. 5329747) on T helper cells showing co-expression of both (CD3+/CD4+). PerCP-conjugated anti-human CD3 (BD Biosciences, USA Cat. no. 95131, lot no. 345766), PE- conjugated anti-human CD4 (BD Biosciences, San jose. Cat. no.555347, lot no.86214). Monoclonal antibodies were added with optimal concentration of 5ug detected after titration experiments for 10 min at room temperature. Then lysis reagent (BD, Biosciences, San jose, USA) for destruction of RBCs was added for 8 minutes before cells were washed with FACS buffer and centrifuged at 500xg. Fifty thousand events were acquired for analysis.Gating strategy: Using forward scatter and side scatter (FS/SS), initial 1st gating was performed on lymphocytes area in the dot plot graph, then quadrant plot was used for CD3 versus CD4 for detection of T helper cell population (CD3+/CD4+). A 2nd gate was set on Th population [CD3+/CD4+]. A single histogram was drawn for detection of percentage of subset positive for CD38+ cells within Th cells (Figure, 1). Data was also expressed as mean fluorescence intensity (MFI) of CD38 using single histogram as illustrated in (Figure, 2).
2.4. Plasma cfDNA Extraction and Quantification
- The cfDNA was extracted from 200 μL of plasma manually using the QIAamp DNA Blood Mini kit (Qiagen, Germany) according to the manufacturer’s protocol and was eluted in 50 µL of elution buffer then concentrations were measured using QIAXpert (Qiagen, Germany).Real – Time Quantitative PCRPlasma cfDNA was determined by real-time quantitative PCR for β-actin gene found in all nucleated cells using Quanti Tect SYBR Green Master Mix (Qiagen, Germany) on a Rotor-Gene Q detection system (Qiagen, Hiden Germany). The primers sequence were: forward (5′–3′): GCGCCGTTCCGAAAGTT; reverse (5′–3′): CGGCGGATCGGCAAA. Total reaction volume was 25ul containing 12.5 ul SYBR green master mix (Qiagen, Hiden, Germany), 1ul of each primer, and 10.5 uL of the extracted plasma supernatant. Fluorescence measurements were made in every cycle. The cycling conditions used were as follows: PCR initial active step at 95°C for 15 min, then 40 cycles which includes denaturation at 94°C for 15s, annealing at 55°C for 30s then extension at 70°C for 30s. Melting curve analysis was performed after the thermal profile to ensure specificity in the amplification. Temperature increased very slowly (from 65°C to 95°C) with monitoring of fluorescence signal. It resulted in detection of single sharp peak for the target.The absolute DNA concentration was calculated according to the standard curve generated using serial dilutions of genomic DNA ranging from 0.00001 to 100 ng/µL.
2.5. Statistical Analysis
- Data were coded and entered using the statistical package SPSS (Statistical Package for the Social Sciences) version 25. Data was summarized using median and interquartile range in quantitative data and using frequency (count) and relative frequency (percentage) for categorical data. Comparisons between quantitative variables were done using the non-parametric Kruskal-Wallis and Mann-Whitney tests. For comparing categorical data, Chi square test was performed. Exact test was used instead when the expected frequency is less than 5. Correlations between quantitative variables were done using Spearman correlation coefficient. ROC curve was constructed with area under curve analysis performed to detect the best cutoff value of cfDNA or CD38% for detection of disease activity. P-values less than 0.05 were considered statistically significant.
3. Results
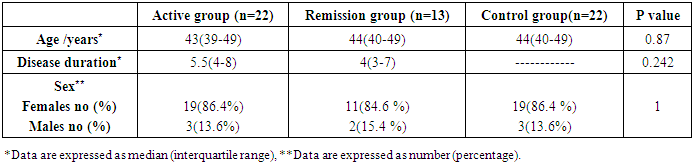
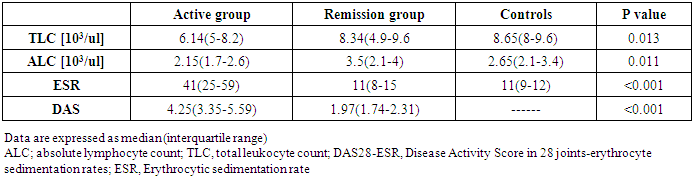
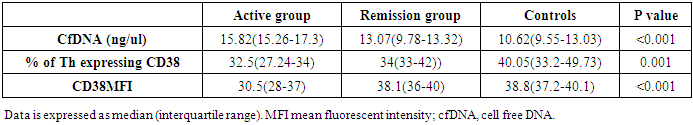
- The study was conducted on 22 active RA patients together with 13 RA patients in remission in addition to 22 healthy controls. No significant difference was observed among groups regarding age, sex or disease duration. Demographic data of study participants is illustrated in (Table, 1). Lab findings of the three study groups are illustrated in (Table, 2).
|
|
|
|
|
4. Discussion
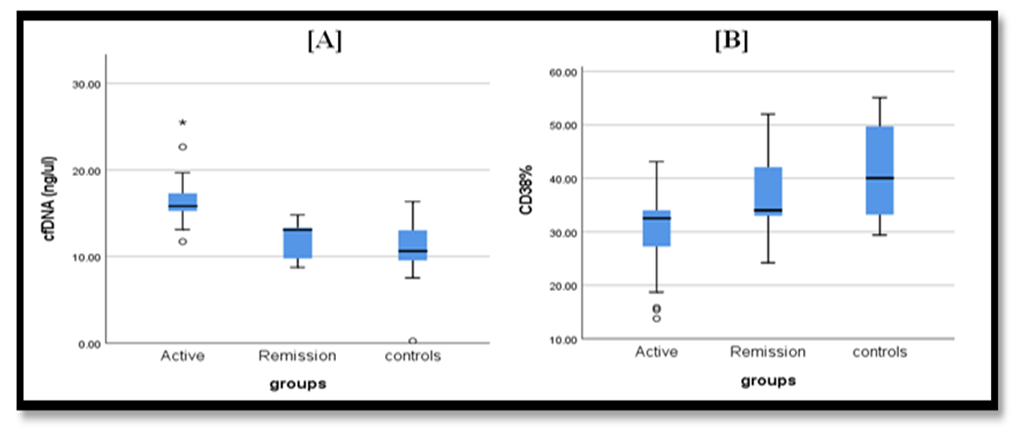
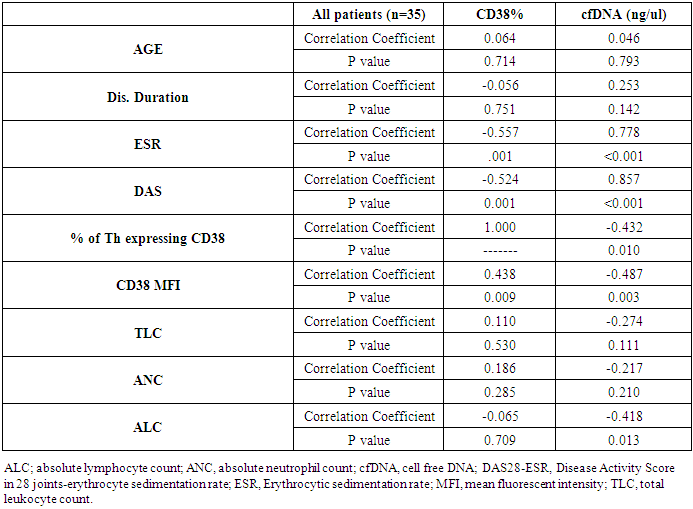
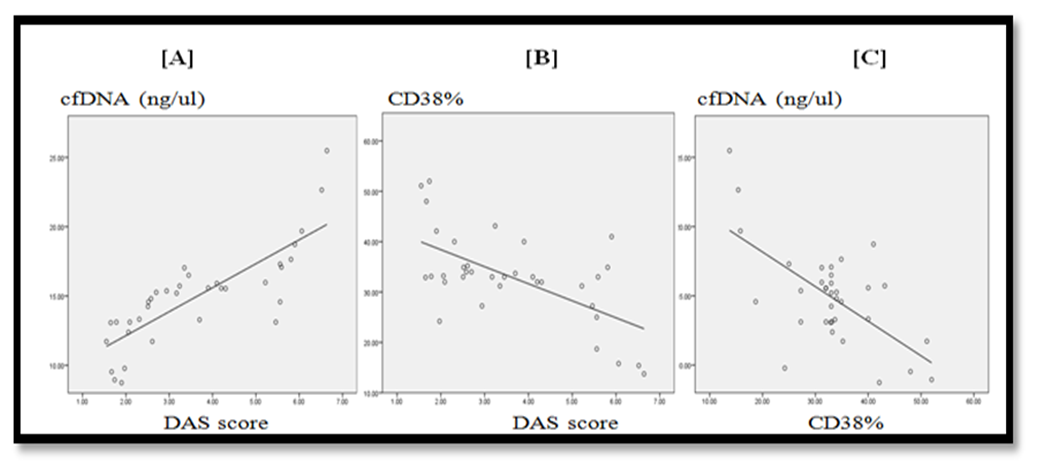
- Rheumatoid arthritis is characterized by chronic inflammation of joints, and erosion of bone. The activity of the disease is associated with decrease physical muscle performance [15], impairing the quality of patient life, and negatively influencing society health resources [1].Successful treatment of RA relies on patients attaining low disease activity or a state of remission [4]. Unfortunately, suggestive patient reported outcomes (PROs) are widely included to evaluate the activity of RA which may influence the precise evaluation of disease state [6]. Accordingly, the precise evaluation of disease activity can be achievable goal with the introduction of objective biological markers to monitor the disease activity and the effect of therapy. The cfDNA and CD38 molecule were linked to cell viability and activation during inflammatory processes that is why both markers were the interest of this current study.Cell-free DNA present in blood and other body fluids received growing attention as a potential biomarker of many conditions like sepsis, pregnancy related complications, cancers and kidney disease and those studies proved that cfDNA originates from apoptotic and damaged cells [16-19]. Normally, phagocytic cells remove apoptotic debris, decreasing the consequences of the presence of dead cells materials [20]. This phagocytic system is overwhelmed in states of diseases when cell death exceeds the clearance capacity [21]. Previous data may suggest a link between the level of inflammation, and the amount of cfDNA released from damaged cells in the circulation, which gives a potentiality of cfDNA for being a biomarker for assessing degree of RA severity.Synovial membrane of small joints is considered the primary site of inflammation in RA. The synovial macrophages, fibroblasts and lymphocytes are critical to the pathogenesis of this disease, in which apoptosis may play crucial roles [22]. Still it was shown that there was a significant, systemic inflammatory changes associated with the disease, and that immunopathological changes in circulating lymphocytes may have an important role in pathogenesis of RA [23].CD38 is a transmembrane receptor expressed in many immune system cells. It is involved in cell activation, and proliferation. CD38 was also linked to both cell viability and survival [13, 24].Comparison of the 3 groups of the study showed significant difference in levels of plasma cfDNA, being highest in active RA patients (p<0.001) (Table, 3) (Figure, 3A). This finding was in agreement with Bartoloni et al. and Zhong et al. They showed a substantial increase in cfDNA concentration in plasma of RA patients [25, 8]. They attributed high levels of cfDNA to increased extrusion of DNA form neutrophil extracellular traps by neutrophils. Also, the results of the present study were in accordance with Hashimoto et al. who reported that serum cfDNA is increased in RA patients owing to the tumour‐like robust proliferation of rheumatoid synovium (Synovial hyperplasia contains different inflammatory cells as lymphocytes, neutrophils and macrophages) [26].On the other hand, Dunaeva et al. (2015) showed a substantial decrease in cfDNA concentration in plasma of old RA patients than early RA patients [27]. This discrepancy may be due to difference in classification of groups of patients studied in current study as both high active and low active were pooled in one group.In the present study, cfDNA was significantly higher in active RA than those in remission (p <0.001). There was no significant difference in cfDNA concentration between RA patients in remission and controls (p=1.000) which may be attributed to great reduction of the inflammatory process in remission phase. Previous data points out the possible role of cfDNA as a biomarker of activity throughout the course of disease and to follow up effect of therapy in patients receiving treatment.Our study showed that both % of T helper cells expressing CD38, and CD38 MFI were significantly lower in RA active group compared to controls and patients in remission (p=0.001) and (p<0.001) respectively (Table, 3). These findings may throw light on the functional roles of human CD38 in autoimmune diseases and that its decrease is linked with autoimmunity.This was in agreement with Malashchenko et al. (2018) who reported that enhancing the proportion of CD38+ in T cell subsets, prevents the development of potentially damaging inflammatory reactivity [12]. Also, Frasca et al. (2006) suggested that CD38 is involved in functions crucial for establishing and regulating the adaptive immune response and that of CD38 multiple interactions with signalling receptors rule innate and adaptive immune responses by tuning dendritic cells (DC) migration, survival, and immunosuppressive Th1-polarization ability [10].In addition, Domínguez-Pantoja et al. (2018) proposed that CD38 deficiency is to accelerate autoimmune processes, and RA activity. They reported that CD38−/− mice exhibited elevated titres of antinuclear antibodies (ANAs) and anti‐double stranded DNA (anti‐dsDNA) antibodies and reduction in survival of CD38−/− mice compared to the wild type. Also, they reported the effect of CD38 expression on B cells and that CD38 was highly expressed by CD1dhigh/CD5+ regulatory B cells, and the agonistic anti CD38 stimulus was able to increase regulatory B cell subset % and its ability to induce IL-10 production [28]. Together, these results suggest that CD38 molecule could play a role in the control of autoimmune diseases through their expression on regulatory B cells.Our study was also in line with Cole et al. (2018) who examined CD38 expression in both circulating and synovial mononuclear cells in RA and they stated that circulating T cells showed very low CD38 expression, whereas the highest CD38 expression was observed on plasma cells, but as they performed immunohistochemistry assessment on synovial tissue they reported CD38 high staining in CD3+ T cells from patients with early phases of RA [29]. This data revealed reduced expression of CD38 on circulating CD3+ T lymphocytes with high expression on local lymphocytes resting in the synovial region, which might be attributed to different pattern of inflammatory mediators.On other hand some reports have shown elevated CD38 expression in patients with autoimmunity [30]. A study by Viegas et al. (2011) reported that in active SLE, the patients had higher numbers of CD38 expressing T and B cells. Still their study also proved that absence of CD38 altered the differentiation of T cells in mice, proving the role of CD38 in differentiation and proliferation of lymphocytes [31]. Discrepancy of levels of expression could be attributed to difference in method of assay, studied species and type of autoimmune disease.The cfDNA was directly correlated with (DAS28-ESR) score (r=0.857, p <0.001) and with ESR (markers of disease activity) (r=0.778, p <0.001) (Table, 5). This finding was in accordance with study made by Abdelal et al. (2016) who reported that the concentration of plasma cfDNA might indicate the extent of cell damage and inflammation [32]. Accumulation of cfDNA in plasma might result from an excessive release of DNA caused by massive cell death or inefficient elimination of dead cells during inflammation.Current study stated that there was inverse correlation between cfDNA and and % of Ths expressing CD38 (r= -0.432, p=0.01) (Table, 5), this could be explained by Romero-Ramirez et al. (2011) study which stated that CD38 is able to induce proliferation, and survival of human lymphocytes [11]. Also, Frasca et al. (2006) stated that decreased CD38 expression is linked to initiation of apoptosis and loss of its stimulatory signalling which induce cytokine production linked to maintaining cell proliferation and regulating cell cycle [10].Smolewska et al. (2003) stated that abnormalities in regulation of apoptosis may have a role in the pathogenesis of autoimmune disorders, including RA. They reported increased apoptosis of circulating lymphocytes despite its inhibition in synovial fluid of Juvenile idiopathic arthritis [33].In addition, cfDNA showed inverse correlation with ALC (r= -0.418, p=0.013) (Table, 5). This may be explained partly by increased apoptosis of total circulating lymphocytes as reported by Moodley et al. (2011). They observed that in RA, circulating lymphocytes showed signs of elevated apoptosis and concluded that the apoptotic status of circulating lymphocytes in RA may be a useful indicator of underlying pathological processes or disease activity [23]. Current data points out the possible role of the lymphocyte death in formation of circulating cfDNA adding to the previous studies about the link between cfDNA and neutrophil death with formation of neutrophil extracellular trap (NET) in many autoimmune diseases, including RA. NETs were said to be extruded by neutrophils and consist of cfDNA combined with antimicrobial peptides and proteases [34].
5. Conclusions
- Up-regulation of plasma cfDNA, and down-regulation of % of Ths expressing CD38 may serve as indicators for RA activity. Using up regulation of cfDNA as a biomarker of RA activity showed higher discriminative ability than down regulation of Ths expressing CD38 in differentiating RA activity from remission. Down regulation of CD38 expression on Ths during RA activity would drag the attention to its protective role and could suggest a potential role in therapy.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML