-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2018; 8(1): 7-12
doi:10.5923/j.ajb.20180801.02

Effects of Canarium schweinfurthii Oil Extract on Some Biochemical Indices on Indomethacin Induced Hepatotoxicity in Rats
M. A. Dogara 1, S. Sarkiyayi 1, H. G. Sheriff 2
1Department of Biochemistry, Modibbo Adama University of Technology, Yola, Nigeria
2Department of Applied Sciences, Kaduna Polytechnic, Nigeria
Correspondence to: S. Sarkiyayi , Department of Biochemistry, Modibbo Adama University of Technology, Yola, Nigeria.
| Email: |  |
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The therapeutic effect of administration of oil extract of Canarium schweinfurthii on Indomethacin induced hepatotoxixity in rats was investigated. Twenty five albino rats were divided into five groups with five rats each. Group 1 served as baseline control, group 2 was administered with the oil extract only. Groups 3, 4 and 5 were administered 200mg/kg of indomethacin. Then, groups 4 and 5 were treated with 1mL/kg and 2mL/kg of oil extract respectively, while group 3 served as indomethacin negative control group. Among indices investigated were some biochemical and haematological parameters. At the end of the study, the results of our findings revealed that the level of haemaglobin and PCV significantly decreases (p< 0.05) and white blood cell increases significantly (p< 0.05) in group 3 (negative control) when compared to group 1 (baseline control) and group 2 (oil control). Also, these parameters shows a gradual significant increase (p< 0.05) when the animals were treated with 1mL/kg and 2 mL/kg of the oil extract, while the white blood cell decreases significantly (p< 0.05) toward normal. Ourfindings shows that the results for alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase there were significant increase (p< 0.05) in the level of these parameters in group 3 (negative control) when compared to group 1(baseline control) and group 2 (oil control). When the animals were treated with 1ml/kg and 2ml/kg of the oil extract, there were significant (p< 0.05) decreases in the parameters levels. The result for the albumin and protein levels decreased significantly (p< 0.05) in group group 3 (negative control) when compared to group 1 (baseline control) and group 2. The levels of bilirubin, urea and creatinine significantly increase (p< 0.05) in group 3 (negative control) as compared to group 1 (baseline control) and group 2 (oil control). But when treated with 1mL/kg and 2 mL/kg of oil extract in groups 4 and 5, the protein and albumin levels significantly increase (p< 0.05) while the bilirubin, urea and creatinine levels decreases significantly (p< 0.05). The results revealed that the oil extract of Canarium schweinfurthii possesses some hepatocurative activity.
Keywords: Canarium schweinfurthii, Biochemical indices, Hepatotoxixity, Indomethacin
Cite this paper: M. A. Dogara , S. Sarkiyayi , H. G. Sheriff , Effects of Canarium schweinfurthii Oil Extract on Some Biochemical Indices on Indomethacin Induced Hepatotoxicity in Rats, American Journal of Biochemistry, Vol. 8 No. 1, 2018, pp. 7-12. doi: 10.5923/j.ajb.20180801.02.
Article Outline
1. Introduction
- Medicinal plants may be defined as those plants that are commonly used in treating and preventing specific ailments and diseases that are generally considered to be harmful to humans (Anselem, 2004). These plants are either “Wild plant species” those grown spontaneously in self-maintaining populations in natural or semi-natural ecosystems and can exist independently of direct human actions or the contrasting “Domesticated plant species” those that have arisen through human actions such as selection or breeding and depend on management for their existence. An example is Aloe barbadensis (Cowley, 2002). Since the dawn history, man has been dependent on medicinal plants for health and food needs. The traditional use of medicinal plants for curing and preventing illness, including the promotion of both physical and spiritually well-being among human beings, has become paramount in almost every house-hold. Several species of medicinal plants have been identified to be naturally distributed in communities. Plants species of medicinal importance include: Asiminatriloba (Paw paw), Citrus autentifolia (Lime) Psidiumguajava (Guava), Zingiberoffinales, AzadirachaIndica (Neem). Others include Aspila Africana (haemorrhage plant), Venoniaamagdalina (Bitter leaf), Gongronemalatifolia (Utazi), Canarium schweinfurthii (Atile), among others. (sofowora, 1986). Many of these plants species are known as a source of medicine for treating a particular ailment, without knowledge that two or more species could be mixed together to produce a more effective medicine. For instance, Azadirachaindica (Neem) is commonly used or known for treating malaria scourge, though it could also use to treat ailments like hepatitis and intestinal problems when mixed with the bark and leaves of Citrus aurantifolia. Modern science has established new frontiers in the human research for knowledge, but there is still cluster of mysteries surrounding human physiology and chemistry which science is yet to discover. However, medicinal plants were found to be a major source of active principles capable of curing diseases and maintaining good health. They continue to be important to people who do not have access to orthodox medicines; hence, the modern pharmaceuticals rely on these plants in compounding their drugs and traditional healers prepare their medicine from local herbs and administer them to their patients. (Cowley, 2002) reported that one of the most important drugs obtained from the bark of several species of cinchana, all of which are small ever green trees with a hard thicky grey bark growing in the valleys of southern America. The most important of the various alkaloids, obtained from cinchona bark is quinine with other numerous important alkaloids obtained from cinchona bark such as cinchonine, chinchonidineand quinidine. The relative proportion of these alkaloids varies in different species. Alaribe (2008) reported that about 80% of Nigerian home, maintain some private family tradition medicine practices. Canarium schweinfurthii is a widely reported plants used in different parts of Africa. Both the leaf, roots, stem barks are used in preparing remedies for the treatment of ailments. The essential oil of Canarium schweinfurthii has been reported to exhibit anti-diabetic, analgesic, antimicrobial, antioxidant, and also ulcer treating properties among others hence the need to assay it hepatoprotective properties. Therefore, the main aim of this research is to investigate the effects of Canarium schweinfurthii oil extract on some biochemical indices on indomethacin induced hepatotoxicity in rats.
2. Materials and Method
2.1. Materials
- Collection and Identification of fruitsFresh fruits of Canarium schweinfurthii (atile) were collected from farm land around kpanshi area in Jos south local government area of Plateau state. The plant was identified and authenticated at the department of plant science, School of Pure and Applied Sciences, Modibbo Adama University of Technology Yola. Nigeria.Experimental AnimalsWhite albino rats of wistar strain were obtained from the animal house unit of the department of pharmacology University of Jos, Plateau state.Chemicals and ReagentAll other reagents and solvents used were of analytical grade.
2.2. Methods
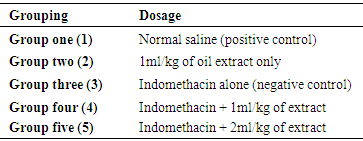
- Extraction of oilFruits of Canarium schweinfurthii was rinsed and the fleshy part was peeled off to remove the seed. The fleshy part was weighed and soaked in n-hexane solution for 72hrs. The oil was filtered and dried at 70°C in an oven to remove excess hexane. Administration of oil extractThe oil extract was given orally at a dose of 1 mL and 2 mL per kg of body weight. This was administered 2 hours after the administration of indomethacin. Experimental designTwenty five (25) white albino rats of wistar strain were housed in a well-ventilated plastic cages, and was acclimatized for 7 days prior to their randomization into various groups. The rats were divided into five groups, each having five (5) rats.
|
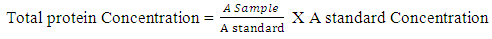 Determination of Serum AlbuminThe determination of serum albumin was carried out using colorimetric method (Modification of Bartholomew and Delaney method, 1966). Briefly, Two test tubes labeled ‘test’ and ‘standard’ have 4 mL of working coloured solution dispensed into them. To the test tube labeled ‘test’, 0.02ml of serum was added and 0.02mL of standard to the ‘standard’ test tube. The test tubes contents was separately mixed and incubated at 37°C for 10minutes. The spectrophotometer was set to zero using working BCG. The absorbance of test and standard were read at 628nm.
Determination of Serum AlbuminThe determination of serum albumin was carried out using colorimetric method (Modification of Bartholomew and Delaney method, 1966). Briefly, Two test tubes labeled ‘test’ and ‘standard’ have 4 mL of working coloured solution dispensed into them. To the test tube labeled ‘test’, 0.02ml of serum was added and 0.02mL of standard to the ‘standard’ test tube. The test tubes contents was separately mixed and incubated at 37°C for 10minutes. The spectrophotometer was set to zero using working BCG. The absorbance of test and standard were read at 628nm.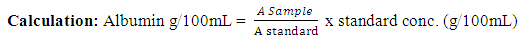 Albumin g/1 = value obtained in g/100mL multiplied by 10Concentration of standard = 3g/100mL or 30g/LDetermination of Serum Alkaline phosphatase (ALP)This method of King-Amstrong (1964) was used in determining the serum alkaline phosphatase. Briefly, to 0.01mL of serum in a test tube labeled “Test”, 0.50mL of reagent (p- nitrophenylphosphate) was added, mixed and the initial absorbance was read and timer was started simultaneously. It was read again after 1, 2 and 3 minutes. Calculation:U/l=2760 × A sample at 450nm/min.Determination of Serum Aspartate aminotransferase (AST)Serum AST activity was estimated according to the method of Reitman and Frankel (1957).Into two clean test tubes, separately labeled ‘test’ and ‘Reagent Blank’ 0.5mL of substrate buffer (solution) was added to each test tube and 0.1 mL of serum was added to the ‘Reagent Blank’ tube, it was mixed and incubated for exactly 30 minutes at 37°C. 0.5mL of solution 2 (2, 4-dinitrophenyl hydrazine) was added to each test tube. Mixed and allowed to stand for 20 minutes at 25°C. 50mL of sodium hydroxide was finally added to each test tube and the absorbance of sample was read against the reagent blank after 5 minutes at 546 nm.Determination of Alanine aminotransferase (ALT)Serum ALT activity was estimated according to the method Reitman and Frankel (1957) Briefly, Into two clean test tubes, separately labeled ‘Test’ and ‘Reagent Bland’ 0.5mL of solution (buffer) was added to each test tube, 0.1 mL of serum was added to the reagent blank tube. It was mixed and incubated for 30 minutes at 37°C. Solution R1 (2,4-dinitrophenylhydrazine). 0.5 mL was added to each test tube, mixed and allowed to stand for 20 minutes at 25°C. Then 5mL of 0.1M NaoH was finally added and reading taken after 5 minutes.Determination of Serum BilirubinColorimetric method based on that described by Jendrassic and Grof (1938). Briefly, two test tube labeled ‘B’ blank and ‘T’ sample, 0.2mL of reagent 1 (sulphanilic acid + hydrochloric) was pipetted in to each of the test tubes. 0.05mL (a drop) of reagent 2 (sodium nitrite) was added to test tube ‘T’ followed by 1 mL of reagent 3 (caffeine = sodium benzoate) added to each of the test tubes. 0.2mL of the sample was then pipetted and added also to each test tube, then allowed to stand for 10 mintues at 20-25°C finally 1 mL of reagent 4 (Tartrate + sodium hydroxide) was pipetted into each of the test tube. Mixed and allowed to stand for 5-30 minutes at 25°C, absorbance of sample and standard was read against the blank.For Direct BilirubinTwo test tubes labeled ‘B’ blank and ‘T’ sample. 0.20 mL of reagent I (sulphanilic acid = hydrochloric acid) was pipetted into each of the test tubes. A drop (0.05mL) of reagent 2 (sodium nitrite) was added to the ‘T’ test tube then 2 mL of sodium chloride was pipetted and added to each of the test tubes. Followed by 0.2mL of the sample also allowed to stand for exactly 5 minute at 20-25°C before reading the absorbance of sample against sample blank.Calculation:Total Bilirubin (umol/L) =185 X ATBTotal Bilirubin (mgldL) = 10.8 X ATBDirect Bilirubin (umol/L) = 14.4 X ADBTo get concentration of unconjugated bilirubin (indirect).= Total bilirubin – conjugated bilirubin (direct) Determination of Serum CreatinineThe method of Hare (1950) was used in the determination of serum creatinine. Serum (1 mL) was added to trichloroacetic acid and 1ml of deproteinizing agent. It was mixed and centrifuged at 2,500rpm for 10 minutes. 0.5ml of solution 1 was pipetted into the ‘standard test tube, 0.5mL of TCA was pipetted into ‘standard’ and ‘blank’ test tubes, 1mL of the supernatant (sample) was pipetted into the ‘test’ test tube, 1ml of reagent mixture was pipetted into each of the three test tubes. Mixed and allowed to stand for 20 minutes at 25°C. The absorbance of sample and standard was measured against the blank at 546nm spectrophotometrically.Calculation:Concentration of creatinine in serum or plasma
Albumin g/1 = value obtained in g/100mL multiplied by 10Concentration of standard = 3g/100mL or 30g/LDetermination of Serum Alkaline phosphatase (ALP)This method of King-Amstrong (1964) was used in determining the serum alkaline phosphatase. Briefly, to 0.01mL of serum in a test tube labeled “Test”, 0.50mL of reagent (p- nitrophenylphosphate) was added, mixed and the initial absorbance was read and timer was started simultaneously. It was read again after 1, 2 and 3 minutes. Calculation:U/l=2760 × A sample at 450nm/min.Determination of Serum Aspartate aminotransferase (AST)Serum AST activity was estimated according to the method of Reitman and Frankel (1957).Into two clean test tubes, separately labeled ‘test’ and ‘Reagent Blank’ 0.5mL of substrate buffer (solution) was added to each test tube and 0.1 mL of serum was added to the ‘Reagent Blank’ tube, it was mixed and incubated for exactly 30 minutes at 37°C. 0.5mL of solution 2 (2, 4-dinitrophenyl hydrazine) was added to each test tube. Mixed and allowed to stand for 20 minutes at 25°C. 50mL of sodium hydroxide was finally added to each test tube and the absorbance of sample was read against the reagent blank after 5 minutes at 546 nm.Determination of Alanine aminotransferase (ALT)Serum ALT activity was estimated according to the method Reitman and Frankel (1957) Briefly, Into two clean test tubes, separately labeled ‘Test’ and ‘Reagent Bland’ 0.5mL of solution (buffer) was added to each test tube, 0.1 mL of serum was added to the reagent blank tube. It was mixed and incubated for 30 minutes at 37°C. Solution R1 (2,4-dinitrophenylhydrazine). 0.5 mL was added to each test tube, mixed and allowed to stand for 20 minutes at 25°C. Then 5mL of 0.1M NaoH was finally added and reading taken after 5 minutes.Determination of Serum BilirubinColorimetric method based on that described by Jendrassic and Grof (1938). Briefly, two test tube labeled ‘B’ blank and ‘T’ sample, 0.2mL of reagent 1 (sulphanilic acid + hydrochloric) was pipetted in to each of the test tubes. 0.05mL (a drop) of reagent 2 (sodium nitrite) was added to test tube ‘T’ followed by 1 mL of reagent 3 (caffeine = sodium benzoate) added to each of the test tubes. 0.2mL of the sample was then pipetted and added also to each test tube, then allowed to stand for 10 mintues at 20-25°C finally 1 mL of reagent 4 (Tartrate + sodium hydroxide) was pipetted into each of the test tube. Mixed and allowed to stand for 5-30 minutes at 25°C, absorbance of sample and standard was read against the blank.For Direct BilirubinTwo test tubes labeled ‘B’ blank and ‘T’ sample. 0.20 mL of reagent I (sulphanilic acid = hydrochloric acid) was pipetted into each of the test tubes. A drop (0.05mL) of reagent 2 (sodium nitrite) was added to the ‘T’ test tube then 2 mL of sodium chloride was pipetted and added to each of the test tubes. Followed by 0.2mL of the sample also allowed to stand for exactly 5 minute at 20-25°C before reading the absorbance of sample against sample blank.Calculation:Total Bilirubin (umol/L) =185 X ATBTotal Bilirubin (mgldL) = 10.8 X ATBDirect Bilirubin (umol/L) = 14.4 X ADBTo get concentration of unconjugated bilirubin (indirect).= Total bilirubin – conjugated bilirubin (direct) Determination of Serum CreatinineThe method of Hare (1950) was used in the determination of serum creatinine. Serum (1 mL) was added to trichloroacetic acid and 1ml of deproteinizing agent. It was mixed and centrifuged at 2,500rpm for 10 minutes. 0.5ml of solution 1 was pipetted into the ‘standard test tube, 0.5mL of TCA was pipetted into ‘standard’ and ‘blank’ test tubes, 1mL of the supernatant (sample) was pipetted into the ‘test’ test tube, 1ml of reagent mixture was pipetted into each of the three test tubes. Mixed and allowed to stand for 20 minutes at 25°C. The absorbance of sample and standard was measured against the blank at 546nm spectrophotometrically.Calculation:Concentration of creatinine in serum or plasma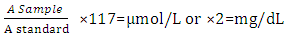 Determination of Serum UreaUrea in serum is hydrolyzed to ammonia in the presence of urease. The ammonia was then measured spectrophotometrically by Berthelots reaction (Weatherburn, 1976). Brifly, three test tubes labeled ‘T’ for test ‘S’ for standard and ‘B’ for blank, to each 10ul of sample, 10uL standard and 10ul of distilled was pipetted into it respectively. Hundred 100ul of solution 2 (sodium nitroprusside + urease) was pipetted into each of the test tube, mixed and incubated at 37°C for 10 minutes. After that, 2.5ml of sodium hypochlorite (solution 4) was pipetted into each of the test tubes. Mixed and incubated at 37°C for 15 minutes. The absorbance of sample and standard were read at 546nm against blank.Calculation:
Determination of Serum UreaUrea in serum is hydrolyzed to ammonia in the presence of urease. The ammonia was then measured spectrophotometrically by Berthelots reaction (Weatherburn, 1976). Brifly, three test tubes labeled ‘T’ for test ‘S’ for standard and ‘B’ for blank, to each 10ul of sample, 10uL standard and 10ul of distilled was pipetted into it respectively. Hundred 100ul of solution 2 (sodium nitroprusside + urease) was pipetted into each of the test tube, mixed and incubated at 37°C for 10 minutes. After that, 2.5ml of sodium hypochlorite (solution 4) was pipetted into each of the test tubes. Mixed and incubated at 37°C for 15 minutes. The absorbance of sample and standard were read at 546nm against blank.Calculation: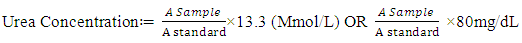
3. Results
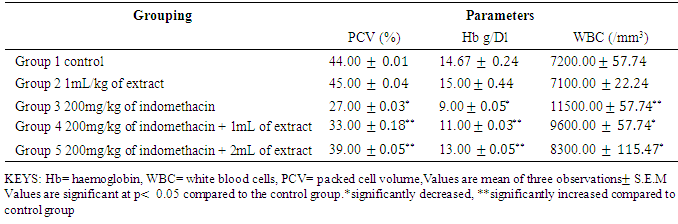
- Table 2: shows the result of serum PCV, Haemoglobin, and White blood cell. The level of haemaglobin and PCV significantly decreases (p< 0.05) and white blood cell increases significantly (p< 0.05) in group 3 (negative control) when compared to group 1(baseline control) and group 2 (oil control). Also, this parameter shows a gradual significant increase (p< 0.05) when treated with 1ml and 2ml of the oil extract while the white blood cell decreases significantly (p< 0.05) toward the normal.
|
|
|
4. Discussion
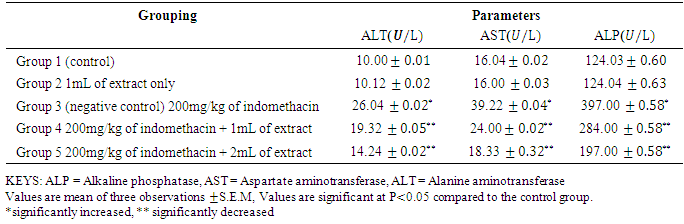
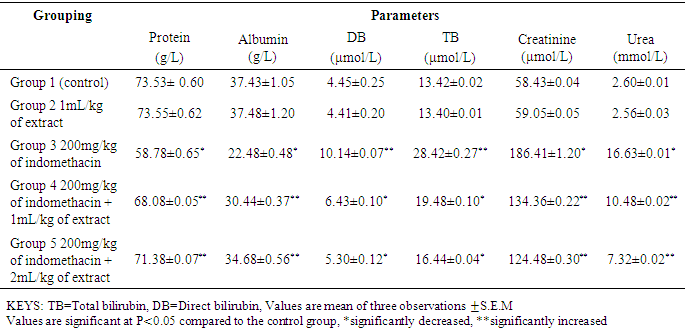
- Drug induced liver injury is a major problem that challenges not only health care professionals but also the pharmaceuticals and drug regulatory agencies. Indomethacin is a common, anti-inflammatory, antipyretic, and analgesic agent which is safe at therapeutic doses but can produce fatal hepatic necrosis in man, rats and mice with toxic doses (Lewis, 2013).The results In Table 2 the results shows that there were significant decrease (p < 0.05) in the PCV and Haemoglobin level of Group 3 (negative control) as compared with group 1 (baseline control) this shows the effect of indomethacin on the group 3 rats. The WBC increases significantly (p < 0.05) in group 1 as compared to group 1 (baseline control) and group 2 (oil control). The white blood cells usually increase in number when there is an infection in the cell or due to a reaction to a drug that increases white blood cell production. Treatment with 1mL/kg and 2mL/kg of oil extract significantly reverses the effect of the indomethacin on the group 4 and 5 rats. Also, administration of indomethacin to rats markedly increases tissue AST, ALT, ALP, total bilirubin, direct bilirubin, creatinine and urea. In a related development, Lin (1991) reported that an increase in the levels of tissue amino tranferases is known to reflect the severity of liver injury. Tissue bilirubin and ALP levels are related to function of the hepatic cell and increase in tissue level of ALP is due to increase synthesis of this enzyme (Moss and Butterworth, 1974). The increase in these liver parameters is clear indications of cellular leakage and loss of functional intergrity of the membrane resulting from liver damage (Saraswat, et al, 1993). The level of serum protein and albumin shows a significant decrease (p < 0.05) in group 3. Albumin accounts for half the serum protein content, a decrease in albumin is often associated with a decrease in total protein. A decrease in serum total protein may reflect decreased protein synthesis or increase protein loss, nearly all proteins are synthesized in the liver; hence, hepatic failure is a cause of decreased serum protein. The group 2 rats which were administered 1mL/kg of oil extract only, showed no deviation in the levels of haematolgical parameters from the control group. There was no significant change in the level of liver enzymes and other biochemical parameters when compared to the control group. However, the protein and albumin levels significantly increased (p< 0.05) as compared to control group. This means that the oil extract of Canarium schweinfurthii has no effect on the rat’s tissue/organ but also boost the immune system of the group 2 rats. Group 4 and 5 were treated with 1mL/kg and 2mL/kg of extract respectively. In a related development, Koudou et al, (2005) reported that administration of 3ml/kg of Canarium schweinfurthii oil shows significant analgesic effects. This study also demonstrated that treatment of rats with oil extract of Canarium schweinfurthii caused a substantial reduction in the levels of AST, ALT, ALP, total bilirubin, direct bilirubin, creatinine, and urea when compared with group 3 (negative control). Also, the level of serum protein and albumin increases significantly (p< 0.05) when compared with the negative control group. The significant change in the levels of AST, ALT, ALP, bilirubin, urea, creatinine, protein and albumin in the treated groups (4&5) point towards an improvement in the function of the renal and hepatic cells.
5. Conclusions
- This study revealed that Canarium schweinfurthii extract possesses somehepatocurative effect against indomethacin induced hepatotoxicity. The oil extract showed significant (p<0.05 hepatocurative activity in the treated groups there by decreasing the elevated levels of liver enzymes AST, ALP, ALT and other liver parameter bilirubin and there were increasein levels of albumin and total protein in groups treated with extract of Canarium schweinfurthii.
6. Recommendations
- The fruit extract of Canarium schweinfurthii has the potential of curing liver damage which suggest its use by people. Additional work should be embarked upon to elucidate the possible mechanism of action of the extract.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML