-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2018; 8(1): 1-6
doi:10.5923/j.ajb.20180801.01

Pre- and Post-Treatment Effectiveness of SRP on Levels of IL-6, IL-10, and CRP in Chronic Periodontitis Patients with and without Diabetes
Abdul Samad Aziz1, MadhavGovind Kalekar1, Adinath Narayan Suryakar2, Rahul Kale3, Tabita Benjamin4, Madhurima Dikshit5
1Department of Biochemistry, Grant Medical College and Sir J J Group of Hospitals, Mumbai, India
2Registrar, Dr. D. Y. Patil Vidhyapeeth, Pune, India
3Department of Periodontology, M. A. Rangoonwala College of Dental Sciences and Research Centre, Pune, India
4Department of Dentistry, Grant Medical College and Sir J J Group of Hospitals, Mumbai, India
5Department of Chemistry, Savitribai Phule Pune University, Pune, India
Correspondence to: Abdul Samad Aziz, Department of Biochemistry, Grant Medical College and Sir J J Group of Hospitals, Mumbai, India.
| Email: |  |
Copyright © 2018 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: The relationship between periodontal disease and diabetes mellitus has been shown to be associated with activation of innate immunity. This is characterised by an altered inflammatory state in the diseased individuals. The present study assesses the effect of SRP (scaling and root planning) on some inflammatory markers in Chronic Periodontitis Patients with and without diabetes. Materials and Methods: A total of 168 individuals with generalized chronic periodontitis (CAL ≥ 3mm; American Association of Periodontology criteria 1999) were divided into two groups. Group I (CP): Non-diabetics (F Glucose≤ 5.5 mmol/L); n= 86; and Group II (CPDM): Diabetics (F Glucose≥ 7.0 mmol/L WHO 2006 criteria); n= 82. Their periodontal status was evaluated on the scale of gingival index (GI), plaque index (PI), probing depth (PD), and clinical attachment loss (CAL). The biochemical inflammatory markers assessed were Interleukins –6, –10, and C-Reactive Protein (CRP). The periodontal therapy (SRP) was performed on the individuals from both the groups and a follow-up was done after three months. Results: Individuals in the CPDM group showed significantly higher inflammation (p≤0.05) than those in the CP group. SRP reveals significant improvement (p≤0.001) in the study parameters compared to their corresponding pre-treatment values in both the groups. The extent of improvement, that is relative percentage change, also differed significantly (p≤0.05) between the groups. Conclusion: The patients with diabetes and periodontitis demonstrated higher oral and systemic inflammation compared to non-diabetics. The SRP therapy was effective in improving clinical and biochemical markers. However, the extent of improvement was lower in diabetic patients than those in the non-diabetic patients.
Keywords: Chronic periodontitis, Inflammatory markers, Scaling and root planing, Type 2 diabetes
Cite this paper: Abdul Samad Aziz, MadhavGovind Kalekar, Adinath Narayan Suryakar, Rahul Kale, Tabita Benjamin, Madhurima Dikshit, Pre- and Post-Treatment Effectiveness of SRP on Levels of IL-6, IL-10, and CRP in Chronic Periodontitis Patients with and without Diabetes, American Journal of Biochemistry, Vol. 8 No. 1, 2018, pp. 1-6. doi: 10.5923/j.ajb.20180801.01.
1. Introduction
- Among various systemic diseases, the interrelationship between periodontal disease and diabetes mellitus (DM) has been extensively investigated [1], and that has shown mutual influence on each other, where glycemic control appears to be an important determinant in this relationship [2, 3]. Hyperglycemia in DM is associated with a number of macro and micro vascular changes along with an activated innate immunity that may have a synergistic effect when it coexists with periodontitis in the host. This is characterized by higher levels of pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, and prostaglandin that may lead to tissue damage. Hyperglycemia leads to accumulation of advanced glycation end-products (AGEs) resulting in a pro-inflammatory state where AGEs bind to receptors to target cell surfaces. AGEs in human gingival tissue are responsible to increase apoptosis and impair bone repair and wound healing [4, 5].Further, the AGE-RAGE (receptor for AGE) interaction mediates long-term effects on key cellular targets of diabetic complication such as macrophages, glomerular mesangial cells, and vascular endothelial cells. These effects include the expression of various cytokines and growth factors by macrophages and mesangial cells. RAGE has been shown to mediate signal transduction through the generation of reactive oxygen species (ROS). Diabetes cause to change in redox balance, increased generation of reactive oxygen and nitrogen species (RONS), and modification of enzymes or structural proteins by glycation. This in turn impairs oxidant: anti-oxidant (AO) balance [6, 7]. Scaling and root planning (SRP): This is the phase I periodontal therapy and has been employed in controlling periodontitis in diabetics. This therapy yielded several clinical benefits such as reduction in probing depth (PD), bleeding on probing (BOP), and gain in clinical attachment level (CAL) [3]. The clinical improvement was also associated with reduction in IL1-β, elastase activity and MMP-8 and -9, TNF-α, hsCRP, and E-selectin levels [5, 8]. Numerous studies have demonstrated beneficial effect of SRP on periodontal clinical parameters and metabolic control in diabetics. Moreover, some studies have addressed the effect of SRP on systemic inflammatory markers in conjunction with clinical and metabolic control in type 2 diabetes mellitus with chronic periodontitis, which is being attempted in this study.
2. Materials and Methods
- The study was conducted jointly by the Institutional Ethics Committee of Grant Medical College and Sir J. J. Group of Hospitals, Mumbai. A written informed consent was obtained from all the subjects enrolled in the study. Study Groups: A total of 168 individuals who visited the department of dentistry, Grant Medical College, Mumbai, participated in the experiment. They were divided into the following study groups:(1) Group I (CP): Non-diabetics with generalized chronic periodontitis (CAL ≥ 3mm, F Glucose≤ 5.5 mmol/L); n= 86 (males=51, females=35), Mean age= 40.9±4.6.(2) Group II (CPDM): Diabetics with generalized chronic periodontitis (CAL ≥ 3mm, F Glucose≥ 7.0 mmol/L; WHO 2006 criteria); n= 82(males=50, females=32), Mean age= 47.7±7.0.The patients in the study groups were clinically evaluated for chronic periodontitis in compliance with the criteria (CAL≥3mm) of the American Academy of Periodontology 1999 [9]. Each patient had a minimum of 20 teeth present, of which at least 30% had a site with probing depth of 5 mm and clinical attachment level ≥3 mm. The patients in group CP were otherwise healthy, with no history of major illness or consumption of antioxidants, antibiotics, anti-inflammatory or any other drugs and they had not received any periodontal therapy in thelast 6 months prior to the inception of the study. The participants with a history of past illness and undergoing any treatment for diabetes and alcoholics were excluded from this group. The patients in the diabetic group (CPDM) were type 2 diabetics (according to the WHO criteria, fasting glucose ≥7.0 mmol/L) [10] with an average duration of diabetes being 7.8 ± 3.2 yrs. About 50% of them reported other complications of diabetes, predominantly retinopathy and nephropathy. They were taking oral hypoglycemic agents along with the diet restriction.Clinical MeasurementsThe periodontal status of the participants was evaluated on the scale of gingival index (GI) developed by Loe and Silness [11], plaque index (PI) as described by Silness and Loe [12], probing depth (PD), and clinical attachment loss (CAL). The PD and CAL measurements were done as prescribed in [13]. These measurements were based on UNC-15 probe introduced by Hu-Friedy from the University of North Carolina.Sample Collection4 ml venous blood was taken as sample from all the participants for standard precautionary measures. The sample was stored at room temperature for 30 minutes and centrifuged at 3,000 rpm for a period of 20 minutes to obtain serum. Serum was then stored at −4°C until further analysis. The blood samples were collected twice, once before treatment and second time after 3 months of SRP therapy.Biochemical StudiesInterleukins -6 and patients -10: The IL-6/-10 were analysed by a quantitative chemiluminescence assay [IMMULITE1000 systems, SIEMENS, Germany], following the instruction of the manufacturer (SIEMENS, Germany). Briefly, 100 µl of serum was loaded in the test units and was allowed to react with the respective IL assay reagents [alkaline phosphatase conjugated to murine monoclonal IL-6/-10 antibodies respectively in buffer]. The standard calibration was done using IL adjustors provided with kit that contains low and high of lyophilized ILs in a non-human serum matrix.C–Reactive Protein (CRP): CRP–Turbilatex (Beacon Diagnostics Pvt. Ltd., India) is a quantitative turbidimetric test to measure CRP in human serum or plasma. Latex particles, coated with certain anti-human CRP, are agglutinated and mixed with the samples that contained CRP. The agglutination shows change in correspondence with the CRP contents of the patient sample that can be quantified by comparison from a calibrator of known CRP concentration [14].Plasma Glucose: Glucose oxidase-Peroxidase (GOD-POD) kit was employed for glucose estimation ensuing manufacturer’s (Accurex Biomedicals, India) protocol [15].The CRP and glucose were measured on calibrated semi-automated analyser BIOTRON BTR-830.Periodontal TherapyGroup I and Group II patients received periodontal therapy that included scaling and root planning (SRP) and oral hygiene instructions. In the instructions, the participants were asked to brush twice daily after meals and were demonstrated on BASS technique of brushing [16]. The SRP was performed by qualified periodontologists using ultrasonic instrument (Electro Medical Systems, Switzerland) and manual Gracey curettes (Hu-Friedy, Avco). Statistical AnalysisThe values for clinical parameters and biochemical markers were analyzed using Statistical Package for Social Sciences (IBM-SPSS version 19) for Microsoft Windows. The values on clinical parameters and biochemical markers were expressed as mean ± SD across the study groups. The normality assumption for each clinical and biochemical parameter was tested using criteria suggested by George and Mallery [17]. Significance of comparative difference of clinical parameters and biochemical markers across the two study groups was done using independent sample t test. The paired sample t test was employed for intra group comparison. The relative percentage change was calculated using the following formula:
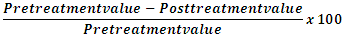 Where P value < 0.05 is considered to be statistically significant for all the statistical tests.
Where P value < 0.05 is considered to be statistically significant for all the statistical tests.3. Results
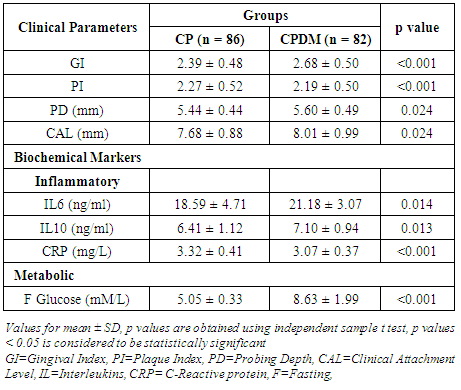
- The observed pre-treatment values indicate a significant difference (p<0.05) in the clinical and biochemical markers in the individuals belonging to the CP and CPDM groups (Table 1).
|
|
|
4. Discussion
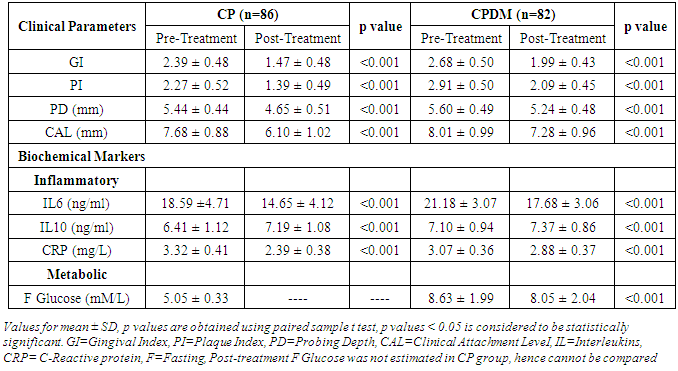
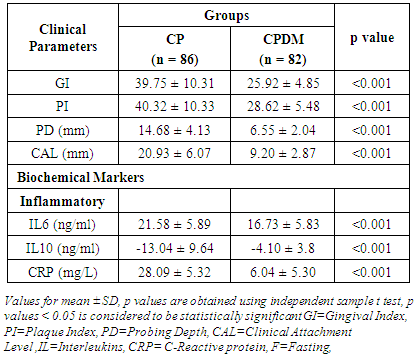
- Diabetes linked with hyperglycemia and the resultant AGEs induce hyperactive innate immune response leading to higher production of pro-inflammatory cytokines. The hyperactive innate immune response may be the precursor of both diabetes and periodontitis, which probably have a synergistic effect when they coexist in the host [4]. The hyperglycemia in diabetes is associated with adverse periodontal outcomes and severe periodontitis adversely affects glycemic control in diabetes [18]. The present study assesses the clinical periodontal parameters and systemic biochemical inflammatory markers in the patients with periodontitis and T2DM (CPDM). Further, the effects of SRP on these markers were observed and an attempt was made to compare the relative percentage changes between the diabetic and non-diabetic groups.The results of the present study demonstrated that the individuals in the CPDM group showed higher clinical damage and systemic inflammation compared to the CP group (Table 1). SRP is the most routinely used non-surgical periodontal therapy and several studies have reported improvement in clinical periodontal variables by the sole use of mechanical treatment [19]. The present study showed significant (p< 0.001) reduction in clinical parameters and significant (p< 0.001) improvement in biochemical markers post SRP (Table 2). Various studies [19-23] have reported improvement in periodontal clinical parameters in the patients with diabetes, after periodontal therapy, which is in accordance with our study.Among various inflammatory cytokines, association of gene polymorphism of IL-6 and IL-10 with T2DM has been reported [24]. These cytokine levels were evaluated in peripheral blood in DM with diabetic retinopathy and were found to be significantly differentfrom controls. IL-6 showed significant differences among different degrees of diabetic retinopathy and the risk of retinopathy in diabetic patients was decreased by an increase in IL-10 levels [25]. Serum levels of IL-6 and IL-10 were also evaluated in the patients with T2DM with soft tissue infection and were found to be highly significant. Furthermore, IL-6 was significantly correlated to glycemia in all the patients [26]. Hyperglycemia was reported to regulate the levels of IL-6, TNF-α in periodontal sites and their concentration was associated with DM [27]. Engebretson SP et al 2004 [28] associated elevated GCF IL-1β with poor glycemic control in the patients with periodontitis and T2DM. Talbret Jennifer J et al 2006 [29] evaluated the effect of SRP on systemic TNF-α and IL-6 along with metabolic control. They showed an improved periodontal measures following SRP; whereas systemic TNF-α and IL-6 did not show significant reduction which is similar to the results of Galhardo CGA et al 2013 [30]. O’Connell PA et al 2008 [21] showed the effect of SRP on the profile of systemic inflammatory markers related to both periodontitis and diabetes. The therapy was effective in significantly improving some pro-inflammatory cytokines and chemokines. Correa FOB et al 2008 [8] reported effectiveness of SRP in reducing the levels of IL-1β, elastase activity, and MMP-8 and-9 in GCF from the patients with T2DM and periodontitis. Similarly, Kardleslar L et al 2010 [20] showed decreased levels of IL-6, TNF-α, CRP, and leptin and increased adiponectin levels after SRP in the patients with T2DM and CP. These studies point to increased inflammation in the patients with diabetes and CP and showed that SRP was effective in reducing local and systemic inflammatory markers in such individuals. In the present study, IL-6 and IL-10 showed significantly (p = 0.014 and 0.013 respectively) higher values in the CP and CPDM groups (Table 1). SRP leads to significant lowering in IL-6 and increasing IL-10 (p<0.001) compared to the respective pre-treatment values (Table 2) in the CPDM group. Similar reduction in inflammatory marker in the CPDM group was also reported by O’Connell PA et al 2008 [21].CRP is used as a systemic inflammatory marker and higher CRP levels are reported in periodontitis patients than in healthy subjects [31-33]. Furthermore, its estimation was reported in diabetic patients with periodontitis. Chen L et al 2010 [34] estimated hsCRP in periodontitis patients with T2DM and showed higher hsCRP with increased clinical damage. Furthermore, the hsCRP was significantly correlated to mean PD in these patients. This clearly indicates that the periodontal damage can affect systemic inflammation in the patients with periodontitis and diabetes. However, Kardesler L et al 2010 [20] reported insignificant change in the CRP levels pre- and post-periodontal therapy. As both diabetes and periodontal disease are multifactorial, other confounding factors for CRP such as BMI, diet, and baseline characteristics affecting glycemic control should be adjusted [35]. The diet and BMI were not taken into consideration in the present study. Although there was no drastic change in the dietary pattern of the participants during the course of the study, we are not sure of the dietary effect on study parameters. In the present study, CRP level in diabetics was significantly (p<0.001) lowered than those in non-diabetics. This finding could be attributed to the effects of oral hypoglycaemic medicine consumed by the diabetic individuals. The oral hypoglycaemic medicine taken by the subjects contained anti-inflammatory agent “metformin” that could have affected the level of the acute phase systemic inflammatory protein, i.e. CRP. The level of CRP may have got adjusted due to prolonged use of metformin by the diabetic individuals. The same medicine may not have affected the levels of interleukins, which are inflammatory mediators at cellular level and are probably being produced constantly due to oral bacterial challenge in the diseased individuals. Further, when SRP was employed, the level of CRP got decreased (Table 2) which is also reported in [20, 36]. This is possibly the beneficial effect of the therapy on the inflammatory markers.When therapy response in terms of relative percentage change was analyzed and compared, it was found that the CPDM group showed significantly lowered therapy response. As the periodontal disease results from excess inflammation; an essential goal of intervention in inflammatory diseases is the return of the tissue to homeostasis defined as an absence of inflammation. Hence, the rapid and complete elimination of invading leukocytes from a lesion is the ideal outcome following an inflammatory event. Accordingly, the inadequate resolution and failure to return tissues to homeostasis results in neutrophil-mediated destruction and chronic inflammation. This results in destruction of both extracellular matrix and bone in periodontium [37]. Thus, the individuals in the CPDM group who represent additional inflammatory burden compared to CP showed relatively lowered therapy response. Though this shows limited benefit of SRP in the CPDM group, it is promising to note that it has significantly contributed to improving systemic inflammation and may have helped in maintaining metabolic status.
5. Conclusions
- In this study, we observed that the patients with diabetes and periodontitis demonstrated higher local (oral) and systemic inflammation compared to the individuals without diabetes and periodontitis. The SRP therapy was effective in improving clinical and biochemical inflammatory parameters. However, the extent of improvement was lower in diabetic than the non-diabetic group. This may be attributed to their health status. The therapy may contribute in checking the systemic inflammation at least those initiated and/or caused by oral infections.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML