-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2017; 7(5): 114-126
doi:10.5923/j.ajb.20170705.04

Relationship between Monocyte Subsets, IL-6 and hs-CRP with the Severity of Coronary Artery Disease in Stable Angina Pectoris Patients
Entsar R. Mokhtar1, Bothayna I. Saleh1, Abdelmohsen M. Aboualia2, Hala M. Nageb3
1Department of Clinical Pathology, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt
2Department of Cardiology, Faculty of Medicine Al-Azhar University, Cairo, Egypt
3Department of Internal Medicine, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt
Correspondence to: Entsar R. Mokhtar, Department of Clinical Pathology, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Monocytes are crucially involved in all stages of atherogenesis as cellular drivers of vascular inflammation hallmarking atherosclerotic disease. CD16+monocytes are proinflammatory cells, whose proportion is related to the occurrence of coronary artery disease (CAD), intma-media thickness and plaque stability. Interleukin-6 (IL-6) and highly sensitive C reactive protein (hs-CRP) were also closely related to atherosclerotic disease. Objective: We investigated the relationship between the monocyte subsets, IL-6, and hs-CRP with the severity of CAD assessed by coronary angiography (CAG) in patients with stable angina pectoris (SAP) through their correlation with Gensini score. Methods: Our study included 45 SAP patients who underwent diagnostic CAG. Thirty two patients of them who diagnosed as CAD were subdivided into 2 groups: 17 patients with multiple-vessel disease (MVD) and 15 patients with single-vessel disease (SVD). The rest thirteen SAP patients without CAD (non-CAD) were considered as a comparative group. Gensini score was used to assess the severity of CAD. Monocyte subsets were analysed by flow cytometry and serum levels of IL-6 and hs-CRP were measured by ELISA. Results: The relative proportion of CD14+ CD16+ and CD14bright CD16+ was significantly higher in CAD patients, MVD and SVD as compared with non-CAD patients and in MVD more than SVD. Serum levels of IL-6 and hs-CRP were significantly increased in CAD patients, MVD and SVD when compared with non-CAD patients, but no significant difference between MVD and SVD. The proportion of CD14+ CD16+ and CD14bright CD16+ monocytes was positively correlated with Gensini score (r = 0.667, P = 0.000, r = 0.695, P = 0.000). Conclusions: Elevated proportion of CD14+ CD16+ monocytes subsets was associated with the severity of CAD in patients with SAP.
Keywords: Monocyte subsets, Coronary artery disease, Coronary angiography, Stable angina pectoris, IL-6, hs-CRP
Cite this paper: Entsar R. Mokhtar, Bothayna I. Saleh, Abdelmohsen M. Aboualia, Hala M. Nageb, Relationship between Monocyte Subsets, IL-6 and hs-CRP with the Severity of Coronary Artery Disease in Stable Angina Pectoris Patients, American Journal of Biochemistry, Vol. 7 No. 5, 2017, pp. 114-126. doi: 10.5923/j.ajb.20170705.04.
Article Outline
1. Introduction
- Cardiovascular diseases (CVD) remain a major leading cause of death worldwide despite major advances in the field of vascular medicine [1]. Although mortality rates related to CAD have continued to decline over past years, CAD remains accountable for about one-third of all deaths over the age of 35 years [2]. Vascular inflammation plays a crucial role the pathogenesis of atherosclerosis and mediates various stages of atherosclerotic plaque development, from lipid streak formation to the plaque rupture and destabilization that precede the clinical syndromes of CVD [3]. An important role in the pathogenesis of atherosclerosis is played by endothelial dysfunction, which results in the appearance of adhesion molecules on the surface of leukocytes. Active macrophages, located in the vascular wall, are the source of proinflammatory cytokines such as interleukin-1 (IL-1), IL-6, IL-18, and tumor necrosis factor-α (TNF-α) [4]. Monocytes, as precursors of macrophages, are known to play an important role in the inflammatory reaction during atherosclerosis [5].Circulating human monocytes had been classified into CD14+ CD16- (classical; CM) and CD14+ CD16+ subsets according to their expression levels of CD14 and CD16. Depending on the level of CD14 expression, the CD14+ CD16+ population is further sub-classified into CD14bright CD16+ (intermediate; IM) and CD14dim CD16+ (non-classical; NC) monocytes. The classical monocyte is the major subset, while the CD14brightCD16+ and CD14dim CD16+ subsets occur in lower numbers than classical monocytes. It has been found that the CD14brightCD16+ monocyte population increases in inflammatory or infectious conditions and, upon lipopolysaccharide stimulation, produces TNF-α, IL-1β, IL-6, and IL-10 [6].CD14+CD16- monocytes are professional phagocytes that ingest native low-density lipoprotein (LDL), and generate reactive oxygen species (ROS). In contrast, CD14+CD16+ monocytes do not generate ROS and are weak phagocytes that preferentially take up oxidized LDL but substantially secrete inflammatory cytokines such as tumor necrosis factor-α [7]. Both IM and NCM are pro-inflammatory cells, and their proportional increase was found to be related to the occurrence of CAD, intima-media thickness, and plaque stability [8]. IL-6 is considered one of the inflammatory markers of local coronary plaque and the peripheral blood cycle, which promote the occurrence of atherosclerosis development. It can be detected with high concentration in atherosclerotic plaque [9]. Hs-CRP is a sensitive indicator of the body's inflammatory response, which is closely related with the progress of plaque formation in patients with stable angina. There is growing evidence that CRP is not merely a marker of inflammation, but also plays an active role in atherogenesis [3]. In the light of these data, the aim of our study was to assess the relationship between monocyte subsets, IL-6 and hs-CRP with the severity of CAD in SAP patients, because identification of patients with high risk, is highly warranted for the purpose of risk stratification and for an appropriate indication for therapy.
2. Patients and Methods
2.1. Study Design
- Between October 2016 and March 2017, we included 45 patients with SAP admitted to cardiology department of Al-Hussein University Hospital for elective coronary angiography (CAG). All included patients gave written informed consent. Thirty two patients of them were diagnosed by CAG as CAD patients; that means that they have stenosis ≥ 50% in a major coronary artery. This group was sub-classified into 2 subgroups: 17 (37.7%) patients with multiple-vessel disease (MVD), and 15 (33.3%) patients with single-vessel disease (SVD). The other thirteen (29%) SAP patients were diagnosed as non-CAD (i.e. having stenosis < 50%) and were considered as a comparative group. To avoid recent acute inflammatory conditions impact; we excluded patients who experienced a recent acute coronary syndrome, defined as ST-elevation or non ST-elevation myocardial infarction, or unstable angina, a history of percutaneous coronary intervention. Further exclusion criteria consisted of valvular heart disease, heart failure, malignant disease, liver, kidney or other acute or chronic inflammatory diseases. Cardiovascular disease risk factors were defined as follows: arterial hypertension (systolic blood pressure ≥ 140 mm Hg and or diastolic blood pressure ≥ 90 mm Hg in at least 2 measurements or the current use of antihypertensive drugs); diabetes mellitus (if treated for insulin or noninsulin-dependent diabetes mellitus or having plasma fasting glucose ≥ 126 mg/dL); dyslipidemia (if serum total cholesterol ≥ 220 mg/dl or LDL cholesterol ≥ 140 mg/dl or on statin treatment); current smoking (defined as smoking within one year of admission) and body mass index (BMI) expressed as mass in kilograms/square of height in meters [7].
2.2. Blood Sampling
- Before sampling, the patient were allowed to rest for at least 5 min, in order to avoid increases of CD16+ monocytes due to stress which increase catecholamine release that can mobilize CD16+ monocytes. Blood was drawn in the morning before CAG to exclude effects of angioplasty on monocyte subset distribution and any diurnal variation [10]. Blood sample was collected from the antecubital vein of each patient using EDTA tube and serum separation tube, the EDTA tube was sent for immediate flow cytometric analysis of monocyte subsets as more prolonged storage may affect monocyte phenotype. The serum separator tube was centrifuged at 3000 rpm for 15 min then serum was divided into three aliquots; first one is sent to chemistry lab for cholesterol, triglyceride, HDL-C and LDL-C, second and third aliquots for IL-6, hs-CRP were stored at -20°C for further analysis. All blood samples were analyzed in the laboratory of Al-Zahraa University Hospital, except for lipid profile, where the results were taken from the files of the patients.
2.3. Flow Cytometric Analysis of Monocyte Subsets
- Cytometric analysis were performed using a flow cytometer (BD FACS Calibur, BD Biosciences, San Jose, USA), and the results were analyzed using Cell Quest pro Software (BD Bioscience). For the monocyte subpopulation analysis, 50μL of EDTA anti-coagulated whole blood was incubated with the following fluorochrome-conjugated monoclonal antibodies (mAbs): 5μL of peridinin chlorophyll protein (PerCP) conjugated mAb for CD45 (BD, Biosciences, San Jose, USA Lot number 6039924), 5μL of fluoresceinisothiocyanate (FITC) conjugated mAb for CD16 (Beckman coulter, Lot number 71), 5μL of Phycoerythrin (PE) conjugated mAb for CD14 (Beckman coulter, Lot number 26). The optimal concentration was determined for each antibody or dye by titration experiment. Matched isotype antibodies were used as negative controls. After incubation for 20 min in the dark, 1mL lysing solution (BD FACS Lysing solution BD Biosciences) was added. After additional 8 min. incubation in dark place, cells were washed 3 times by adding 1 mL phosphate-buffered saline and centrifugation at 820 rpm for 5 min. each. Cells were then resuspended in 1 mL fixative solution (FACS Flow, reagent-grade water and BD Cell fix) for FACS-analysis. Monocytes were first gated morphologically in a forward scatter /side scatter dot plot, then by using PerCP–conjugated mAb for CD45. The gated monocytes were sub-classified according to CD14 and CD16 expression into 3 groups: CD14+ CD16–, CD14bright CD16+, and CD14dim CD16+ (figure 9, 10, 11).
2.4. Measurements of IL-6, hs-CRP and Lipid Profile
- Interleukin-6 was measured by using commercially available ELISA kits; supplied by DIA source Immunoassay S.A., catalog No. (KAP1261), Lot No. (152701). Hs-CRP was done by ELISA kits supplied by Immunospec Corporation, catalog No. (E29-056), Lot No. (317041701). Measurement was done on ELISA system (reader; A31851 and washer; 909 from DAS (Italy), according to manufacturer’s instructions. Total cholesterol, triglycerides, HDL-C levels were measured by enzymatic colorimetric assay, using Cobas Integra plus 400070 auto analyzer. Reagents were supplied by Roche diagnostics. LDL-C was calculated using the Friedewald equation.
2.5. Angiographic Analysis
- Coronary angiography was performed according to the Judkins technique and images of the coronary tree were obtained in routine standardized projections with the digital quantitative Philips Allura Xper FD system (Philips, The Netherlands) in all patients. All stored angiographic images were reviewed separately by 3 independent experienced cardiologists who were unaware of the patient’s clinical characteristics and biochemical results. Coronary stenosis was considered if there was ≥ 50% reduction in lumen diameter of coronary artery [11]. In each coronary angiogram, the number of major coronary arteries presenting stenosis was assessed and patients were subdivided into 3 groups: those without CAD, if no coronary artery showed a reduction in lumen diameter ≥ 50%; those with SVD, if stenosis was detected in only 1 coronary artery; and those with MVD, if stenosis was detected in 2 or more coronary arteries.
2.6. Gensini Score
- The Gensini scoring system was used to evaluate the severity of CAD [12]. The Gensini score was calculated for each patient from the stored coronary angiographic images by assigning a severity score to each coronary stenosis according to the degree of luminal narrowing and its geographic importance taking into consideration concentric and eccentric plaques. Reductions of 25%, 50%, 75%, 90%, 99%, and complete occlusion were given Gensini scores of 1, 2, 4, 8, 16 and 32 respectively. Each coronary segment was assigned a multiplier according to figure (1) based on functional significance of the myocardial area supplied by that segment. Scoring was performed by 3 observers and averaged.
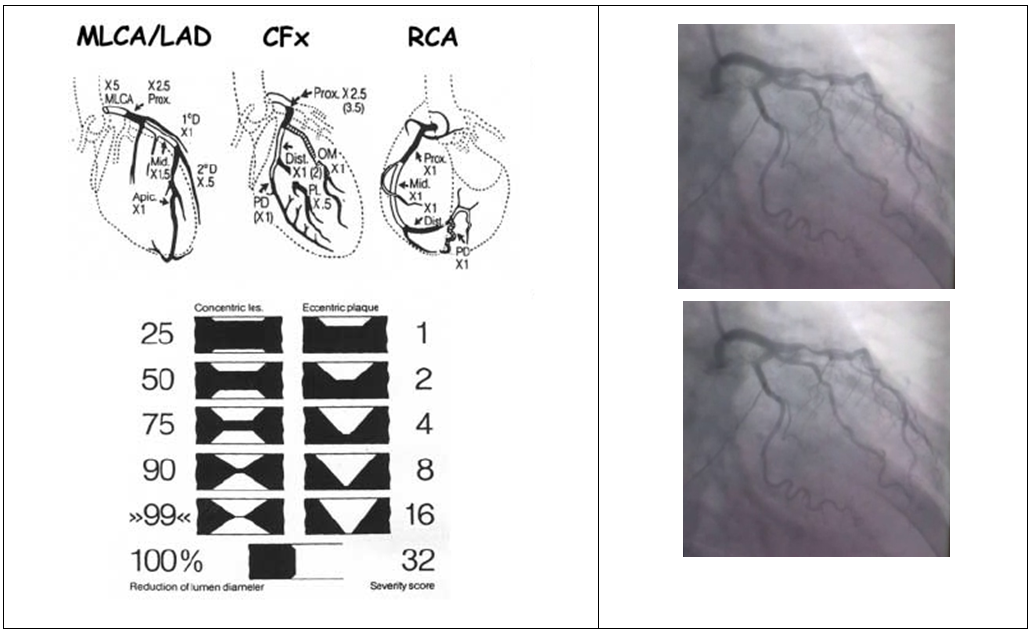 | Figure (1). Example of Gensini score calculation in patient No. 26 with 3VD |
3. Statistical Analysis
- Data were collected, coded, revised and entered to the Statistical Package for Social Science (IBM SPSS) version 20. The data were presented as number and percentages for the qualitative data, mean, standard deviations and ranges for the quantitative data with parametric distribution. Chi-square test was used in the comparison between two groups with qualitative data. Independent t-test was used in the comparison between two groups with quantitative data and parametric distribution. The comparison between more than two groups with quantitative data and parametric distribution were done by using one way analysis of variance (ANOVA) test. Spearman correlation coefficients were used to assess the significant relation between two quantitative parameters in the same group. Receiver operating characteristic curve (ROC) was used to assess the best cut off point between two groups with its sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and area under the curve (AUC). The confidence interval was set to 95% and the margin of error accepted was set to 5%. So, the P value was considered as the following: P > 0.05: non significant; P < 0.05: significant; P < 0.01: highly significant.
4. Results
- The results of this study showed no significant differences between CAD and non-CAD patients in terms of age, sex, BMI, history of diabetes, hypertension, hyperlipidemia or smoking habits (table 1). As regard lipid profile and Gensini score, no significant differences between CAD and non-CAD patients except for serum total cholesterol (P = 0.006), and Gensini score (P < 0.001) (table 2). There was a highly significant increase in the relative proportion of CD14+CD16+ in CAD patients, MVD and SVD as compared with non-CAD patients (P < 0.001, P1 < 0.001, P2 <0.001) respectively, and in MVD than in those with SVD (P3 < 0.001) (table 3, 5, figure 2). Furthermore the relative proportion CD14bright CD16+ was significantly higher in CAD patients, MVD and SVD as compared with non-CAD patients (P < 0.001, P1 < 0.001, P2 < 0.001) respectively, and in MVD than in those with SVD (P3 < 0.001) (table 3, 5, figure 3). Serum levels of IL-6 were significantly increased in CAD patient, MVD and SVD when compared with non-CAD patients (P = 0.021, P1 = 0.009, P2 = 0.043) respectively, but no significant difference between MVD and SVD (P3 = 0.229) (table 3, 5). Serum levels of hs-CRP showed a highly significant increase in CAD patients, MVD and SVD when compared with non-CAD patients (P < 0.001, P1 < 0.001, P2 < 0.001) respectively, but no significant difference between MVD and SVD (P3 = 0.163) (table 3, 5). The proportion of CD14+ CD16+ and CD14bright CD16+ was positively correlated with Gensini score (r = 0.667, P = 0.000, r = 0.695, P = 0.000) and hs-CRP (r = 0.590, P = 0.000, r = 0.531, P = 0.002) respectively, and no significant correlation was found between IL-6, hs- CRP and Gensini score (P = 0.306, r = 0.088 P = 0.205, r = 0.230) (table 6, figure 4, 5, 6, 7). ROC curve analysis of the proportion of CD14+ CD16+ monocytes, IL-6 and hs-CRP for detection of severity of CAD showed that, at a cutoff point >10.5% for CD14+ CD16+ monocytes, AUC was 1.000, with 100% sensitivity and specificity, and at a cutoff point of IL-6 > 83.5 pg/ml, AUC was 0.620, with 78.12% sensitivity and 46.15% specificity. At a cutoff point > 5 mg/L for hs-CRP, AUC was 0.938, with 93.7% and 100% sensitivity and specificity respectively (table 7, figure 8).
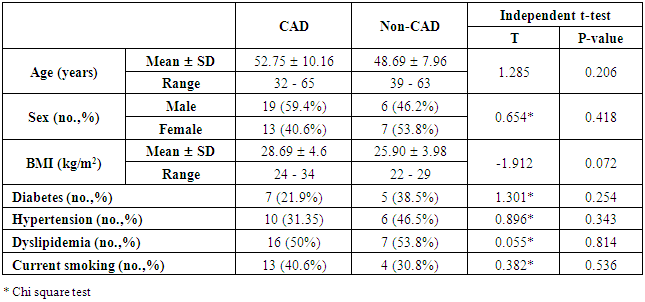 | Table (1). Comparison between CAD and non-CAD patients as regard demographic data and coronary risk factors |
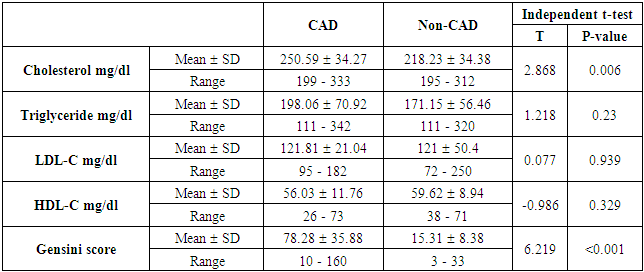 | Table (2). Comparison between CAD and and non-CAD patients as regard lipid profile and Gensini score |
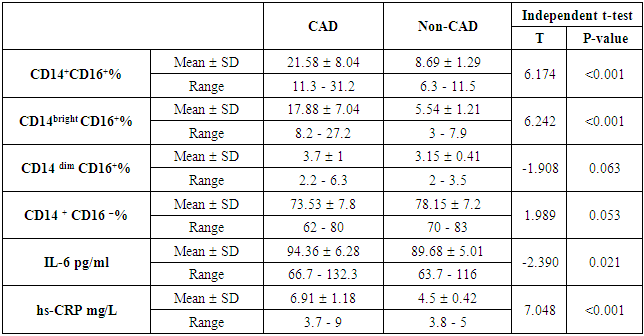 | Table (3). Comparison between CAD and and non-CAD patients as regard monocyte subsets, IL-6 and hs-CRP |
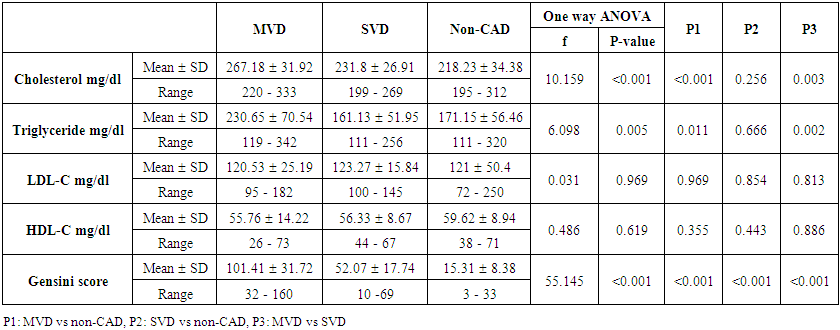 | Table (4). Comparison between MVD, SVD and non-CAD patients as regard lipid profile and Gensini score |
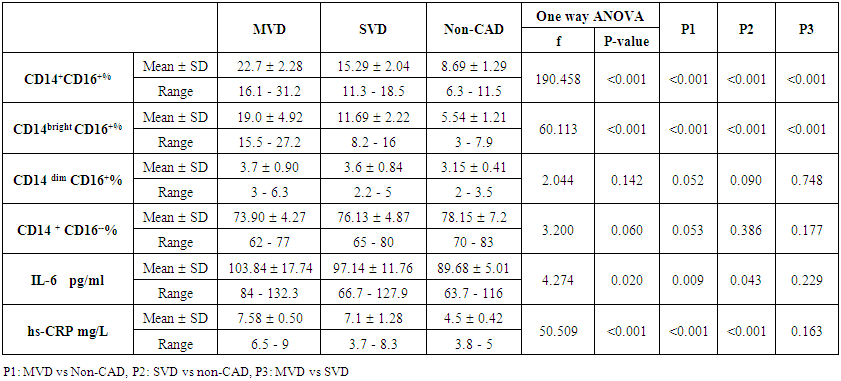 | Table (5). Comparison between MVD, SVD, and non-CAD patients as regard monocyte subsets, IL-6 and hs-CRP |
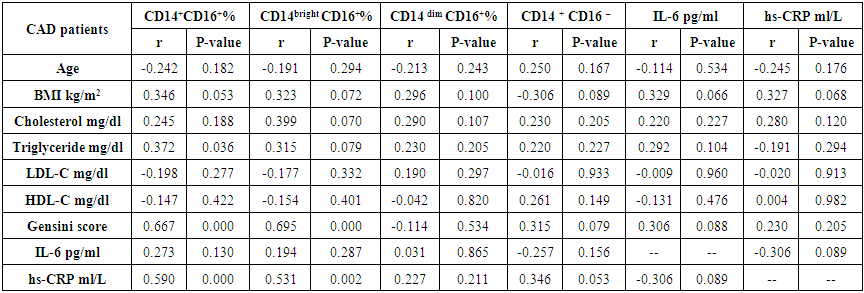 | Table (6). Correlation between monocyte subsets, IL-6, hs-CRP and the studied parameters in CAD patients |
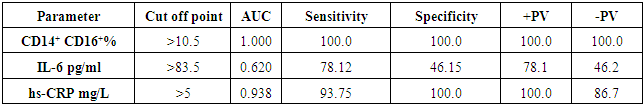 | Table (7). ROC curve analysis of CD14+CD16+, IL-6 and hs-CRP |
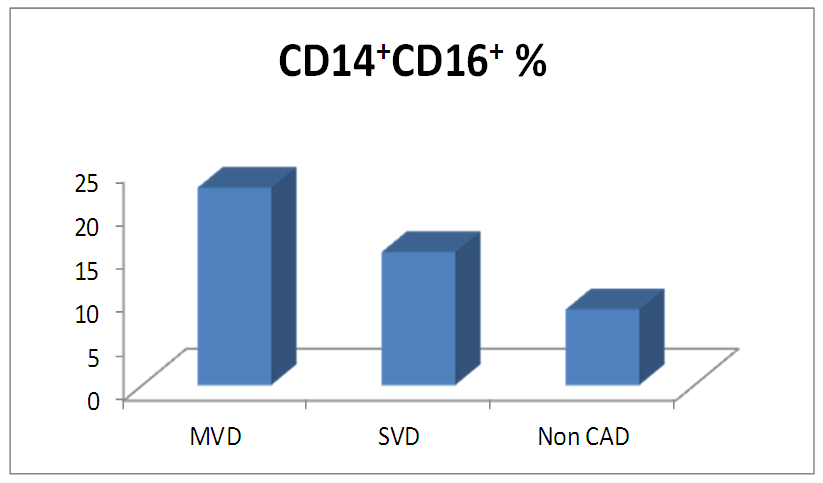 | Figure (2). Comparison of proportion of CD14+CD16+monocytes among MVD, SVD and non-CAD patients |
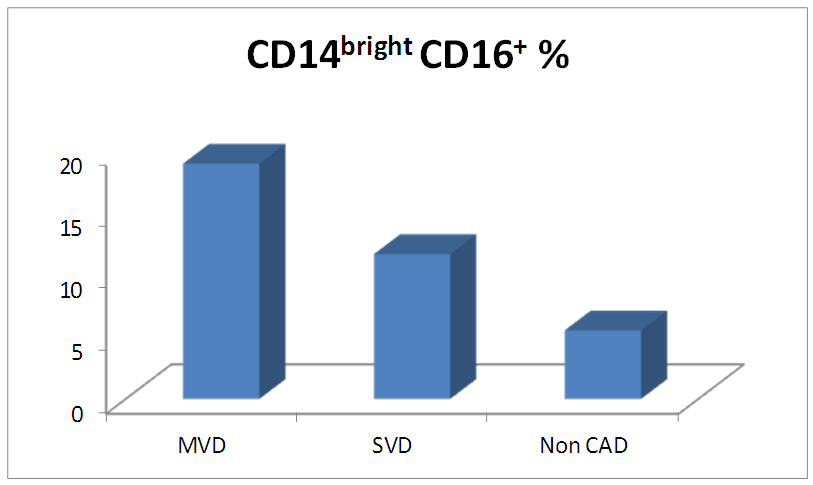 | Figure (3). Comparison of proportion of CD14bright CD16+ monocytes among MVD, SVD and non-CAD patients |
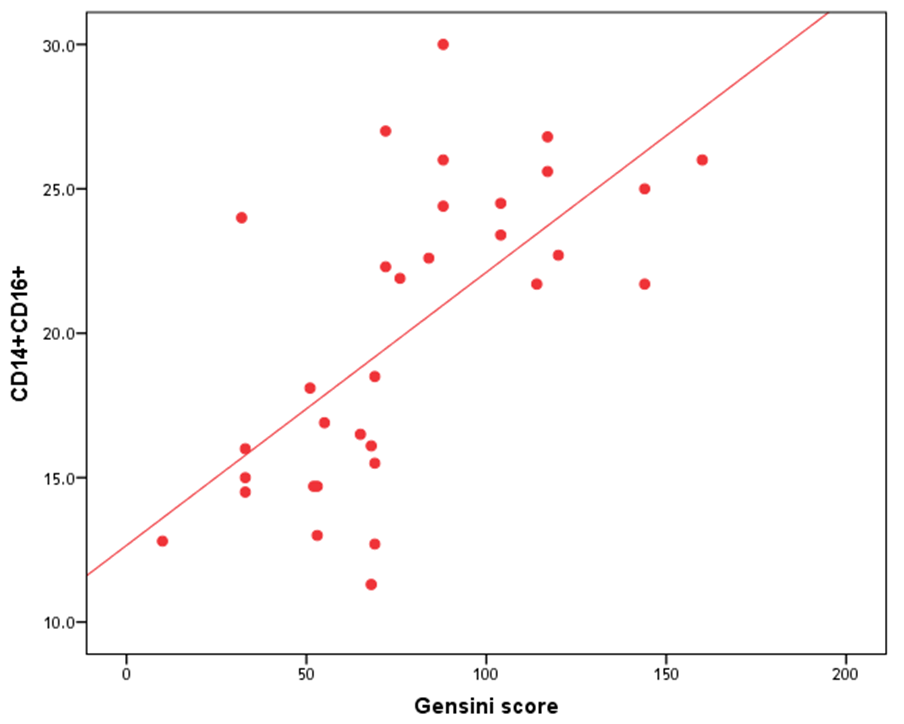 | Figure (4). Correlation between proportion of CD14+CD16+ monocytes and Gensini score |
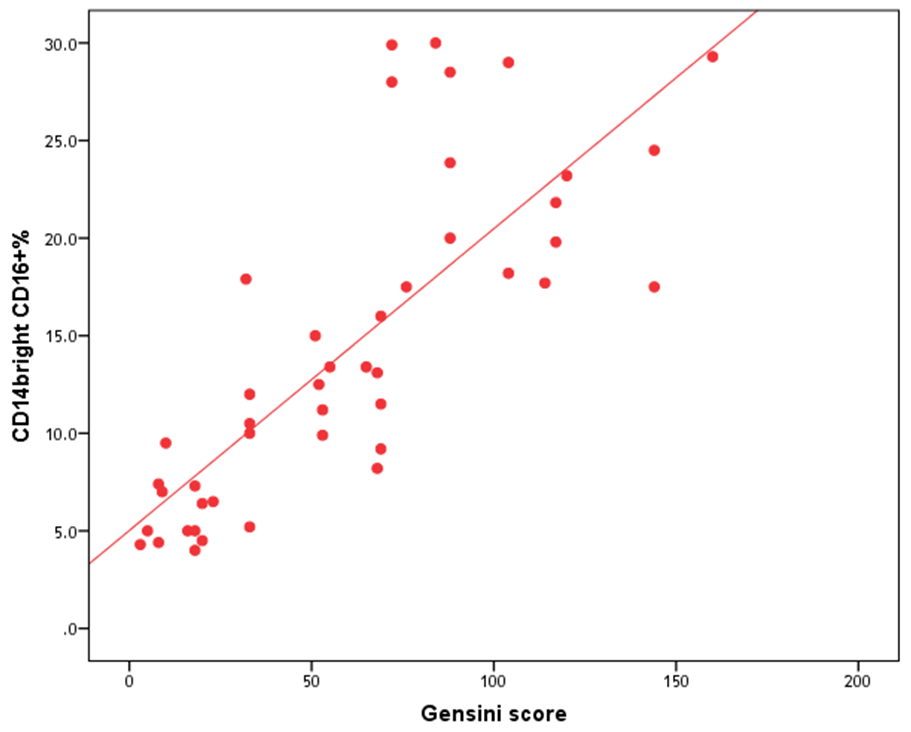 | Figure (5). Correlation between proportion of CD14brightCD16+ monocytes and Gensini score |
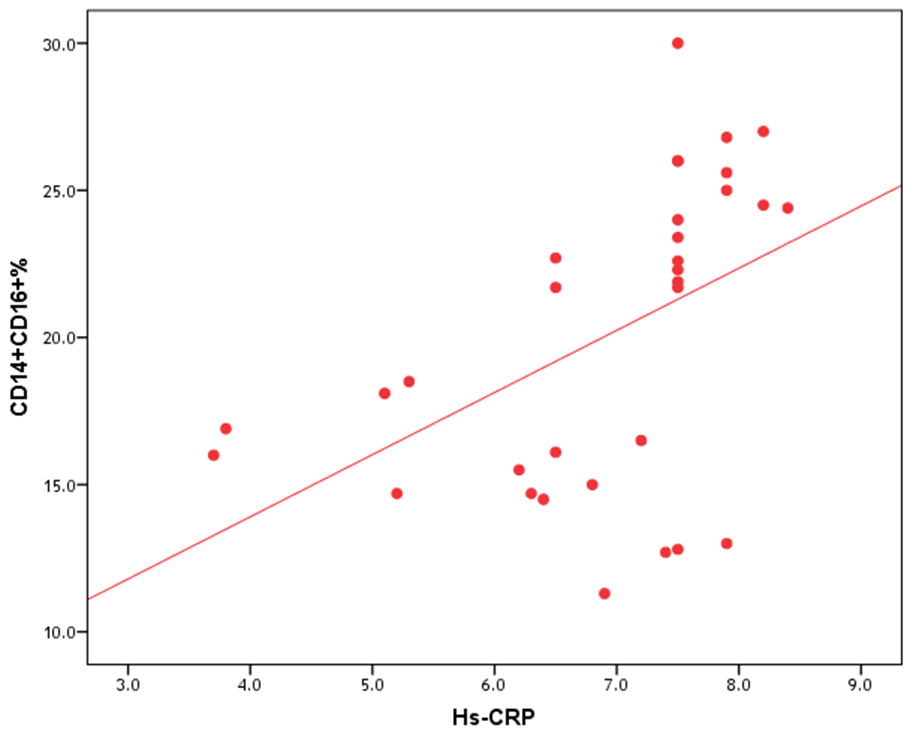 | Figure (6). Correlation between proportion of CD14+CD16+ monocytes and hs-CRP |
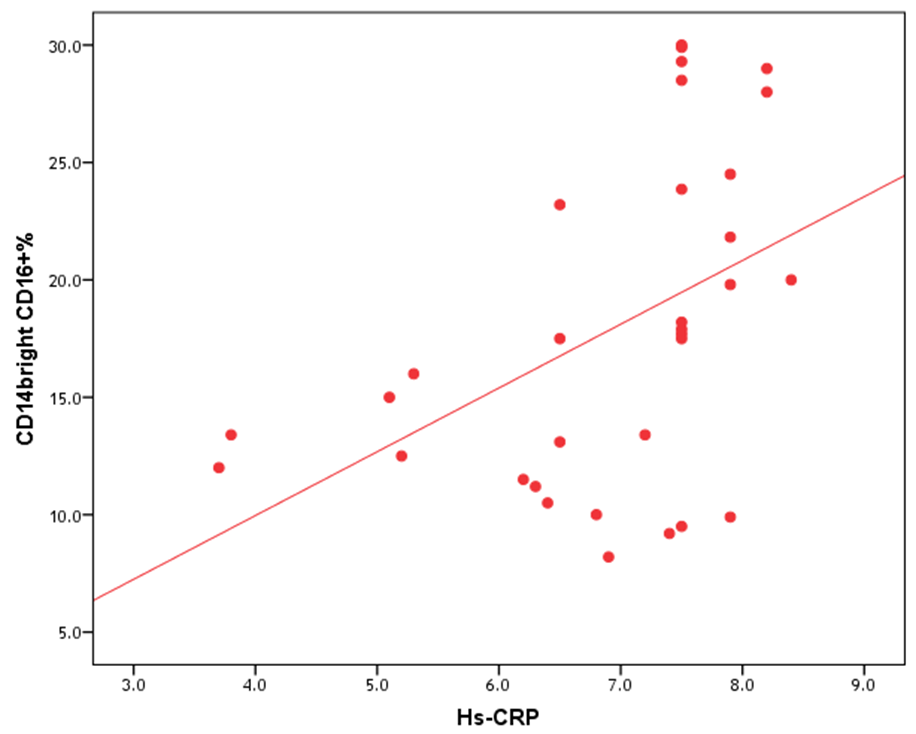 | Figure (7). Correlation between proportion of CD14brightCD16+ monocytes and hs-CRP |
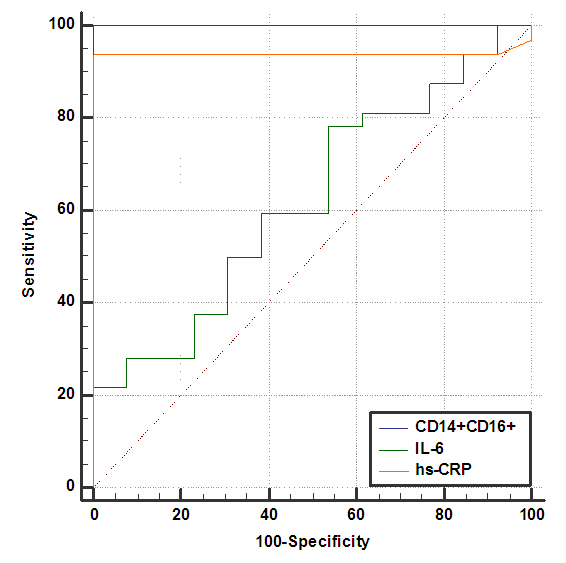 | Figure (8). ROC plot for CD14+ CD16+, IL-6 and hs-CRP |
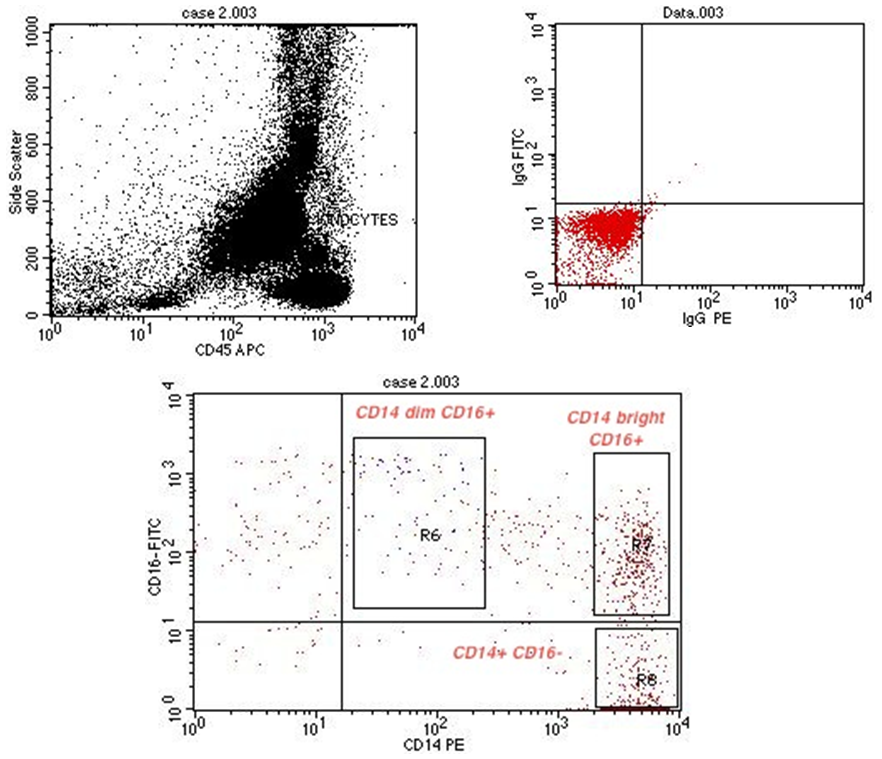 | Figure (9). Flow cytometric analysis of monocyte subsets in MVD patient |
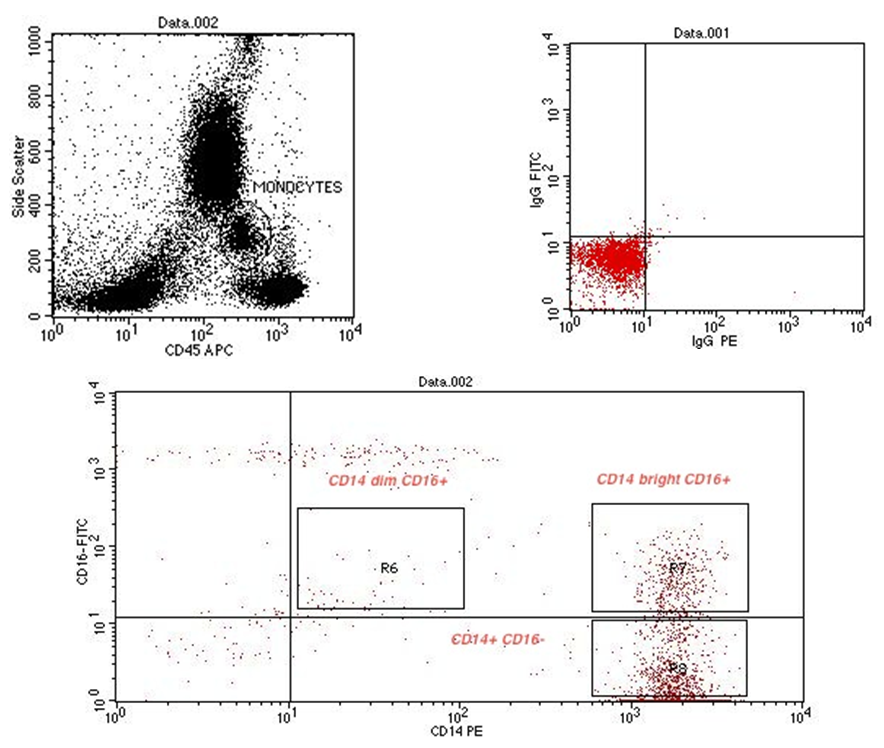 | Figure (10). Flow cytometric analysis of monocyte subsets in SVD patient |
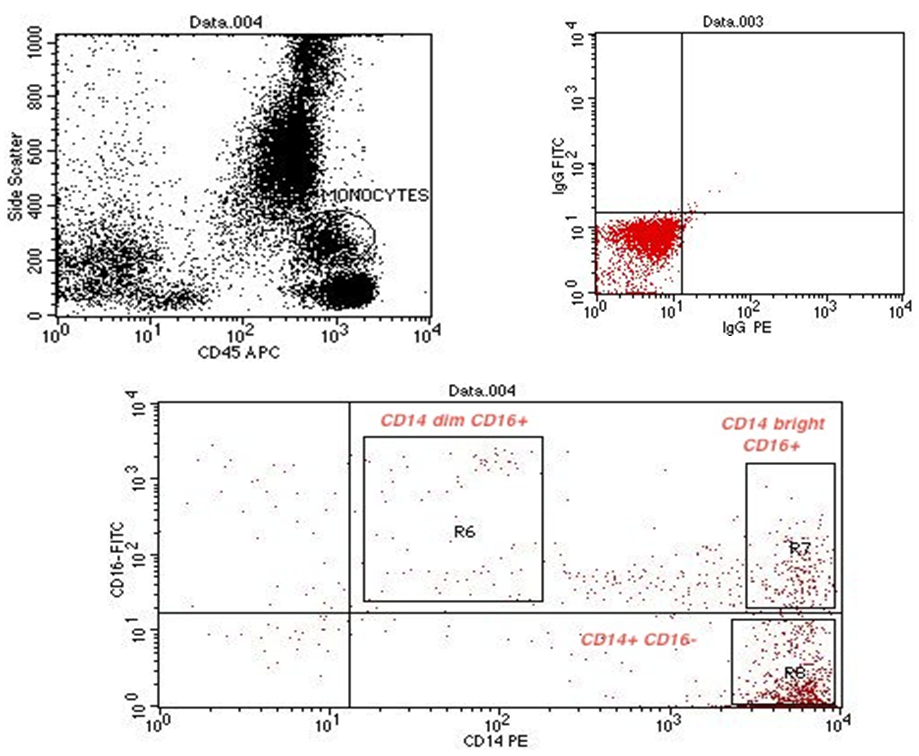 | Figure (11). Flow cytometric analysis of monocyte subsets in non-CAD patient |
5. Discussion
- The evaluation of the atherosclerotic plaque and severity of CAD is a matter of continuous research for detection, prediction and prevention of coronary artery disease. The development of atherosclerosis is related to dysregulation of complex interactions between inflammatory, metabolic and vascular mechanisms. The inflammatory processes underlying development of atherosclerosis are mainly associated with recruitment and activation of monocytes and monocyte-derived macrophages [13]. Monocytes as cells of the innate immunity are prominently involved in the development of atherosclerotic lesions. Monocytes in peripheral blood are no longer considered to be a homogeneous population; the heterogeneity of monocytes and its associations with atherosclerosis has been confirmed by the detection of expression of surface markers CD14 and CD16. The relative contribution of the monocyte subsets in atherosclerosis, its risk factors and complications has attracted major interest [14, 8].There is growing evidence demonstrating a significant role of CD16+ monocytes (both IM and NC) in the development of atherosclerosis [15]. In the present study, we found significant increase in relative proportion of CD14+CD16+ monocytes in CAD as compared with non-CAD patients. This was matched with previous studies of Kashiwagi et al., Ozaki et al. and Imanishi et al. [16, 7, 17] who found a shift towards CD16+ monocytes was associated with the presence of CAD, vulnerable atherosclerotic plaque, or fibrous cap thickness. Interestingly in a cohort analysis of more than 900 subjects with CAD, Rogacev et al. [18] found that increased CD14+ CD16+ monocytes predicted future cardiovascular events. This could be explained by that CD16+ monocytes are proinflamatory cells and their number is increased during chronic inflammation and atherosclerosis. They are rapidly activated when triggered by inflammation, exhibiting an increased production of inflammatory cytokines [8]. By contrast Czepluch et al. [19] found that, the relative proportion of the CD14+CD16-monocyte subset was significantly increased and the relative proportion of CD14+ CD16+ subset was significantly reduced in SAP patients. Also, Berg et al. [20] reported that the percentage of classical CD14+ CD16- monocytes was increased in individuals with an ischaemic cardiovascular event. These discrepancies between studies may arise from different study population sizes, racial variability and different patients' characteristic (e.g., age and disease duration) and presumably, by the treatment they received which may affect the proportion of each subset. When we compared MVD with SVD patients we found significant higher proportion of CD14+CD16+ monocytes in MVD as compared with those with SVD and non-CAD patients. Among CD16+monocytes, CD14bright CD16+ monocytes can be viewed as proatherogenic, as they selectively express C-C chemokine receptor type 5 which has been associated with atherosclerosis [18]. Accordingly, we found that the proportion of CD14bright CD16+monocytes was significantly higher in patients with MVD than in those with SVD and non-CAD patients, and this was in agreement with Ozaki et al. [7] who found that circulating CD14+CD16+ and CD14brightCD16+ monocytes were more frequently observed in patients with MVD than those with SVD or those without CAD. They demonstrated a close relationship between CD14+ CD16+monocytes and the severity of CAD in SAP patients. Our results also matched with Lo et al. [21] who found that CD14bright CD16+ percentage was increased in patients with severe stenosis compared with those with non-severe luminal narrowing, thus, CD14bright CD16+ could independently predict severe CAD and improve risk stratification. Also, they showed that CD14bright CD16+ promotes the plaque calcification that may be due to the potent pro-osteochondrogenic effect of TNF- α produced by them.In patients with CAD, elevated circulating IL-6 concentrations may result from various clinical risk factors that lead to the release of IL-6 from numerous cell types, including smooth muscle cells and macrophage / foam cells found in atheromatous plaques [22]. In our study we found significant increase in serum level of IL-6 in CAD patients when compared with non-CAD patients. This was in consistent with Tang et al., Caselli et al. and Sarrafzadegan et al. [23-25] who reported elevated concentrations of IL-6 in SAP patients and that IL-6 may play an important role in reflecting the degree of inflammation and the severity of coronary arterial atherosclerosis in CAD.When comparing serum level of IL-6 in MVD, SVD patients, we found significant increase in serum level of IL-6 in MVD and SVD patients as compared with non-CAD patients, while no significant difference between MVD and SVD. This is in accordance with Liu et al. [22] who showed that, serum level of IL-6 was significantly increased in single, double and triple vessel groups as compared with control with no difference between the three patients groups.Serum hs-CRP is a sensitive indicator of inflammation and is closely related with the progression of plaque formation [9]. In our study we found highly significant increase in serum level of hs-CRP in CAD patients, MVD and SVD when compared with non-CAD patients, but no significant difference between MVD and SVD patients. This was in agreement with chio et al. [26] who showed that patients with ≥ 50% coronary stenosis have higher hs-CRP levels than patients with < 50% stenosis and a high hs-CRP level was related to the severity of coronary atherosclerosis. This was explained by Yousuf et al. [27] who showed that necrotic debris and inflammatory mediators from coronary plaque are released into the systemic circulation, with a subsequent increase in the hepatic release of hs-CRP. In our study there was significant positive correlation between the proportion of CD14+ CD16+, CD14bright CD16+monocytes and Gensini score,while no association between hs-CRP and Gensini score. This was matched with Ozaki et al. [7] who found that the proportions of CD14+ CD16+ and CD14bright CD16+ monocytes were positively correlated with Gensini score. They demonstrated a significant association between increased proportion of CD14+ CD16+ monocyte and the severity of CAD in SAP patients. Razban et al. and Ozaki et al. [28, 7] had found no significant correlation between serum level of hs-CRP and severity of CAD assessed by Gensini score which was similar to our result. Also, no association was found between IL-6 and Gensini score; however, Tanindi et al. [29] had found that IL-6 was associated with more extensive and severe CAD as represented by Gesini score. Also, we found significant positive correlation between the proportion of CD14+ CD16+, CD14bright CD16+ monocytes and hs-CRP which was matched with Rogacev et al. [18] who showed that CAD patients who had higher CD14bright CD16+ monocytes proportion in conjunction with higher CRP levels had the worst cardiovascular outcomes.
6. Conclusions
- Elevated proportion of CD 14+ CD16+ monocytes subsets was associated with the severity of CAD and superior to IL-6 and hs-CRP for determining CAD severity in SAP patients. Further studies are needed to verify these results and elucidate the mechanisms underlying these associations which allow specific targeting of therapeutic intervention.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML