-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2017; 7(4): 73-82
doi:10.5923/j.ajb.20170704.03

Biochemical Study of the Effect of Insulin Resistance on Adiponectin, Lipid Profile and Some Antioxidants Elements with Relation to Obesity in Type 2 Diabetic Patients /Basrah-Iraq
Adnan J. M. Al-Fartosy, Nadhum A. Awad, Douaa J. Abdalemam
Department of Chemistry, College of Science, University of Basra, Basra, Iraq
Correspondence to: Adnan J. M. Al-Fartosy, Department of Chemistry, College of Science, University of Basra, Basra, Iraq.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: The prevalence of diabetes complications and its effects on type 2 diabetic patients has been extensively studied in several population groups; however, there is scarce information and no scientific report on diabetes complications and its effects on type 2 diabetic patients in Basrah province (southern of Iraq). Hence, the aim of the present study was designed to assessment the effect of insulin resistance on adiponectin, lipid profile and some antioxidants elements with relation to obesity in men and women type 2 diabetic patients of the population of Basrah province (southern of Iraq). Subjects and Methods: In this case-control study, fifty patients suffering from type II of diabetes (25 men and 25 women), and controlled with (50) healthy individuals (26 men and 24 women), in the age group of 36-62 years who attended the diabetes and endocrine center in Al-Mowany general Hospital in the province of Basrah in the period between August 2016 and May 2017. The two groups were matched for their fasting blood sugar, body mass index (BMI), insulin hormone; c-peptide, adiponectin, lipid profile and insulin resistance parameters as homeostasis model assessment (HOMA2-IR, HOMA%B and HOMA%S) were calculated using HOMA2 calculator software. Results: There was a highly significant increase (p < 0.01) in levels of serum BMI, C-peptide, glucose, HOMA2-IR and insulin, while there was highly significant decrease (p < 0.01) in level of serum adiponectin. Furthermore, this study showed, a significant elevation (p < 0.01) was seen in levels of serum triglycerides, total cholesterol, low density lipoprotein cholesterol (LDL-C), very low density lipoprotein cholesterol (VLD-C) and LDL-C/HDL-C ratio, while a significant reduced (p < 0.01) was seen in serum HDL-C level. Moreover, data obtained, clearly showed that concentrations of selenium in whole blood, serum zinc and serum magnesium were found to be significantly lower (p < 0.01) in men and women (NIDDM) patients compared to control. Conclusion: From the present study, we can conclude that insulin resistance plays an important role in the pathogenesis of many human diseases, such as diabetes, obesity, and metabolic syndrome. On the other hand, the baseline of serum adiponectin concentration was statistically significanty and inversely associated with risk of NIDDM. Therefore, a reduced level of serum adiponectin seems to be not just a mere biomarker of the ailment, but may also play a causal role in the development of insulin resistance, NIDDM, metabolic syndrome and dyslipidemia. Also, these findings underscore the importance of obesity as a significant predictor of incident metabolic syndrome and can be used to identify at-risk individuals from both sex men and women. Hence, taking into consideration the high world prevalence of obesity, insulin resistance, NIDDM, metabolic syndrome and dyslipidemia, the possibility of a defined and unique therapeutic target to simultaneously combat their development becomes increasingly important.
Keywords: Insulin Resistance, Obesity, Adiponectin, Lipid Profile, Type 2 Diabetes Mellitus
Cite this paper: Adnan J. M. Al-Fartosy, Nadhum A. Awad, Douaa J. Abdalemam, Biochemical Study of the Effect of Insulin Resistance on Adiponectin, Lipid Profile and Some Antioxidants Elements with Relation to Obesity in Type 2 Diabetic Patients /Basrah-Iraq, American Journal of Biochemistry, Vol. 7 No. 4, 2017, pp. 73-82. doi: 10.5923/j.ajb.20170704.03.
Article Outline
1. Introduction
- Diabetes mellitus (DM) is a metabolic disorder resulting from a defect in insulin secretion, insulin action, or both. Insulin deficiency, in turn, leads to chronic hyperglycemia with disturbances of carbohydrate, fat and protein metabolism [1]. Abdominal obesity is a strongly correlated phenotypic companion for a cluster of metabolic abnormalities characterized by insulin resistance [2]. However, the associations with abdominal obesity and features of the metabolic syndrome have been reported to vary with gender [3], and with different degrees of obesity [4]. Although abdominal obesity is the best obesity-related predictor of type 2 diabetes, it is not clear to what extent abdominal obesity can be used as a surrogate measure for insulin resistance in subjects who have already developed type 2 diabetes mellitus [5]. Insulin resistance can be described as a reduced biological response to insulin, primarily to its acute effects on glucose and lipid metabolism. This is pertinent as to whether the insulin is produced endogenously or administered exogenously, eventually leading to hyperglycemia. Insulin resistance occurs in several tissues, mainly as decreased insulin dependent glucose uptake in skeletal muscle, decreased ability of insulin to inhibit lipolysis in adipose tissue, impaired insulin-mediated inhibition of endogenous glucose production in liver, and decreased insulin enhanced glucose-induced insulin secretion in pancreas [6]. Adiponectin is a 244 amino acid, collagen-like protein, a member of a new family of obesity related hormones, the adipocytokines, which are produced solely by white adipose tissue, and which may be linked to both insulin resistance and endothelial dysfunction [7]. Adiponectin modulates a number of metabolic processes via the activation of 50-adenosine monophosphate-activated protein kinase (AMPK) and peroxisome proliferator activated receptor-a (PPAR-a) [8]. Type 2 diabetes is associated with a cluster of interrelated plasma lipid and lipoprotein abnormalities [9]. The lipoprotein abnormalities commonly present in type 2 diabetes include an abnormally high level of triglycerides (TG), a high proportion of small dense low density lipoprotein cholesterol (LDL), low high density lipoprotein cholesterol (HDL), and postprandial lipemia [10]. Direct associations of trace macro elements with diabetes mellitus have been observed in many research studies [11]. Insulin action on reducing blood glucose was reported to be potentiated by some trace elements such as chromium, magnesium, vanadium, zinc, manganese, molybdenum and selenium [12]. The prevalence of diabetes complications and its effects on type 2 diabetic patients has been extensively studied in several population groups; however, there is scarce information and no scientific report on diabetes complications and its effects on type 2 diabetic patients in Basrah province (southern of Iraq). Hence, the aim of the present study was designed to assess the effects of insulin resistance on adiponectin, lipid profile and some antioxidants elements with relation to obesity in men and women type 2 diabetic patients of the population of Basrah province (southern of Iraq).
2. Materials and Methods
- This study consisted of (50) patients suffering from type II of diabetes (25 men and 25 women), and controlled with (50) healthy individuals (26 men and 24 women), in the age group of 36 -62 years who attended the diabetes and endocrine center in Al-Mowany general Hospital in the province of Basrah in the period between August 2016 and May 2017. Written informed consent was obtained from each study participant. Blood samples were collected and a brief clinical interview including age, duration of diabetes, health habits (smoking, alcohol consumption and exercise), medical history and current medications was recorded from all the participants. Many of patients were excluded from this study because they belong to the class of secondary diabetes due to pancreatic diseases, hormonal abnormalities, drug induced, genetic syndromes …etc. Also, the control group was healthy Individuals, not taking any drug believed to alter serum glucose level. After verifying the questionnaire, body mass index (BMI) was calculated as body weight (in kilograms) divided by the square of body height (in meters), where the height and weight were measured (without heavy clothing and shoes), for each participant. Every participant was advised to fast overnight for 12 hours, and 10 ml of venous blood was collected in a disposable syringe the next morning (before breakfast). All the samples were analyzed for biochemical parameters by standard procedures as follows: Glucose and lipid profiles including total cholesterol (TC), triglycerides (TG) and high density lipoprotein (HDL) were measured quantitatively in serum by various procedures [13, 14]. The LDL-cholesterol level was calculated by using the formula: LDL-C= Total cholesterol-(HDL-C + (Triglyceride/5)), where (Triglyceride/5) = VLDL-cholesterol [15]. Serum insulin hormone and C-peptide were estimated by ELIZA kit (demeditec / Germany) [16, 17]. Adiponectin hormone was determined by ELISA method. Available kit for adiponectin was supplied from DRG Company / Germany [18]. The concentration of blood selenium (Se) was determined using a hydride generation method, while serum magnesium (Mg) and zinc (Zn) were measured by flame atomic absorption spectrometry (AAS) [19, 20]. Insulin resistance parameters [insulin resistance (HOMA2-IR), beta cell activity (HOMA%B) and insulin sensitivity (HOMA%S)] were calculated from fasting insulin and fasting blood glucose (FBG) using HOMA2 calculator software [www.dtu.ox.ac.uk/homa].
3. Statistical Analysis
- Data were collected, reviewed and fed into the computer where statistical analysis was done using the Statistic Package for Social Science Version 18 (SPSS 18.0) for windows. Comparing groups was done using Student's t-test and f-test. The level of significance was taken at p- value of <0.05 and high significant at p- value of < 0.01.
4. Results
- A total of 100 subjects were included in the present study. There were 50 patients with type 2 DM and 50 healthy individuals considered as control group. In patients, body mass index as a clinical parameter can be used to determine the extent of glycemic control. Results in (Table 1) show that there was a significant increase in BMI level (27.851±5.21 vs. 26.530 ± 5.08 and 28.322 ± 3.19 vs 27.462 ± 4.17 Kg/m2, p< 0.01), significantly increases in C-peptide level (2.101±0.32 vs. 0.632 ± 0.15 and 2.819 ± 0.19 vs. 0.668 ± 0.21 ng/ml, p< 0.01) and highly significantly increases in serum glucose level (195.23 ± 6.99 vs. 106.29 ± 2.92 and 182.45 ± 4.40 vs. 99.55 ± 3.31 mg/dl, p< 0.01), respectively, in men and women NIDDM patients compared with controls.Data obtained in Table 2, show a significant increase in HOMA2 IR level (4.0 ± 0.121 vs. 1.38 ± 0.043 and 4.12±0.15 vs. 1.57 ± 0.05, p< 0.01) and increases significantly in level of insulin (26.84 ± 5.16 vs. 10.22 ± 1.31 and 28.130 ± 6.24 vs. 11.91 ± 3.22 µU/ml, p< 0.01), respectively, in NIDDM patients compared with controls. Table 2 also shows the level of serum adiponectin with their significant decreases (10.955 ± 3.19 vs. 16.015 ± 4.26 and 12.761 ± 2.58 vs. 17.728 ± 2.76 µU/ml, p<0.01) respectively, in men and women NIDDM patients with compared to controls. Furthermore, as this study showed (Table 3), a significant elevation (p < 0.01) was seen in serum triglyceride level in type 2 DM subjects (180.69 ± 3.15 mg/dl) in men and (195.85 ± 5.56 mg/dl) in women compared with that of control were (130.21 ± 4.41 mg/dl) in men and (176.560 ± 6.49 mg/dl) in women and a significant increase (p < 0.01) was seen in serum total cholesterol in type 2 DM subjects (205.61 ± 4.61 mg/dl) in men and (221.13 ± 5.95 mg/dl) in women compared with that of control were (155.26 ± 3.12) in men and (176.56 ± 4.21 mg/dl) in women, respectively. Also, Table 3 showed a significantly reduced (p < 0.01) was seen in serum in serum HDL-C level in type 2 DM subjects (40.963±3.25 mg/dl) in men and (38.549±3.84 mg/dl) in women compared with that of controls (45.752±4.52 mg/dl) in men and (43.251±6.61 mg/dl) in women. In this work (Table 4), there was a significant increase (p < 0.01) was seen in serum LDL-C level (128.509 ± 5.28 vs. 83.766 ± 4.91 and 143.411 ± 4.61 vs. 120.897 ± 3.52 mg/dl) and increases significantly (p < 0.01) in level of serum VLDL-C (36.138 ± 4.24 Vs. 26.042 ± 2.16 and 39.170 ± 3.49 Vs. 27.846 ± 3.95 mg/dl) respectively, in NIDDM patients compared with controls. Same Table 4 also reflects the level of LDL-c/ HDL-C with their significant increases (3.137±0.416 vs. 1.830±0.328 and 3.720±0.537 Vs. 2.795 ± 0.642, p < 0.01), respectively, in men and women NIDDM patients with compared to controls.Table 5, clearly showed that Se concentration was found to be significantly lower in whole blood (78.431±3.28 vs. 99.215 ± 2.19 and 65.214 ± 1.93 vs. 80.890 ± 5.21 ng/ml, p < 0.01), significant decreases in the concentration of Zn (0.882 ± 0.0252 vs. 1.401 ± 0.0301 and 0.627 ± 0.0283 vs. 1.024 ± 0.0314 µg/ml, p < 0.01) and significantly decreases in concentration of Mg (16.992 ± 4.16 vs. 22.458 ± 3.27 and 13.365 ± 3.05 vs. 19.210 ± 4.45 µg/ml, p < 0.01) respectively, in NIDDM patients compared with control.
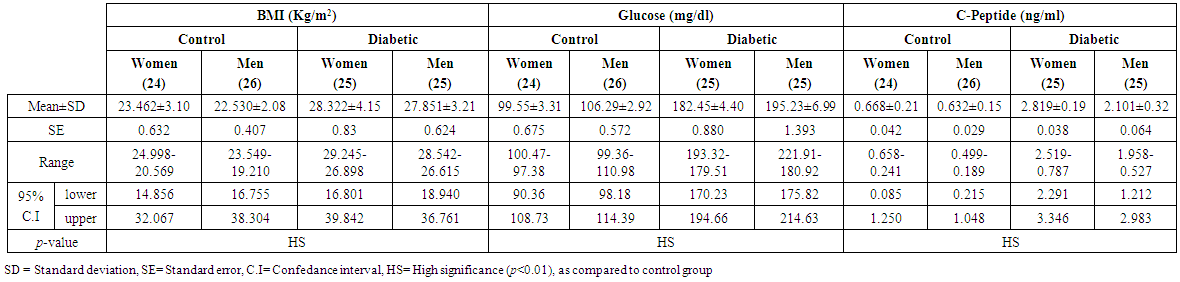 | Table (1). Levels of BMI, glucose and C-peptide in men and women of control and patients with NIDDM |
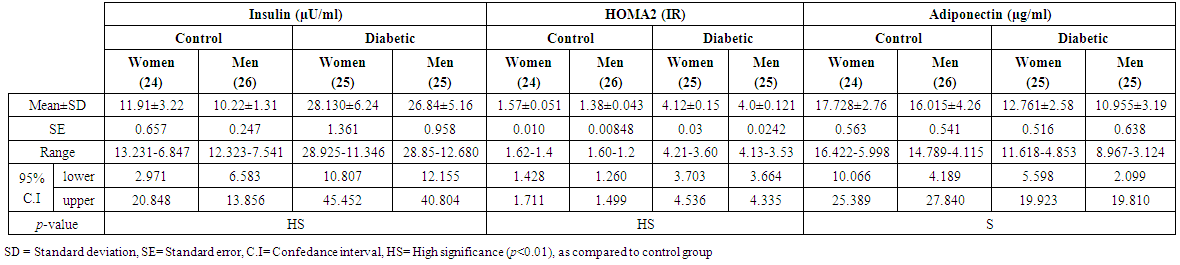 | Table (2). Levels of Insulin, HOMA2 (IR) and adiponectin in men and women of control and patients with NIDDM |
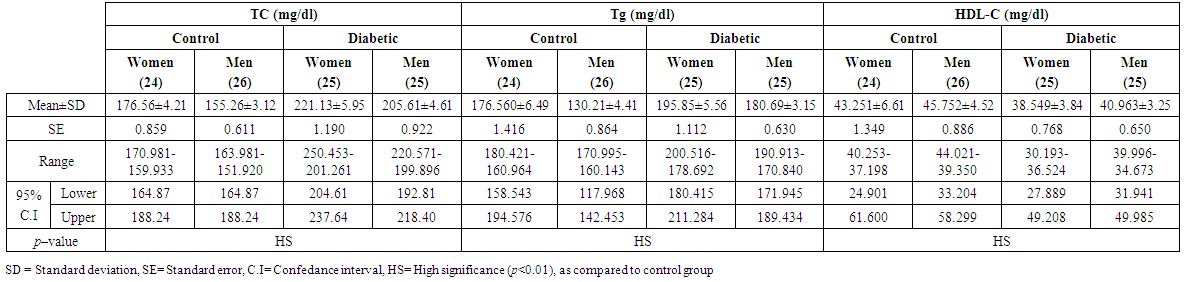 | Table (3). Levels of TC, Tg and HDL-C in men and women of control and patients with NIDDM |
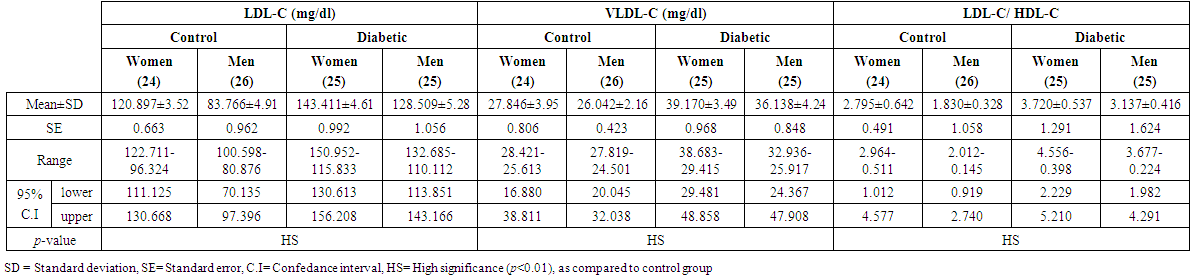 | Table (4). Levels of LDL-C, VLDL-C and LDL-C/ HDL-C in men and women of control and patients with NIDDM |
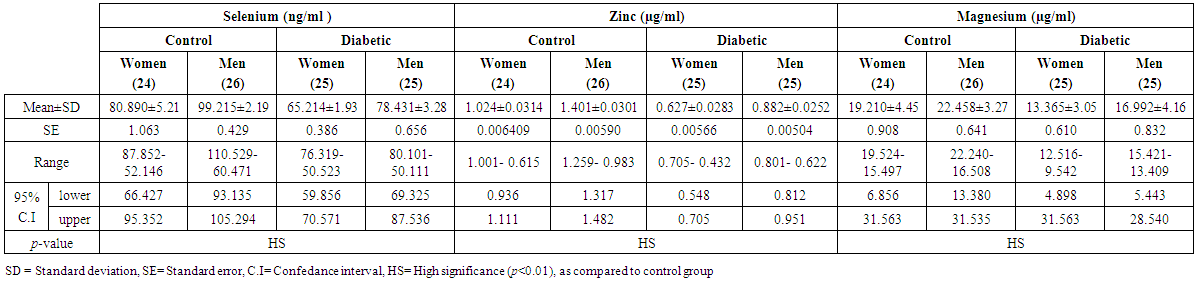 | Table (5). Concentrations of Se, Zn and Mg in men and women of control and patients with NIDDM |
5. Discussion
- Chronic hyperglycemia causes glycation of apolipoproteins and interferes with the normal pathways of lipoprotein metabolism [21]. Free fatty acid (FFA) levels especially from abdominal deposits, with direct delivery to the liver, as well as hyperinsulinemia, and hyperglycemia are all stimulators of VLDL-C production in the liver. Turnover of plasma VLDL-C particles may be increased. The consequence may be elevation of plasma VLDL-C concentrations and reduction of plasma HDL-C concentrations [22]. Furthermore, hepatic insulin resistance may result in increased lipoprotein secretion. It has also been identified that insulin can inhibit the assembly and secretion of VLDL-C by increasing posttranslational degradation of apoB and reducing the expression of microsomal TG transfer protein in the liver [23]. These metabolic abnormalities induce not only hyperglycemia, but also dyslipidemia (elevated VLDL-C and reduced HDL-C values) [22]. Diabetic patients are characterized by abnormalities in glucose metabolism in several organs. Skeletal muscle glucose disposal is reduced, hepatic glucose production is increased and insulin-independent glucose uptake into the lens and neural tissue are increased [24]. Although the actual mechanisms of insulin resistance in type2 diabetes remain unknown, several steps in the uptake and intracellular handling of glucose are probably affected [25]. Measuring blood glucose is one way of monitoring diabetes. High levels of blood glucose of diabetic patients may be due to lack of or resistance to insulin, results that were also found by others [26]. In those studies of a diabetic population, the authors concluded that, the fasting blood glucose level is also elevated, and this indicates poor control of DM. In fact, DM is characterized by hyperglycemia together with biochemical alterations of glucose [27]. When insulin resistance is present, or when insulin secretion is diminished in the later stages of the disease, free fatty acids (FFAs) are released in large quantities followed by an increased production of glucose and triglycerides, and secretion of VLDL. In addition, FFAs also reduce insulin sensitivity in muscles by inhibiting insulin-mediated glucose uptake. On the other hand, increased blood glucose concentration, and to some extent circulating FFAs, increase insulin secretion, leading to even more increased hyperinsulinemia. It is obvious that insulin resistance causes blood glucose concentrations and FFA levels to rise, therefore worsening the insulin resistance. In addition, hyperglycemia with released FFAs further increases insulin secretion, forming a vicious circle [28]. It has been hypothesized that triglyceride accumulation in skeletal muscles plays a direct role in the etiology of insulin resistance [29]. The results of some studies have shown that the degree of insulin resistance is positively correlated with intramuscular triglycerides content [30]. According to Poitout and Robertson, chronic hyperglycemia and dyslipidemia in type 2 DM can produce harmful effects on both β-cell structure and function. Although inter-relationships between glucotoxicity and lipotoxicity have not yet been elucidated, it is presumed that glucotoxicity leading to β-cell apoptosis occurs independently of dyslipidemia, whereas lipotoxicity additionally damaging β-cells occurs only in the presence of hyperglycemia. In the case of normoglycemia, elevated FFAs should be readily oxidized in the mitochondrion and should not harm the β-cell [31]. Elevated levels of serum C-peptide in type 2 diabetic patients may be due to associated with metabolic syndrome in subjects with type 2 diabetes and in subjects with nephropathy and vascular disease; C-peptide is eliminated from the body by the kidneys [32]. In the period of insulin resistance and early type 2 diabetes, elevated levels of C-peptide circulate through the glomeruli and could deposit in the juxtaglomerular apparatus, and from there could have a mitogenetic effect on mesangial cells [33]. Some of the recent studies suggest that C-peptide may promote lesion development in subjects with type 2 diabetes and insulin resistance, while the application of C-peptide in type 1 diabetic subjects who lack C-peptide has been shown to improve diabetic microvascular complications such as diabetic neuropathy [32]. The potential proatherogenic action of C-peptide is not in conflict with the clinical benefits of C-peptide treatment in subjects with type 1 diabetes. Comparing situations, supplementation of C-peptide in type 1 diabetic subjects may be beneficial, whereas in subjects with insulin resistance and type 2 diabetes mellitus, increased levels of C-peptide may be harmful [33]. Moreover, other studies suggest that as BMI increases C-peptide also increases in type 2 diabetes [34]. The present results indicated that the lower levels of serum adiponectin in type 2 diabetic patients may be due to that adiponectin levels are inversely associated with degree of insulin resistance [35] and lower adiponectin is also associated with higher risk of type 2 diabetes [36]. In concordance, hypoadiponectinemia is observed in insulin resistance-related conditions such as metabolic syndrome, hypertension, dyslipidemia, and oxidative stress [37]. However, it remains a question whether the inverse relationship between adiponectin levels and degree of insulin resistance represents a causal relationship. In the liver, adiponectin lowers glucose levels by suppressing gluconeogensis and sensitizes hepatic cells to the effects of insulin by a reduction in lipid content [38]. This is achieved by the inhibition of the expression of gluconeogenic enzymes and the phosphorylation of acetyl coenzyme-A carboxylase (ACC). In skeletal muscle, it activates Adenosine monophosphate activated protein kinase (AMPK), thereby stimulating phosphorylation of ACC, fatty acid oxidation and glucose uptake [39]. In particular, high molecular weight adiponectin has been suggested to be the bioactive form having stronger associations with insulin sensitivity and suppression of hepatic glucose production than other forms of adiponectin [40]. In obese or obese type-2 diabetes individuals, activation of AMPK signaling and fatty acid oxidation by globular adiponectin is reduced, not due to the expression of adiponectin receptors, but as a result of downstream signaling after receptor binding [41]. APPL1, an adaptor protein containing pleckstrin homology domain, has been suggested to be a key molecule in signal transduction linking adiponectin receptors and AMPK activation [42]. On the other hand, data obtained was shown that females had a significantly higher adiponectin serum levels than males. A possible explanation for this gender based difference in adiponectin serum levels might be due to the following reasons; first, is the effect of sex hormones on the production of adiponectin rate [37]. Experimental studies have proved that androgens (testosterone) have an inhibitory effect on adiponectin secretion [43]. Second, is the different body fat distribution between males and females. It has been reported that the number of fat cells and their size are possible determinants of adiponectin production rates since it is mainly secreted from adipocytes [44]. Decreased levels of insulin in type 2 diabetic patients may lead to increased activity of lipase in adipose tissue, which converts the triglycerides into glycerol and free fatty acids. Therefore, increased the release of free fatty-acid from insulin-resistant fat cells may be the cause of the increased flux of FFAs into the liver in the presence of adequate glycogen stores [45]. High triglycerides levels cause increased transfer of cholesteryl esters from HDL-C and LDL-C to very VLDL-C via cholesteryl ester transfer protein, thus forming cholesteryl ester depleted, small dense LDLC particles [46]. These small dense lipoprotein particles are taken up by arterial wall macrophages, resulting in atherogenesis [47]. In type 2 DM, enhanced lipolysis leads to high FFA levels in plasma and consequent accumulation of fat in liver. Due to this, more acetyl-COA is now available which cannot be efficiently oxidized by the tricarboxylic acid (TCA) cycle because the availability of oxaloacetate is limited. The stimulation of gluconeogensis is responsible for the depletion of oxaloacetate. The excess of acetyl-COA therefore is diverted to cholesterol leading to hypercholesterolemia. There is hyperlipidemia, especially an increase in non-esterified FFAs, triglycerides and cholesterol. Other factors which are responsible for hypercholesterolemia are low fiber diet, lack of exercise, sedentary and inactive life style. High energy intake leads to obesity, stress, and other changes [48]. Diabetics are more prone for myocardial infarctions and stroke because the cholesterol levels are elevated causing atherosclerosis. This is due to an increase in LDL and VLDL levels in the plasma, probably because of either augmented production of VLDL in the liver or decreased removal of LDL and VLDL from the blood stream. In the present study, type2 diabetes patients showed a low serum HDL-C level in comparison to controls. HDL acts by enhancing the removal of cholesterol from the peripheral tissues and so reduces the body’s cholesterol pool. Type 2 DM was usually associated with low plasma levels of HDL-C [36]. Low HDL-C concentrations are often accompanied by elevated TG levels as seen in this study and others. The combination has been strongly associated with an increase in risk of coronary heart disease (CHD) [49]. The relative insulin deficiency that occurs in type 2 diabetes impairs the action of lipoprotein lipase and results in lower HDL-C levels and higher TG levels, which may improve with improved glycemic control [50]. Thus, HDL hypocholesterolemia in type 2 diabetes patients is mainly due to insulin resistance-linked lipoprotein lipase deficiency [51]. Hyperlipidaemia as a metabolic abnormality is frequently associated with DM. Its prevalence depends on the severity of diabetes, glycemic control, nutritional status and other factors. Type 2 diabetic patients have a high probability of developing cardiovascular disease. Lipids abnormalities that occur in these situations are hypertriglyceridemia and low levels of HDL. Increases of LDL-C exposed to glucose undergo peroxidative damage. Glucose can undergo autoxidation and produce free radicals, which can damage vascular function [48]. This study reports that high levels of serum LDL-C in diabetic patients are indicating the auto oxidation of glucose results in the formation of hydrogen peroxide which inactivates SOD. Therefore, the accumulation of H2O2 might be one of the explanations for decreased SOD in these patients. Also, with longer disease duration, SOD induction and consequently its activity progressively decreases, since non-enzymatic glycation, the other cause of hydrogen peroxide production, later predominates and further inhibition Cu/Zn SOD occurs. These results go with the result of Rahbani-Nobar et al. [52]. It has been proposed that increased plasma insulin levels promote VLDL synthesis resulting in elevated plasma TG levels, whereas, increased elimination of lipids and apolipoproteins from VLDL particles results in the increased production of intermediate density lipoprotein (IDL) and LDL [53]. In insulin resistance, the effects on adipose tissue are similar, but in the liver, the increased FFA flux tends to promote hepatic very low density lipoprotein (VLDL) production whilst ketogenesis typically remains suppressed by the compensatory hyperinsulinaemia. Furthermore, since lipoprotein lipase activity is insulin-dependent and impaired by insulin resistance, peripheral uptake of triglycerides from VLDL is also diminished. These mechanisms contribute to the observed hypertriglyceridaemia of insulin resistance [54]. Increased levels of VLDL-transported triglyceride enable (CETP) to promote further transfer of triglyceride to LDL in exchange for LDL-transported cholesteryl esters. The triglyceride-rich LDL undergoes hydrolysis by hepatic or lipoprotein lipase, which results in lipid-depleted small dense LDL particles. Large buoyant LDL particles are cleared rapidly by LDL receptors while small dense particles do not bind readily to LDL receptor and thus persist longer in the circulation. They are also readily modified by oxidation and particularly in NIDDM glycation and become atherogenic [46]. In this work, selenium levels were found to be significantly lower in diabetic patients when compared to healthy control group. It is the main element in glutathione peroxidase (an active enzyme against oxidative stress) that reduces formation of free radicals and peroxidation of lipoproteins. The low concentration of selenium in serum may be due to expose the subject to oxidative stress which is known to be associated with the pathogenesis of diseases such as diabetes mellitus [55]. On the other hand, low concentration of this element in blood might be an indication of active production of free radical and increased scavenging activity of either selenium or glutathione peroxidase. This decrease in serum selenium levels could contribute to oxidative stress and low selenium level has been shown to reduce insulin secretion and increased insulin resistance in some experimental models, thereby possibly playing a causal role in the development and pathogenesis of type 2 diabetes [56]. Our study indicated that a Zn level in diabetics was lower than the control group. The possible explanation of the present results come as follows: in the mammalian pancreas, Zn is essential for the correct processing, storage, secretion, and action of insulin in beta (β)-cells. Insulin is stored inside secretory vesicles or granules, where two Zn+2 ions coordinate six insulin monomers to form the hexameric-structure on which maturated insulin crystals are based [57]. It is also known that like, most other chronic disorders, diabetes increases the excretion of more minerals such as Zn in urine than non-diabetics or may be decrease gastrointestinal absorption of Zn [58]. Also, hyperglycemia in diabetes is usually associated with hyperzincuria, which is of renal origin, and increased urinary loss of Zn++and decreases of its concentration in total body Zn+2 [59]. Renal tubular defect in handling zinc and glucose-induced, osmotic diuresis are other possibilities. Zn deficiency is associated with metabolic disturbances including impaired glucose tolerance, insulin degradation, and reduced pancreatic insulin content. Furthermore, Zn may improve glycemia, and a restored Zn status in patients with type 2 diabetes may counteract the deleterious effects of oxidative stress, helping to prevent complications from beneficial antioxidant effects in persons with type 2 diabetes. Zn has been reported to have particular importance in light of the deleterious consequences of oxidative stress in persons with diabetes [60]. Zn has antioxidant properties; thus, it can stabilize macromolecules against radical induced oxidation. Moreover, it plays a key role in the synthesis, secretion and action of insulin in both physiological and pathological situations. In addition, recent studies have highlighted Zn’s dynamic role as a “cellular second messenger” in the control of insulin signaling and glucose homeostasis [61]. Finally, there was some evidence which shows that Zn acts as an antioxidant. Under Zn deficiency, free radicals are activated due to an impaired antioxidant defense system and imbalances in the production of free radicals. High oxidative stress conditions created this situation are involved in the pathogenesis of diabetes and its related complications [62]. In our present study, the serum value of Mg showed statistically significant decreases in diabetics when compared to healthy subjects. Mg is necessary for several enzymes that play an important role in glucose metabolism. The hypomagnesaemia in diabetic nephropathy might be due to poor dietary intake, impaired absorption of Mg, increased urinary loss due to hyperglycemia, osmotic diuresis, defective Mg reabsorption from renal tubules, and/or loss of plasma protein bound Mg. Mg depletion is said to reduce insulin sensitivity, thereby increasing the risk of secondary complications. Hyperglycemia leads to decreased cellular Mg levels. Hypomagnesaemia leads to collagen and ADP-induced platelet agreeability and also decreased function of Mg dependent enzymes, kinases and oxidative stress [63]. Mg deficiency also has a role in the perturbation of lipid metabolism of diabetic patients. Hypomagnesaemia inhibits prostacyclin receptor function, producing an imbalance between prostacyclin and thromboxane effects. Hypomagnesaemia can increase platelet reactivity, increase vascular and adrenal responses to angiotensin II, enhance thromboxane A2 (TXA2) release, and lead to organ damage from free radicals [64]. Hypomagnesaemia causes dyslipidaemia by decreasing activity of lipoprotein lipase, LCAT (lecithin cholesterol acyl transferase) and increasing (HMG-COA) reductase enzyme. The lipid changes are attributed to increased FFAs flux secondary to insulin resistance [65].
6. Conclusions
- In conclusion, our findings indicated that insulin resistance plays an important role in the pathogenesis of diabetes, obesity, and metabolic syndrome. On the other hand, the baseline of serum adiponectin concentration was statistically significant, inversely associated with risk of NIDDM. Therefore, a reduced level of serum adiponectin seems to be not just a mere biomarker of DM but may also play a causal role in the development of insulin resistance, NIDDM, metabolic syndrome and dyslipidemia. Also, these findings underscore the importance of obesity as a significant predictor of incident metabolic syndrome and can be used to identify at-risk individuals from both sex men and women. Hence, taking into consideration the high world prevalence of obesity, insulin resistance, NIDDM, metabolic syndrome and dyslipidemia, the possibility of a defined and unique therapeutic target to simultaneously combat their development becomes increasingly important.
ACKNOWLEDGEMENTS
- Author is highly thankful to the head of Chemistry Department, College of Science, University of Basrah for providing their kind support and facilities to accomplish the present research project within time.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML