-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2017; 7(4): 63-72
doi:10.5923/j.ajb.20170704.02

Effects of High Doses of Exogenous Taurine, Caffeine, and Taurine-Caffeine Combination on Biochemical, Haematological and Histologic Parameters of Adult Rabbits
Oluwakemi I. Taiwo, Abdulfatai A. Adesokan
Department of Biochemistry, Faculty of Basic Medical Sciences, Ladoke Akintola University of Technology, Ogbomoso, Nigeria
Correspondence to: Oluwakemi I. Taiwo, Department of Biochemistry, Faculty of Basic Medical Sciences, Ladoke Akintola University of Technology, Ogbomoso, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The potential interactions of taurine and caffeine in ‘energy drinks’ resulting in adverse events in humans have been major concern to many international bodies. Yet, few experimental studies have tested the effects of these interactions in animal models, hence this study. Twenty four adult rabbits were randomly divided into 4 groups of 6 rabbits (3 males, 3 females). Groups 1, 2, 3 and 4 (control) received daily, 1000 mg/kg taurine, 80 mg/kg caffeine, 1000 mg/kg taurine + 80 mg/kg caffeine and water, respectively for 21 days. Venous samples were collected on days 1 (baseline), 14, 21 and 28, and animals sacrificed on days 21 and 28. Assessment of hepatic and renal functions, muscle activity and lipid profiles were done by spectrophotometry, in addition to haematological and histological studies. There were no significant changes in parameters for rabbits in group 1 and control, except for increase in plasma level of high density lipoprotein and in red cell count in group 1. Rabbits in group 2 showed mild reversible derangement in hepatic and renal functions, while rabbits in group 3 showed gross derangements in hepatic, blood and muscle functions as well as lipid profiles. Histology showed congested hepatocytes and intraventricular haemorrhage with mortality of 33.3% recorded in this group. This result showed that high doses of taurine and caffeine can cause serious adverse effects in local strains of rabbits. Because these are usually present in most ‘energy drinks’, there is need for caution in consumption of large amount of these drinks.
Keywords: Exogenous, Taurine, Caffeine, Invivo, Adverse effect, Rabbit
Cite this paper: Oluwakemi I. Taiwo, Abdulfatai A. Adesokan, Effects of High Doses of Exogenous Taurine, Caffeine, and Taurine-Caffeine Combination on Biochemical, Haematological and Histologic Parameters of Adult Rabbits, American Journal of Biochemistry, Vol. 7 No. 4, 2017, pp. 63-72. doi: 10.5923/j.ajb.20170704.02.
Article Outline
1. Introduction
- In recent years, consumption of “energy drinks” which represent a category of beverages that appeared in the European, North American and African markets have become increasingly popular. These drinks contain stimulating substances such as caffeine, taurine, guarana, ginseng or other plant extracts, in combinations with other substances such as carbohydrates, vitamins and minerals. They are designed to give the consumer new energy in form of physical and/or intellectual boost. The major stimulatory component of energy drinks is caffeine, which is consumed as a natural constituent of coffee, tea and other natural sources such as guarana and yerba mate. Caffeine is also used as food additive and is present in certain carbonated soft drinks such as Coca Cola as well as in some therapeutic products such as cold remedies and allergy drugs [1]. A single serving of c affeinated beverage containing 40-120 mg of caffeine produces a stimulatory biological effect, and promotes alertness and physical endurance, enhances cognitive performance and relieves fatigue [2-4]. This single serving can also cause transient adverse effects such as insomnia, headaches and nervousness in caffeine-sensitive individuals [5, 6]. The principal mode of action of caffeine is as a competitive antagonist of adenosine receptors in the brain [7] resulting in increased activity of the neurotransmitter dopamine, that largely account for caffeine stimulatory effects. Caffeine is also a known competitive inhibitor of the enzyme cAMP-phosphodiesterase (cAMP-PDE), which converts cyclic AMP (cAMP) to its non-cyclic form, allowing cAMP to build up in cells [7]. Cyclic AMP participates in the messaging cascade produced by cells in response to stimulation by epinephrine, so by blocking its removal, caffeine intensifies and prolongs the effects of epinephrine and epinephrine-like drugs such as amphetamine, methamphetamine or methylphenidate [8]. In an extensive review of 300 studies that examined the potential health effect of caffeine [5], it was concluded that a healthy adult could tolerate a maximum intake of 400 mg caffeine per day which is equivalent to 6.7 mg/kg body weight/day for a 60 kg adult.The second major stimulatory component of energy drink, taurine, is naturally present in the diet, specifically meat and seafood, and was first identified as 2-aminoethanesulfonic acid [9]. Although taurine is generally referred to as an amino acid, it lacks a carboxyl group and is not incorporated into protein [10]. Daily human intake of taurine is estimated to be in the range 40 to 400 mg [11]. These dietary levels of taurine are relatively low compared with 1000 mg of taurine in a single serving of a typical energy drink. Taurine is one of the most abundant amino acids in the human body present in relatively high amounts in the brain, retina, gall bladder, skeletal and cardiac muscles, and organs throughout the body. Felines especially cats lack the ability to synthesize taurine and therefore require supplements in their food. Human also have limited ability to synthesize taurine, making it conditionally semi-essential. Especially in infants, taurine is considered essential for normal development and consequently, it is a standard ingredient supplement in infant formula. Taurine plays a role in several biological processes including the formation of bile salt, cell membrane stability, inflammation associated with oxidative stress, modulation of calcium flow and neuronal excitability [12, 13]. It serves a wide variety of functions in the central nervous system, from development to cytoprotection, and taurine deficiency is associated with cardiomyopathy, renal dysfunction, developmental abnormalities and severe damage to the retinal neurons [14]. Despite its many functional properties however, the cellular and biochemical mechanisms mediating the actions of taurine are not fully known, as neither the intracellular link nor a taurine-specific receptor is yet to be identified at the molecular level. Health hazard data on energy drinks are extremely limited. Hazard assessments of these drinks are therefore usually based on assessment of individual ingredients and their interaction. In several human studies of adult, child and infant subjects involving exogenous administration or intake of high doses of taurine of up to 3000 to 6000 mg for periods of up to one months or longer, no demonstrable safety concerns have been raised [15]. However, a dose-related behavioral changes (increased activity, self-injury) were observed in a 90-day study of rats in which doses of 300 to 1000 mg/kg body weight/day of taurine were administered to rats [16], although a repeat of this study in 2009 [15] did not confirm these findings. There is also concern about the potential interactions of constituents of energy drink especially caffeine and taurine with possible synergistic or antagonistic effects on cardiovascular, renal and central nervous system [15]. One double-blind study [17] involving 14 human volunteers showed that a combination of caffeine and taurine induced a decrease heart rate and an increase in mean arterial blood pressure, a finding that is totally unexpected since caffeine generally stimulates heart rate. It is postulated that some of the adverse effects reported from consumption of energy drinks may be due to yet unknown interactions between caffeine and taurine rather than individual effects of caffeine or taurine. Further investigation of these interactions is warranted. The objective of this study therefore is to assess the biochemical, haematological and histological effects of administration of high doses of exogenous taurine, caffeine and taurine-caffeine combination in a 28-day repeated dose toxicodynamic study on local strains of adult rabbits.
2. Materials and Method
- Study areaThis study was conducted at the Department of Biochemistry, Ladoke Akintola University of Technology (LAUTECH), Ogbomoso, Nigeria.Study designThe research is a repeated dose (28-day) toxicodynamic study of the effects of taurine, caffeine and taurine-caffeine combination on biochemical, haematological and histologic parameters in adult rabbits.Reagents and consumablesTaurine (white crystalline powder CAS No 107-35-7, BBI Canada) containing 100 g pure taurine, Canada) and 500 g caffeine (white crystalline powder CAS No 58-08-2, BBI Canada), were purchased from Biocom Biotech Diagnostics, South Africa. Analytical grade commercially prepared biochemical reagents were purchased from Randox Diagnostics, United Kingdom and kept frozen at -20°C until use.Stock solutions of taurine was prepared by measuring 20 g of taurine powder using analytical weighing balance and dissolving in 100 ml of reagent graded (de-ionized) water to give a concentration of 200 mg/ml of taurine in accordance with the manufacturer’s recommendation. Similarly, stock solutions of caffeine were prepared by dissolving 20 g of caffeine powder in 1000 ml of reagent graded (de-ionized) water to give a concentration of 20 mg/ml of caffeine.Experimental and control rabbitsLocal strains of adult rabbits (a total of 24, 12 males and 12 females) with average weight of 1.8 kg were obtained from the Animal House of the College of Health Sciences, LAUTECH, Osogbo, Nigeria. All rabbits were housed in metal cages of a maximum of 2 rabbits (of the same sex)/cage in a well-ventilated experimental room to acclimatize for 7 days at temperature of 20-26°C, relative humidity of 40-60% and exposure to 12 h light and dark cycles. Ethical approvalThe approval of the Ethical Review Committee of the College of Health Sciences, Ladoke Akintola University of Technology, Osogbo, Nigeria was obtained. All experiments were performed in accordance with Good Laboratory Practice (GLP) regulations and the ethical guidelines of the Organization for Economic Cooperation and Development [18] for the use of laboratory animals. Study protocolThe rabbits were divided into 4 groups of 6 rabbits (3 males and 3 females) each, after 7 days of acclimatization. Rabbits in each group were fed the same standard laboratory diet and water ad libitum throughout the period of the experiment. In addition, rabbits in group 1 received 1000 mg/kg body weight/day of taurine (1800 mg taurine) by oral drenching daily for 21 days. Rabbits in group 2 received 80 mg/kg body weight/day of caffeine (144 mg caffeine) daily for 21 days. Rabbits in group 3 received combined 1000 mg/kg body weight/day of taurine (1800 mg taurine) and 80 mg/kg body weight/day of caffeine (144 mg caffeine) per day for 21 days. Rabbits in group 4 served as control.Observation of behavioural changesChanges in the behaviour of animals in the test and control groups were noted by daily observation of physical or mental status before and 1 hour after administration of the substance. Blood sample collection for biochemical and haematological analysesApproximately 5 milliliters of blood were collected from the ear lobe vein of each rabbit in all three groups using sterile needle and syringe on day 1 of the study for evaluation of baseline parameters. The blood samples were collected into Lithium Heparin bottles for biochemical analysis and Ethylene Diamine Tetra acetic acid (EDTA) bottles for haematological parameters. Blood samples were also collected on days 14, 21 and 28. Blood samples for biochemical analysis were first centrifuged at 5000 x g for 3 min in a bucket centrifuge (Model 800 D CE) to separate plasma which was then pipetted into separate plain bottles, and kept frozen at -20°C until time of analysis. Tissue collection for histopathologyOn day 21, test substances was withdrawn from all rabbits in the test groups, and two rabbits (1 male and 1 female) in each of the test groups and 2 rabbits in the control group were sacrificed by cervical dislocation. All other rabbits were sacrificed on day 28. Relevant organs (liver, lung, kidney, heart, stomach and intestine) were harvested and fixed with neutral buffer 10% formalin for histopathological analysis.Biochemical assessment of liver functionLiver functions was assessed by testing for plasma proteins (total protein and albumin) and liver enzymes (Aspartate Transaminase, Alanine Transaminase and Alkaline Phosphatase). Total plasma protein was estimated using Biuret method [19]. Plasma albumin was measured using routine method of Tietz [20]. The kinetic determination of ALT activity was according to the coupling reaction involving pyridoxal-5’-phophate and lactate dehydrogenase at 37°C with decrease in absorbance measured at 340 nm by continuous monitoring while that of AST activity was based on coupling reaction involving pyridoxal-5’-phophate and malate dehydrogenase at 37°C with decrease in absorbance measured at 340 nm by continuous monitoring [21]. Alkaline Phosphatase (ALP) activity was measured by the modified method of Bowers and McComb [22].Biochemical assessment of renal functionAssessment of renal functions was done by measuring plasma creatinine, urea and electrolytes (sodium, potassium, chloride and bicarbonate ions). Creatinine in plasma was estimated by the Kinetic Jaffe reaction [21]. Urea was estimated in the plasma by the colorimetric Urease-Berthelot method [23]. Plasma concentrations of sodium, potassium, chloride and HCO3- were measured using Ion Selective Electrode (ISE, 6000 analyzer, SFRI).Biochemical assessment of muscle activityCreatine kinase activity in the blood, as a function of muscle activity, was based on the reaction that CK catalyzes the transfer of a phosphate group from the creatine phosphate substrate to adenosine diphosphate (ADP). The subsequent formation of adenosine triphosphate (ATP) was measured through the use of two coupled reactions catalyzed by hexokinase (HK) and glucose-6-phosphate dehydrogenase (G6PD) which results in the production of β-Nicotinamide Adenine Dinucleotide (reduced form) (NADH) from β-Nicotinamide-Adenine Dinucleotide (NAD), measured by decreased absorbance at 340 nm [21].Determination of lipid profileLipid profiles were determined by measuring plasma total cholesterol (TC), high density lipoproprotein (HDL) cholesterol and triglyceride. Total cholesterol was determined based on enzymatic hydrolysis and oxidation by cholesterol oxidase. Triglyceride was determined by colorimetric method based on enzymatic hydrolysis with lipase. HDL–C was determined by precipitation method while LDL-C was determined by differential subtraction of the sum of the cholesterol functions from total cholesterol using the Friedewald calculation formula; LDL-C = Total Cholesterol – {HDL-C + (TG/5)} mmol/L [21].Haematological analysisThe automated haematology analyzer (Shanghai Utrao Medical Equipment Company, Model SH800Plus, China) was used to analyze complete blood count (CBC) and different leukocyte count (DLC) with the estimation of the following parameters; red blood cell (RBC) count, haemoglobin concentration, packed cell volume (PCV), white blood cell (WBC) count, platelets count and red cell indices {mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC) and red blood cell distribution width (RWD).Histopathological assessmentHistopathological processing of organ specimens and examination of slides were done at the Histopathology Laboratory of the LAUTECH Teaching Hospital, Ogbomoso, Nigeria with the help of a Histopathologist. The fixed organs were dehydrated in ascending grades of alcohol, cleared and embedded in paraffin blocks. Sections (about 4-6 μ thickness) of paraffin-embedded tissue were cut using microtome and slides prepared and stained with Haematoxylin and Eosin (H&E). The stained sections were then examined under compound light microscope at 1000 x magnification for general histological features [24]. Photographs were taken with a camera. Statistical analysisData were calculated and reported as mean ± standard error of mean (SEM) for both male and females in each group (n = 3). Statistical comparisons were done between and among groups. Comparison of baseline data (day 1) with day 14 and 21 for experimental groups was performed using one way analysis of variance (ANOVA) while comparison of day 21 data with day 28 was done using Students “t” test. Significance level was fixed at p < 0.05.
3. Results
- MortalityMortality of 33.3% (2 of 6 rabbits) was recorded in group 3, involving one male and one female rabbit that died on day 18 and day 12 respectively. No death was recorded in groups 1 and 2, and the control group. Effects of administered substance on behaviour of rabbits in the test groupsRabbits in group 1 that received 1000 mg/kg of taurine, when compared with the control, showed increased activity at the initial stage but this was soon followed by calmness and somnolence. Rabbits in group 2 that received 80 mg/kg of caffeine showed marked aggression and restlessness for several hours after intake. Rabbits in group 3 that received combined doses of taurine and caffeine also showed increased activity and aggression as those in group 2.Effects of taurine, caffeine and taurine-caffeine on liver and muscle enzyme activitiesTable 1 shows the activities of liver (AST, ALT and ALP) and muscle (CK) enzymes of all the rabbits over the 28-day study protocol. These enzyme activities did not change significantly (p > 0.05) over these periods for rabbits in group 1 and group 4 (control). However, the ALT activity significantly increased (p < 0.05) on days 14 and 21 over baseline (day 1) in both male and female rabbits in group 2 that received 80 mg caffeine but reverted for the female rabbits upon withdrawal on day 21. Additionally, ALP activity significantly increased on days 14 and 21 over baseline for female rabbits in this group, however the AST activity was unaffected in both. For male rabbits in group 3 that received taurine-caffeine, AST, ALT, ALP and CK activities were significantly increased on days 14 and 21 over baseline while only AST was significantly affected in the female rabbits.
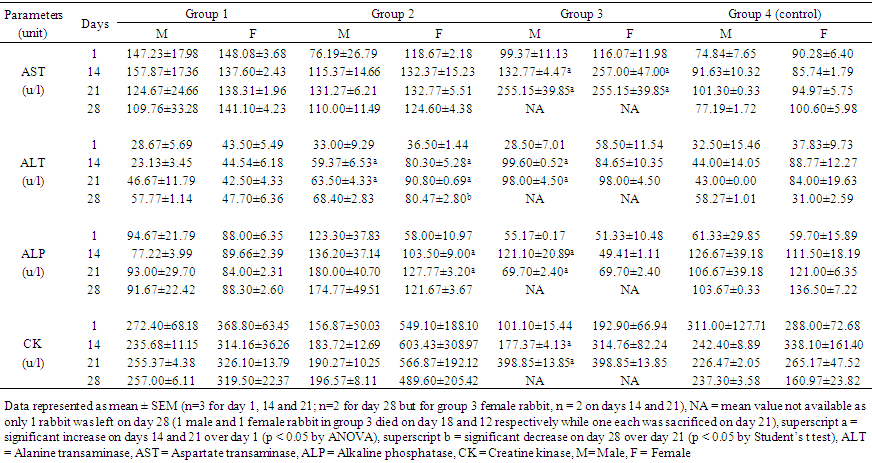 | Table 1. Effects of taurine, caffeine and taurine-caffeine on AST, ALT, ALP and CK activities of adult rabbits |
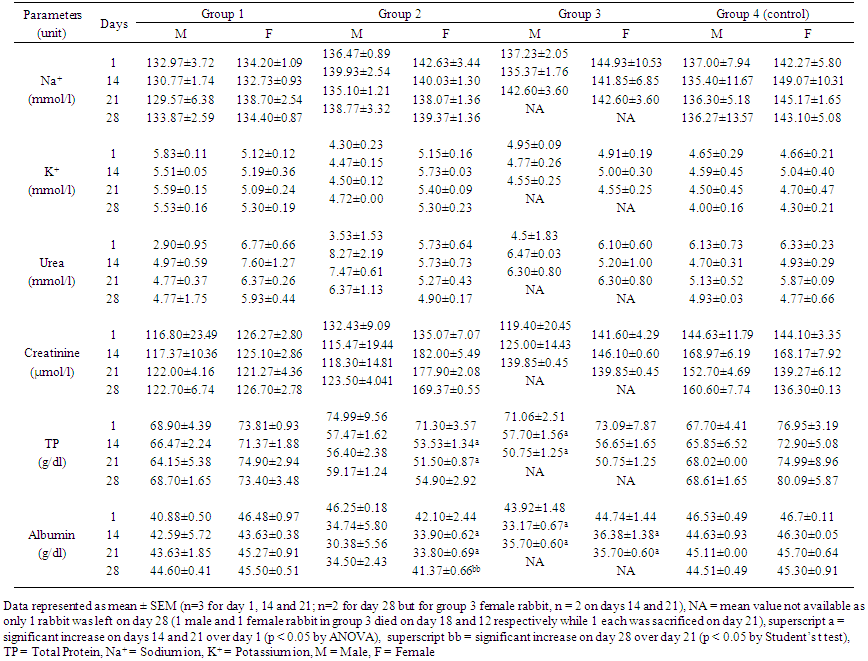 | Table 2. Effects of taurine, caffeine and taurine-caffeine on plasma proteins, Na+, K+, urea and creatinine of adult rabbits |
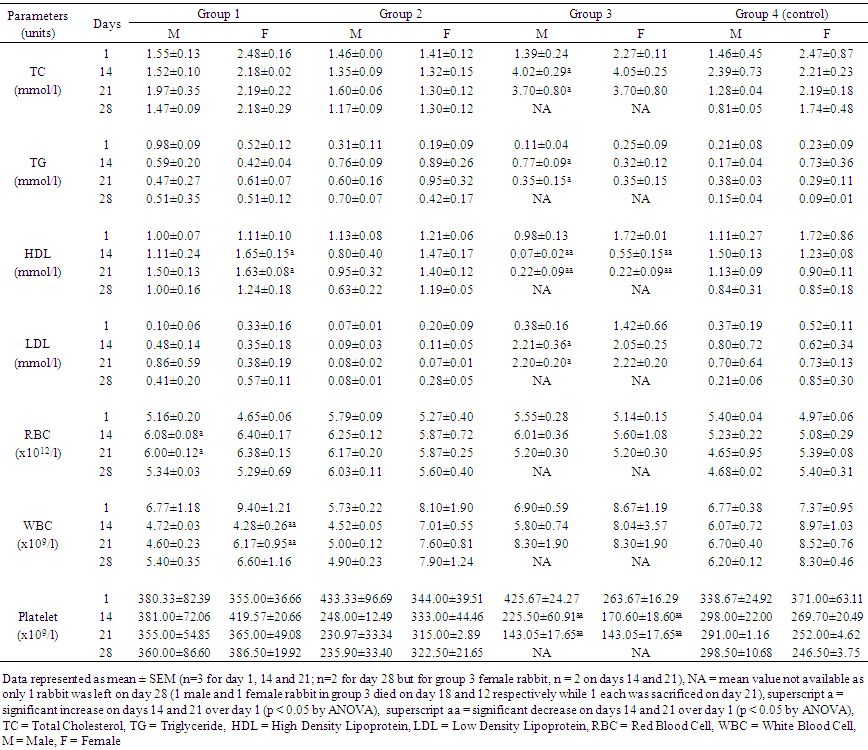 | Table 3. Effects of taurine, caffeine and taurine-caffeine on lipid profile and haematological parameters of adult rabbits |
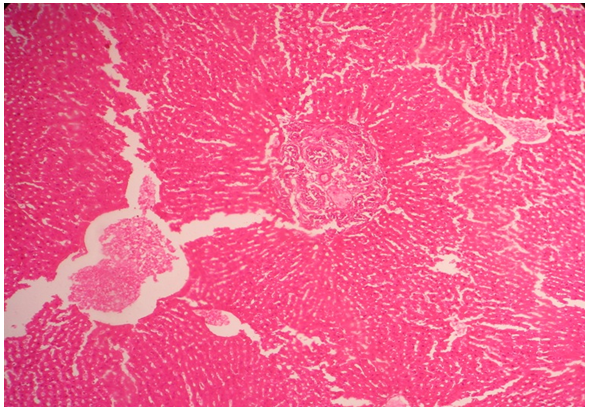 | Figure 1. Histology of liver of rabbit (group 1) showing normal architecture (1000x magnification) |
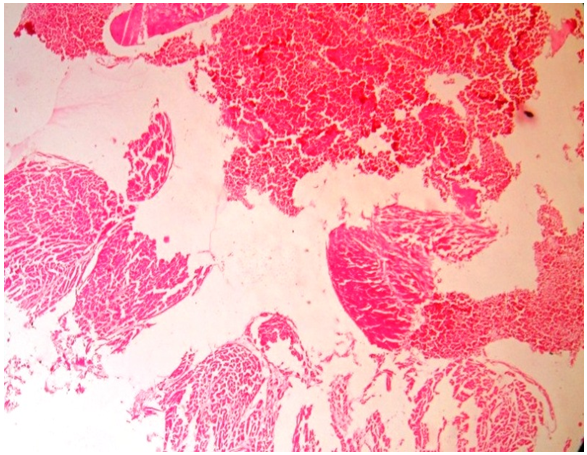 | Figure 2. Histology of heart of rabbit (group 2) sacrificed showing normal cardiac architecture (1000x magnification) |
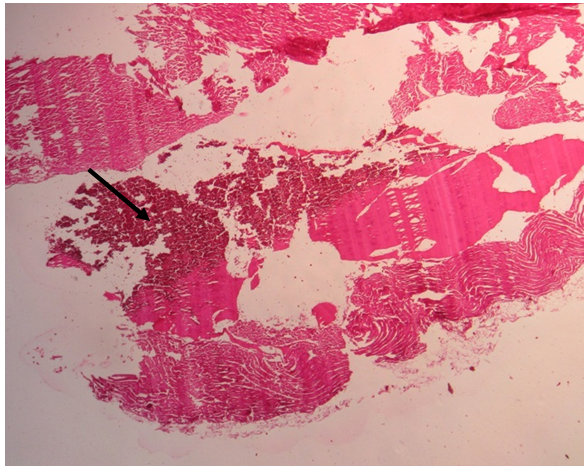 | Figure 3. Heart histology (group 3 rabbit) showing intraventricular haemorrhage (arrow) (1000 x magnification) |
4. Discussion
- The adverse effects reported from consumption of energy drinks have been attributed to two major constituents of these drinks, taurine and caffeine. Although a lot of studies evaluating the effects of taurine and caffeine individually in laboratory animals have been done by pharmaceutical companies producing these drinks [25-27] and by other researchers [1, 28], very few have studied the interaction of the two substances in humans [29-31], and experimental animal studies of these interactions are particularly sparse in the literature. This present research evaluated the toxicodynamic interactions of these two compounds in a repeated dose (28-day) study in order to determine possible adverse effects that they may have on local strains of rabbit.In the experimental group that received 1000 mg/kg taurine (group 1), there were no significant changes in the biochemical and haematological parameters or histopathologic changes in organs or tissues of the rabbits except for a reversible increase in red blood cell counts in both sexes and non-reversible increase in HDL-C levels in the female rabbits. Numerous publications have described different effects of taurine in different animal models on behavior, blood pressure, blood chemistry, serum glucose and cholesterol, and organ histology [32-35]. Some of these effects, which typically occurred following administration of taurine at the dose of 1000 mg/kg or more include behavioral changes such as increased activity and self-injury in rats, and impaired motor activity mediated possibly through pharmacological action of taurine on central nervous system [35]. Increased activity was typically and consistently observed in group 1 rabbits given 1000 mg/kg taurine in this study. In one previous study [33], administration of 0.4% taurine in drinking water to guinea pigs for 2 weeks corresponding to 462 mg/kg/day of taurine led to fatty infiltration of the liver while in another study on rats [34], administration of 1% taurine in drinking water (about 2600 mg/kg/day of taurine) for 2 weeks caused changes in neutral lipids, phospholipids and enzyme activities related to lipid metabolism in liver microsomal membranes. These changes have been found in a recent study on human liver model HepG2 cells [36] to be due to taurine inhibitory action on TG synthesis and reduction in secretion of apolipoprotein B-100 (apoB) that is a major protein component of VLDL-C. In this present study, there were no significant changes in lipid profiles of the rabbits given 1000 mg/kg taurine except for a significantly raised HDL-C level in the female rabbits, which interestingly, is generally regarded as anti-artherogenic and cardio-protective lipid [14, 21]. From the findings of this current study on group 1 rabbits and reports of similar studies using different animal models [33-35], it can be safely concluded that in a short term, there are no adverse biochemical, haematological or histopathologic changes in organs or tissues associated with high intake (≥1000 mg/kg) of taurine. The no-observed-adverse-effect level (NOAEL) of at least 1000 mg/kg/day taurine intake for pathological changes established in a previous study on rats [35] and upheld by the Scientific Committee on Food of the European Commission [15, 16], is supported by the results of this current study on rabbits. However, the long term effects of continuous high intake of taurine on rabbits cannot be predicted since this study was short term. The hyperactivity, marked aggression and restlessness observed for several hours in rabbits given 80 mg/kg/day caffeine (group 2) in this study mirrored the effects of acute overdose of caffeine in humans, which usually occurs when the dose taken is in excess of 350 mg. At this dose of caffeine in humans, there is CNS overstimulation resulting in symptoms that may include restlessness, nervousness, excitement, insomnia, flushing of the face, increased urination, gastrointestinal disturbance, muscle twitching, a rambling flow of thought and speech, irregular or rapid heartbeat and psychomotor agitation [37]. In extreme caffeine overdose, death can occur, for example, the median lethal dose (LD50) of caffeine in rats is estimated to be between 150 and 192 mg/kg [38, 39]. Although, the dose of caffeine administered to rabbits in this group (which is equivalent to giving an adult human 4.8 g of caffeine daily) was extremely high, there were no deaths recorded in this group. This may imply that rabbits possess certain biological mechanism which enable them tolerate high dose of caffeine. The biochemical parameters were deranged in both sexes of rabbits in this group, but more in the females. Increased ALT activity was the only significant change observed in the male rabbits but for the female rabbits, TP and albumin levels were reduced while K+ and creatinine levels were raised, and ALT and ALP activity increased. Histology of rabbits in this group did not show any organ pathology however. The biochemical changes observed in the rabbits in this group may suggest mild hepatic injuries that typically occur following acute consumption of caffeine containing food or drink [39]. The elevated creatinine and K+ concentration in the female rabbits may also suggest renal injury, although caffeine is known to cause short-term diuresis, by directly acting on renal tubules, such as ionic reabsorption and renal perfusion, probably mediated via adenosine receptor blockade [40]. The finding in this present study may suggest a more serious effect of caffeine on the kidneys at high dose especially for the female rabbits that appeared to be more susceptible to these injuries. The reason for this is not apparent but may be related to hormonal differences between male and female rabbits that can differently affect enzymatic activities in the body. The findings in group 3 rabbits which received high doses of taurine (1000 mg/kg) and caffeine (80 mg/kg) combination were remarkable. The behaviour of rabbits was similar to those in group 2 that received caffeine alone. However, there was marked derangement in the parameters that measured hepatic functions as shown by increase in the activity of all three liver enzymes assayed (ALT, AST and ALP) and reduction in albumin concentration in both male and female rabbits. This may imply a more serious injury to the liver. Equally, liver histology of the rabbits showed congestion and balloon degeneration of hepatocytes, which are features of hepatic injury [41]. The activity of the muscle enzyme, creatine kinase (CK), was also increased, although the exact source of the enzyme here cannot be clearly ascertained as the activity of its isoenzyme was not measured. Nevertheless, increased CK activity may imply possible injury to skeletal or cardiac muscle, but the histological finding of suspected intraventricular haemorrhage in the heart of a female rabbit at necropsy, points more to a cardiac injury. The significant reduction in platelets observed in both the male and female rabbits in this group may be contributory to the cardiac injury that resulted in intraventricular haemorrhage, and possibly death. The lipid profile of male rabbits in this group showed increase in levels of total cholesterol, triglyceride and LDL-C, while HDL-C level decreased over the baseline values. Consumption of naturally occurring caffeine rich substances such as coffee, guarana and kolanut is known to be associated with increase in total cholesterol, triglyceride, LDL-C and VLDL-C [39, 42, 43, 44]. While taurine has been shown to inhibit TG synthesis and reduce secretion of apolipoprotein B-100 (apoB) that is a major protein component of VLDL-C [36], consumption of caffeinated coffee of two to three cups per day has been correlated with higher levels of apolipoprotein B [44], in addition to increasing LDL-C, TG and total cholesterol levels. Taurine and caffeine therefore appear to exact opposing effects on cholesterol synthesis probably mediated through different receptor binding or enzyme induction, with taurine reducing VLDL-C while caffeine increases VLDL-C levels. The net effect of combined taurine and caffeine on cholesterol metabolism as shown in this rabbit study is tilted towards overriding caffeine effect of increasing artherogenic lipids, therefore, taurine does not ameliorate the adverse effects of caffeine in relation to cholesterol metabolism, unlike what it has been suggested to do with the cardiovascular or central nervous system [16, 45]. Surprisingly, none of the biochemical parameters used to assess renal functions (urea, creatinine, electrolytes) in this test group 3 was deranged in both male and female rabbits, although these traditional markers of renal functions have low sensitivity and specificity in detecting acute kidney injury [46]. The effects of taurine and caffeine on the kidneys are usually limited to short-term diuresis with loss of body water and sodium. While taurine act via inhibition of central release of the anti-diuretic hormone, vasopressin, to achieve diuresis [47], caffeine act directly on renal tubules to decrease ionic reabsorption and renal perfusion, possibly via adenosine receptor blockade [40]. This diuretic action appear additive but since the effect is usually short-term it may not cause serious renal dysfunction that could lead to derangement of electrolytes, urea and creatinine assayed in this study. However, when this observation is juxtaposed with the findings of elevated creatinine and K+ levels in group 2 rabbits that received caffeine alone, it may be plausible to suggest that taurine ameliorate the adverse effect of caffeine on the kidneys.
5. Conclusions
- The findings in this short term toxicodynamic study showed that most of the adverse effects observed occurred in rabbits that received caffeine (group 2) and taurine-caffeine group (group 3), and not in the group that received taurine (group 1), with more severity observed in the taurine-caffeine group. It can therefore be concluded that the major individual constituents of energy drink, taurine and caffeine, tested for interaction at high doses in this study showed that caffeine is responsible for most of the adverse effects observed. While taurine was not associated with any significant adverse effect at high dose (NOAEL of 1000 mg/kg), it was found to potentiate the adverse effects of caffeine on hepatic and bone marrow functions, and lipid metabolism, while ameliorating its adverse effects on the kidneys.
ACKNOWLEDGEMENTS
- The authors acknowledge the contributions of medical laboratory scientists in the College of Health Sciences, Ladoke Akintola University of Technology, Osogbo during the laboratory aspects of the research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML