-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2017; 7(3): 43-46
doi:10.5923/j.ajb.20170703.02

Screening for Glucose 6 Phosphate Dehydrogenase Deficiency among Mangalorean Population
Sushith P.1, Suriyan S. Nair1, Prathima M. B.1, Reshma S.1, Leeshma James1, Aarathi Devan2
1Department of Biochemistry, A.J. Institue of Medical Sciences and Research, Mangaluru, Karnataka, India
2Department of Medicine, A.J. Institute of Medical Sciences and Research, Mangaluru, Karnataka, India
Correspondence to: Suriyan S. Nair, Department of Biochemistry, A.J. Institue of Medical Sciences and Research, Mangaluru, Karnataka, India.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

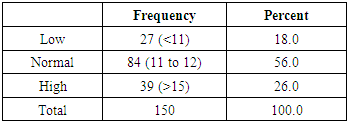
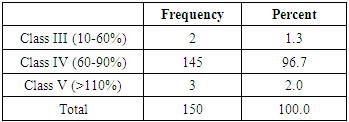
Background: Aim of this study was to quantitatively estimate the G6PD to determine the frequency of G6PD deficiency among the population of Mangaluru, Karnataka, India. Material and Method: This cross sectional study consisting of 150 healthy individuals who attended the A.J. Hospital and Research Centre, Mangaluru, Karnataka, India. Blood samples were collected from all the individuals and used for the analysis of Hemoglobin (Hb), serum total bilirubin and G6PD activity. Result: Hundred and forty five out of 150 subjects had normal enzyme activity, two had low and three have high G6PD activity. The Pearson correlation between G6PD activity and Hb concentration showed statistically significant correlation. Conclusion: In our study out of 150 individuals, only two samples tested were G6PD deficient. Thus according to this study screening for the enzyme deficiency need not be included under the routine tests.
Keywords: Glucose-6-phosphate 1-dehydrogenase, NADPH, G6PD deficiency, Enzyme activity
Cite this paper: Sushith P., Suriyan S. Nair, Prathima M. B., Reshma S., Leeshma James, Aarathi Devan, Screening for Glucose 6 Phosphate Dehydrogenase Deficiency among Mangalorean Population, American Journal of Biochemistry, Vol. 7 No. 3, 2017, pp. 43-46. doi: 10.5923/j.ajb.20170703.02.
Article Outline
1. Introduction
- Glucose-6-phosphate 1-dehydrogenase (G6PD) (EC 1.1.1.49) is the first and rate-limiting enzyme in the hexose monophosphate (HMP) shunt pathway and is important for its role in the regeneration of the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) and the production of ribose [1]. G6PD is a critical metabolic enzyme under complex control that resides at the centre of an essential metabolic nexus that affects many physiologic processes. In the red cell this pathway is the only source of NADPH, which is necessary to protect the cell and its haemoglobin from oxidation in view of their role in oxygen transport. During cellular oxidative stress, whether endogenous in origin or initiated by drugs or environmental chemicals, collectively referred to as xenobiotics, NADPH is critical for maintaining glutathione (GSH) in its reduced form, which is essential for detoxification of reactive free radicals and lipid hydro-peroxides [3]. G6PD deficiency affects every cell in the body, and its primary effects are haematological because the red cell has no alternative source of NADPH. G6PD deficiency is the most common gene mutation in the world and the numerous mutations have been classified by the World Health Organization [3]. G6PD deficiency is inherited in a sex linked fashion, being fully expressed in hemizygous males and homozygous females, but in only a proportion of female heterozygotes. Hereditary deficiencies in G6PD are the most common enzymopathy known, affecting well over 400 million people worldwide, and particularly those from the Mediterranean region and selected African and Asian countries wherein the incidence of G6PD deficiency may approach 60% of some populations. The degree of G6PD deficiency varies from negligible to severe according to both whether one or two alleles are affected and the nature of the gene mutation and protein/enzyme variant [4]. Haematological problems arising in these G6PD-deficient populations from exposure to oxidizing xenobiotics have been well characterized ranging from haemolysis of red blood cells and hereditary non spherocytic haemolytic anaemia to sepsis and life-threatening kernicterus in the new-born [5, 6]. The antioxidant system depends on production of NADPH for proper function. Its three major components in cells are the glutathione system, catalase, and superoxide dismutase [7]. Another system that is dependent on NADPH from G6PD activity is the family of enzymes called NADPH oxidases. These enzymes have many essential physiological roles (e.g normal cell growth, white blood cell function) and many pathophysiologic ones have been determined (e.g, in diabetes, cardiovascular disease) as a major source of reactive oxygen species (ROS). Another set of enzymes that are dependent on NADPH are the nitric oxide synthases [8, 9].G6PD-deficient individuals are at risk for haemolysis due to antimalarial drug- primaquine administration. The mechanism of red cell destruction during the haemolytic crisis is still poorly understood but it is clear that oxidative damage is due to leading denaturation and precipitation of haemoglobin to form Heinz bodies, which cause the red cells to become trapped in the spleen, where they are destroyed. The reaction may vary from transient mild anemia to rapidly progressing anemia with back and abdominal pain, jaundice and haemoglobinuria, and transient splenomegaly. Mangalore city is also endemic area for malaria and the general population is not routinely screened for G6PD deficiency before antimalarial treatment and there is no proper neonatal screening programme for G6PD deficiency at present in this region. Hence, we would like to screen the general population for G6PD deficiency status, so that a general awareness can be created regarding screening for G6PD deficiency by simple biochemical method which can be done at hospital without much cost bearing on patient. The prevalence of G6PD deficiency has been extensively studied in several population groups; however, there is scarce information about G6PD deficiency from Mangaluru, Karnataka and the last study was conducted in 1974.Hence, the aim of the present study was to quantitatively estimate the G6PD to determine the frequency of G6PD deficiency among the population of Mangaluru, Karnataka, India.
2. Materials and Methods
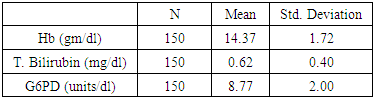
- This cross sectional study consisting of 150 healthy individuals (92 male and 58 female), in the age group of 15 -65 years who attended the A.J. Hospital and Research Centre, Mangaluru, Karnataka, India in the period between April 2015 and March 2016. Written informed consent was obtained from each study participant and the study was approved by the Institutional Ethics Committee. Blood samples were collected and a brief clinical data including age, ethnic group, place of residence, and history of past illnesses including fever and episodes of recurrent jaundice was recorded from all the participants. Adults who are diagnosed with malaria and on antimalarial medications were excluded from the study. All the samples were analyzed for Hemoglobin (Hb), serum total bilirubin and G6PD. Serum total bilirubin estimation was done in fully automatic chemistry analyser [10]. G6PD was determined by the UV kinetic method [11, 12].Statistical Analysis: The values were expressed as mean with standard deviation. The correlation among the parameters were analyzed by Karl Pearson’s Correlation Analysis.
3. Results
- A total of 150 subjects were enrolled in this study. The age of the patients ranged from 17 to 63 years with a mean age of 29.36 years. Age wise distribution of total subjects is given in graph 1. There were 58 females (38.7%) and 92 males (61.3%) in the study group.
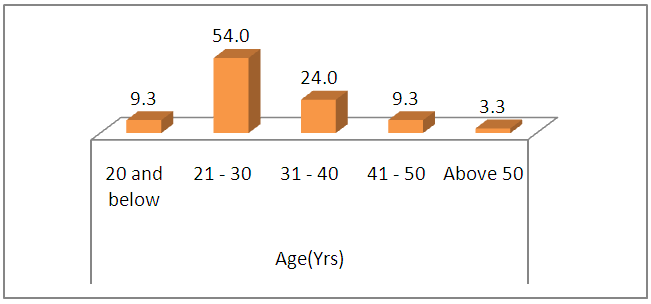 | Graph 1. Bar diagram showing the age wise distribution of total subjects |
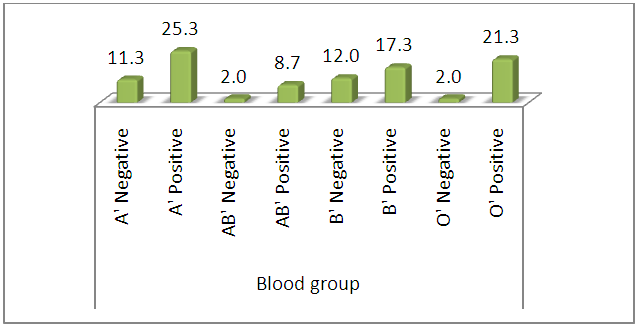 | Graph 2. Blood group distribution among subjects |
|
|
|
|
4. Discussion
- This study was attempted to screen for the presence of glucose6-phosphate dehydrogenase (G6PD) deficiency in asymptomatic population represented by the healthy individuals and to infer whether the screening test should be added as a routine test. But the data collected showed that prevalence of G6PD is very low. Our results differ from a few previous studies which have shown a higher prevalence of the disorder among the general population indicating the need for screening as a routine test.In the study by A M Shanthala Devi et al [13], sixteen out of 2005 screened by methhaemoglobin reduction test were found to be G6PD deficient. Among these, two donors were from West Bengal, one from Kerala and the rest of the 13 subjects were from Karnataka. This makes the incidence of the disorder to be 0.6% in the sampled population from Karnataka. However the study concluded that the prevalence of G6PD is very low in the study. Many people remain asymptomatic unless challenged with oxidative drugs or infection. Screening the whole population for G6PD deficiency was not warranted, but screening of blood to be 2 transfused for select population like neonates was advisable. In another study by Ching-Shan Huang et al [14], 88 out of 90 individuals tested were confirmed to be G6PD deficient at the DNA level. But since the technique used was molecular biology, our test fails to match its specificity and sensitivity. But again the study concluded that it was unnecessary to screen G6PD activity for donors of adult recipients in Taiwan, since no significant haemolytic reactions in the recipients were found post-transfusion. However in a study done by H. Amoozegar et al [15] among blood donors in Shiraz, Iran, a prevalence of 6% was noted (27 out of 450 blood donors were G6PD deficient). The study concluded the prevalence to be noteworthy and recommended screening the blood bags for this enzyme prior to use for simple or exchange transfusion. Similarly, in a study conducted by E. O. Akanni et al [16] among the blood donors in Osogbo, Nigeria, a prevalence of 19.5% (39 out of 200 donors) was obtained for the disorder. The study conducted by us therefore concluded that the necessity of including G6PD should be more with respect to geography, ethnicity, the number of samples screened and the study period. Even the tests used in some of these studies (methemoglobin reductase test) are different from ours where we have quantatively determined the G6PDH activity by UV kinetic method which is a very sensitive assay. Our test results coincide with the data by WHO which states the prevalence of the disorder in various parts of the world. According to this, the disorder is very prevalent in individuals of Africa, America, Mediterranean, and East Asia. In India the incidence of G6PD has been variably reported as 0–37% in different castes and communities. Higher incidence of G6PD deficiency is seen in north and west India (15%) as compared to south India where it is only 1–2 percent. The very low prevalence of the disorder in South India and also the short period of study (14 months) accounts for a portion of the cause behind the negative results.
5. Conclusions
- This study was conducted to determine the prevalence of G6PD deficiency in general population and to see whether screening for G6PD deficiency should be included as a routine especially in blood banks. In our study out of 150 individuals, only two samples tested were G6PD deficient as determined by the UV kinetic method. Thus according to this study screening for the enzyme deficiency need not be included under the routine tests but need to be confirmed with a large sample size. A continued study for longer periods, involving a larger number of individuals from different geographical areas, castes and communities may be required to ascertain the prevalence of the enzyme disorder and the necessity of routinely screening for it in healthy population as well as in blood donors. In vitro tests able to predict risk of haemolysis in G6PD-deficient subjects should be perfected and applied to all new drugs to be introduced in areas where G6PD deficiency is prevalent.
List of Abbreviations
- 1. G6PD - Glucose-6-phosphate 1-dehydrogenase 2. HMP- Hexose monophosphate 3. NADPH- Nicotinamide adenine dinucleotide phosphate4. GSH- Glutathione 5. ROS -Reactive oxygen species6. Hb- Hemoglobin 7. T. Bilirubin- total bilirubin
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML