Seham Mohamed Saied El Nakeeb1, Reem Mohamed Ahmed Ali1, Rasha Elsayed Mohamed Abd El-Aziz1, Giehan Hussien Tawfik Ewida1, Ahmed Abdel Kader Nemr2
1Biochemistry Department, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
2Neurology Department, Maadi Military Hospital, Cairo, Egypt
Correspondence to: Reem Mohamed Ahmed Ali, Biochemistry Department, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Background: Inflammation of central nervous system is the primary cause of damage in multiple sclerosis (MS). The specific elements that start this inflammation are still unknown. Studies have suggested that genetic, environmental and infectious agents may be among the factors influencing the development of MS. The interleukin-2 (IL-2) gene is a powerful functional candidate that is involved in immune regulation and operation. We aimed in this study to find the association between IL-2 plasma level, its -330 promoter polymorphism and multiple sclerosis. Subjects and Methods: This study was conducted on 90 patients with relapsing remitting multiple sclerosis and 60 normal volunteers as controls. Multiple sclerosis (MS) patients were diagnosed according to McDonald criteria 2010. Plasma IL-2 was estimated by ELISA and IL-2 -330 promoter polymorphism was genotyped by PCR-REFLP. Results: There was a significant increase in plasma IL-2 in M.S group (91.7±74.7 pg/ml) as compared to control group (51.5±18.9 pg/ml). Regarding the IL-2 -330 promoter polymorphism, the T/T genotype was statistically significant more frequent in MS patients than the control group (44.4% versus 23.3%), on the contrary the G/G genotype was statistically significant lower in MS patients than the control group (21.2% versus 40%), but there was no statistically significant difference between MS patients and control group in the G/T genotype (34.4% versus 36.7%). By comparing of IL-2 plasma concentration between different genotypes in MS patients group, there was an increase in concentration of IL-2 in carriers of the T/T genotype than other genotypes. Conclusion: The frequency of T allele was significantly higher in MS patients and the T/T genotype was more frequent in MS patients. In MS patients having the T/T genotype, IL-2 plasma concentration was higher than measured in other genotypes.
Keywords:
Multiple sclerosis, Interleukin-2, Promoter polymorphism
Cite this paper: Seham Mohamed Saied El Nakeeb, Reem Mohamed Ahmed Ali, Rasha Elsayed Mohamed Abd El-Aziz, Giehan Hussien Tawfik Ewida, Ahmed Abdel Kader Nemr, The Association of Interleukin-2 Gene Polymorphism with Its Plasma Concentration in Egyptian Multiple Sclerosis Patients, American Journal of Biochemistry, Vol. 7 No. 3, 2017, pp. 37-42. doi: 10.5923/j.ajb.20170703.01.
1. Introduction
Multiple sclerosis (MS) is a chronic, demyelinating disease of the central nervous system (CNS), affecting young adults with a female preponderance. MS is extremely heterogeneous in its initial presentation, rate and severity of relapses, underlying immunopathology, radiological appearance, and response to the disease modifying treatments [1]. Several lines of studies suggested that IL-2 is implicated in the pathogenesis of MS [2]. Knowledge of the frequencies of interleukin polymorphisms in a particular population may contribute to understanding of the pathogenesis of inflammatory diseases. To determine the biological significance of genetic polymorphisms; population genetic studies are a useful tool. Genetic polymorphisms can also be used as genetic markers in anthropological analysis [3].A single base change polymorphism at position −330 (T → G) in the 5′ region of the human IL-2 gene was characterized by John et al. [4] they evaluated the frequencies of the T and G alleles in 79 unrelated healthy Caucasians from Manchester, UK. They suggested that this polymorphism could be useful as a marker to diagnose susceptibility to inflammatory diseases.We aimed to know the effect of IL-2-330 promoter polymorphism on its plasma level in Egyptian multiple sclerosis patients.
2. Subjects and Methods
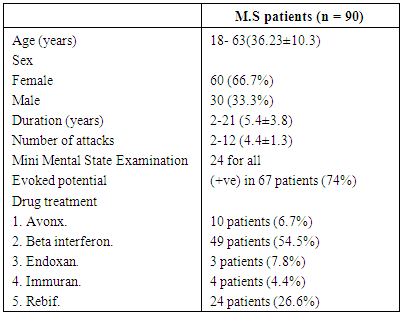
The present study was conducted on 150 subjects divided into two groups the first group included 90 patients with relapsing remitting multiple sclerosis (30 males & 60 females) with mean age (18-63 year, 36.13±10.3), attending the clinic of neurology departments in Al–Maadi military hospital and they are in between attacks. They were with a definite diagnosis of multiple sclerosis, according to the McDonald criteria (2010) [5], supported by clinical examination, MRI brain and/or cervical spine and evoked potentials. The second group included 60 healthy subjects as a control group (20 males & 40 females) with mean age (19-54 year, 35.75±10.1), they were matched to the cases by age, sex and ethnic origin. All subjects underwent a complete history taking, clinical examination, including general and neurological examination. Exclusion criteria involved: infections prior or complicating the attack, patients with clinical evidence of other autoimmune disease or arthritis, patients with clinical evidence of myocardial infarction (To avoid another potential source of cytokines up-regulation).The protocol of this study was approved by the medical ethics committee and a written informed consent was obtained from all patients and healthy controls.Table (1). Clinical characteristics of M.S patients
 |
| |
|
2.1. Immunochemical Assay
Three ml of peripheral blood were collected from all subjects on EDTA tubes and used for estimation of IL-2 plasma level by double antibody sandwich ELISA.
2.2. Genomic DNA Extraction
Genomic DNA was extracted from EDTA whole blood samples in two steps; lymphocyte separation step and the DNA extraction step using gene JET whole blood genomic DNA purification MiniKit#k0781, DNA was stored at -20°C till the time of use.
2.3. Amplification of IL-2 -330T/T Promoter Polymorphism
IL-2 -330 promoter polymorphism was genotyped by PCR-RFLP. The region surrounding this polymorphism was amplified with the following forward primer 5`d ATTCACATGTTCAGTGTAGTTCT 3` and reverse primer 5`d GTGATAGCTCTAATTCATGC3`. PCR was carried out in genomic DNA, 100 pmol of each PCR primer and 1x PCR mix (Dream Taq Green PCR Master Mix (Fermentas life sciences, #k0181) containing [200μM of each dNTP, 2x Dream Taq PCR buffer, 1.25 units Taq DNA polymerase and 4 mmol of (MgCl2)]. Thermal cycling conditions were 94°C for 4 minutes, followed by 40 cycles under the following conditions: 94°C for 1 min., at 60°C for 30s and at 72°C for 1min. A final extension step was carried at 72°C for 4 min. After PCR analysis, PCR product was visualized, also, 1 μl of Bfa. 1 restriction endonuclease was added to PCR product at 37°C for 15 min, then separated on 3% agarose gel stained with ethidium bromide and visualized by UV light. As shown in figure (2) IL-2 -330 allelic size after enzyme digestion were (131 bp) for the T/T genotype, (110 + 21bp) for the G/G genotype and (131, 110+21bp) for the T/G heterogeneous genotype.
2.4. Statistical Analysis
Data was analyzed by Microsoft Office 2003 (excel) and Statistical Package for Social Science (SPSS) version 16. The description of data as done as frequency and proportion for qualitative data and Mean ± SD for normally distributed quantitative data. The analysis of data done to test statistical significant difference between groups for qualitative data [frequency & proportion] chi-square test was used, for quantitative data normally distributed (mean ± SD), ANOVA (Analysis of Variance) and post Hoc correction was used to compare the three groups. Parametric data was expressed as mean ± SD, and non-parametric data was expressed as number and percentage of the total.P value > 0.05 is considered non-significant. P value < 0.05 is considered significant. For allelic and genotypic frequencies Hardy-weinberg equation was used.
3. Results
3.1. Plasma Concentration of IL-2
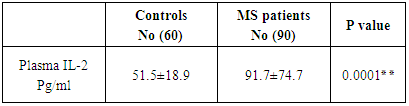
A highly significant increase in plasma IL-2 in patients group (91.7±74.7 pg/ml) as compared to control group (51.5±18.9 pg/ml) as shown in table (2).Table (2). Concentration of plasma IL-2 in MS patients and controls
 |
| |
|
3.2. Relation between IL-2 Plasma Levels and the Drugs Used
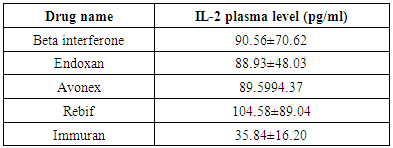
The Mean±SD of plasma level of IL-2 was of lowest values in patients who had immuran (35.84±16.2 pg/ml) and highest values in patients who had rebif (104.58±89.04 pg/ml) as shown in table (3).Table (3). Plasma IL-2 with different drugs used in the treatment of MS
 |
| |
|
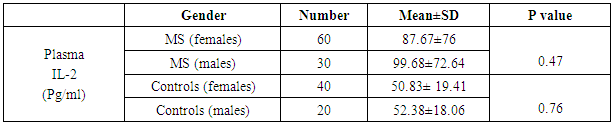
3.3. Gender Variation in Plasma IL-2
There was no significant difference between males and females in plasma IL-2 level and this finding was noted in both MS patients and controls as shown in table (4).Table (4). Gender variation in plasma IL-2
 |
| |
|
3.4. IL-2 -330 Gene Polymorphism in the Studied Groups
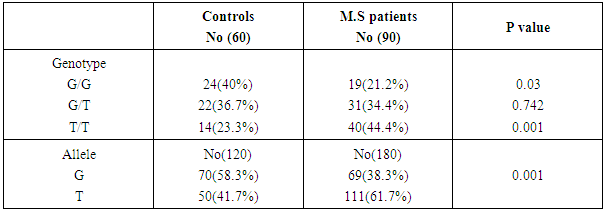
Regarding the distribution of different genotypes between patients and controls, the frequency of T allele was statistically significantly higher in MS patients than controls (p value <0.01). The T/T genotype was statistically significant more frequent in patients than controls (44.4% versus 23.3% and p= 0.001), the G/G genotype was statistically significant lower in MS patients than controls (21.2% versus 40% and p= 0.003). As regard the G/T genotype there was no statistically significant difference between patients and controls (34.4% versus 36.7% and p= 0.74) as shown in table (5).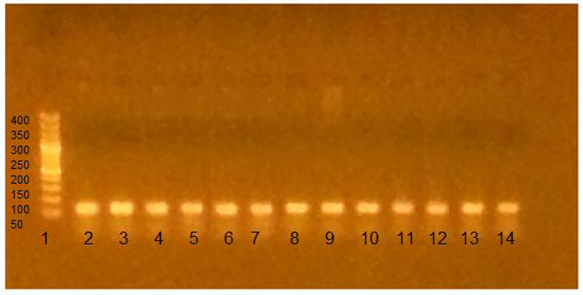 | Figure (1). Agarose gel electrophoresis showing PCR product-based RFLP analysis of IL-2 -330 gene amplification guided by marker in the first lane: 1- The first Lane is: 50 bp ladder (marker). 2- Lanes 2-14: one band 131 bp PCR product before the action of restriction enzyme Bfa-1, performed on 2% agarose, being visualized by UV trans-illumination |
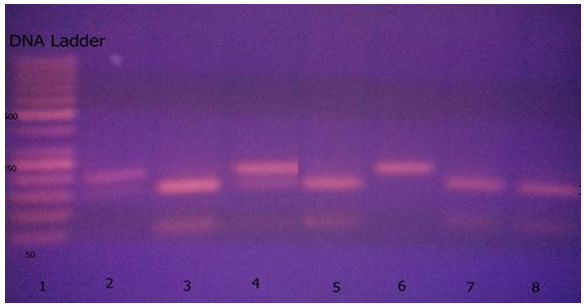 | Figure (2). Agarose gel electrophoresis showing PCR product-based RFLP analysis of IL-2-330gene polymorphism digested by Bfa-1guided by marker in the first lane: 1- The first Lane is: 50 bp ladder (marker). 2- T/T genotype: lane 6 (one band 131 bp). 3- G/T genotype: lanes 2, 4 (there were 3 bands 131, 110, 21 bp). 4- G/G genotype: lanes 3,5,7,8 (there were 2 bands 110, 21 bp) |
Table (5). Genotypes distribution among MS patients and controls
 |
| |
|
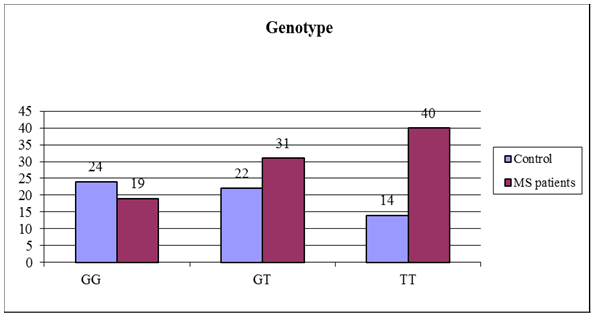 | Figure (3). Different genotypes of IL-2 -330 promoter polymorphism in both MS patients and controls |
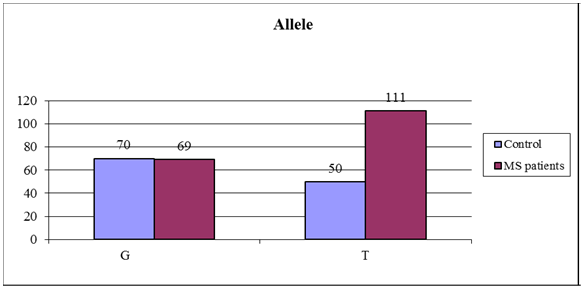 | Figure (4). Frequencies of IL-2 -330 alleles in both MS patients and controls |
3.5. Relation between IL-2 Plasma Level and Its Genotypes in the Studied Groups
IL-2 plasma level was higher in patients having the homozygous T/T allele (129.88±82.14 pg/ml) than measured in control group with T/T allele (76.84±16.67 pg/ml) P value < 0.01. Also, IL-2 plasma level was higher in patients having the heterozygous allele G/T (71.75±63.62pg/ml) versus (46.31±11.26 pg/ml) in control group P value < 0.05. There was no statistically significant difference in carriers of homozygous G/G between patients and control groups P value > 0.05 as shown in table (6).Table (6). Relation between IL-2 plasma level and its genotypes in the studied groups
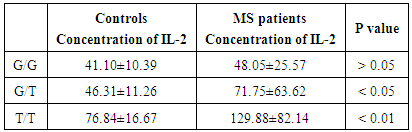 |
| |
|
3.6. Comparison of IL-2 Concentration between Different Genotypes in MS Patients
There was significant increase in carriers of T/T than other genotypes, but there was no significant difference between G/G versus G/T in patients group as shown in table (7).Table (7). IL-2 plasma level in MS patients with different genotypes
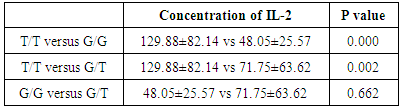 |
| |
|
4. Discussion
Multiple sclerosis (MS) is an autoimmune chronic inflammatory disease of the central nervous system that severely affect people worldwide leading focal degradation of the myelin sheath [6]. Although multiple sclerosis was discovered about a hundred and fifty years ago; neither the etiology nor the mechanism of the disease is fully understood [7]. Kikuchi et al. [8] said that the association between genetic polymorphisms and MS susceptibility may vary with ethnicity. Racial and ethnic differences may affect not only susceptibility, but also the phenotypic expression of MS, including clinical manifestations, site of lesions, disease course and prognosis.IL-2 is produced by activated T cells and promotes the proliferation of lymphocytes, macrophages and NK cells. It promotes an inflammatory response through the generation of Th1 and Th2 effector cells [9]. IL-2 physiologically plays an important role in T-lymphocyte activation and acts as autocrine actor to stimulate T-cell proliferation [10]. It has been demonstrated that the concentration of IL-2 increases in cerebrospinal fluid (CSF) as well as in sera of MS patients that indicates a role of IL-2 in MS disease [11].To our knowledge, this is the first study conducted in Egyptian MS patients to investigate the relationship of IL-2 -330 promoter polymorphism with MS susceptibility and the association between this polymorphism and IL-2 plasma concentration in those patients.Our study was conducted on 90 patients with relapsing remitting multiple sclerosis and 60 normal volunteers as a control group, patient and control groups did not differ regarding their age and gender distribution (P value > 0.05).Results of our study revealed that plasma IL-2 in MS patients was statistically higher (P<0.01) compared with its plasma level in healthy controls. This was in accordance with a study conducted by Sayad et al. [12], they found increased plasma IL-2 level in MS patients compared to controls and they suggested that measurement of plasma IL-2 concentration may provide an objective marker of disease in patients with MS.In a large study conducted by Martins et al. [13], they measured the levels of cytokines and other markers in 833 patients with confirmed MS and 117 healthy control subjects. Pro-inflammatory cytokines were elevated in patients of MS, included IFN-γ, IL-2, IL-1β, and TNF-α. They suggested that elevated concentrations of the pro-inflammatory cytokines are consistent with the damage of neuronal tissue found in patients with MS.In our study IL-2-330 promoter polymorphism of relapsing remitting MS patients and control group was studied. Regarding the distribution of different genotypes between patients and controls, T allele was significantly higher in MS patients than controls (P value < 0.01). Also T/T genotype was statistically significant more frequent in MS patients than controls. However, G/G genotype was statistically significant more frequent in controls than MS patients, but no statistically significant difference was found in G/T genotype between MS patients and controls.This was in accordance with a study conducted by Sayad and Movafagh [14], they found that the frequency of T allele was significantly higher in MS patients than controls. Moreover, the T/T genotype was more frequent in MS patients than in controls.The study conducted by Sayad et al. [15] included 100 relapsing remitting multiple sclerosis (RRMS), 30 secondary progressive MS (SPMS) and 125 healthy control. They found that the -330 T/T IL-2 genotype were significantly more frequent in MS patients compared to controls. Also, the G/G IL-2 genotype was significantly more frequent in control individuals than MS patients. These implies that IL-2 -330 gene polymorphism may influence on the course of the disease.Shahbazi et al. [9] revealed that the IL-2-330 T allele and the G/T and T/T genotypes were associated with a higher risk of developing MS in the studied population.In this study carriers of T/T genotype in the MS patients group have significantly higher plasma IL-2 concentration in comparison to other genotypes, it seems that may be there are mechanisms that affect or control the relation of T/T genotypes and IL-2 concentration in MS patients. Indeed, -330 T/T genotype of IL-2 gene may have an effect on increasing IL-2 plasma concentration in control group also.This was in accordance with a study done by Sayad and Movafagh [14] their study demonstrated that the −330 T/T genotype, which was significantly more frequent in MS patients and it was related to a higher level of IL-2 plasma concentration in comparison to controls. Matesanz et al. [2] evaluated the expression of the −330 IL-2 and T alleles in vivo and in vitro. Their work, which was performed on Jurkat cell line, demonstrated an unlikely promoter relation between the G and T alleles. The promoter with the G allele was twice more effective than the one with the T allele. On the contrary, quantification of allelic expression in lymphocytes showed that the −330 T allele was associated to a higher level of transcription than the −330 G allele. Moreover, they showed higher level of IL-2 mRNA expression in cases of individuals with −330 T/T and G/T genotypes than controls with the −330 G/G genotype. Matesanz et al. [2] proposed that the distinction between the in vitro and in vivo effect of the IL-2 −330 promoter polymorphic locations indicated the existence of additional unknown polymorphisms that affected gene adjustment.These results were not in accordance with those of Hoffman et al. [16], they described an increase in IL-2 production in controls genotyped as −330 G/G.
5. Conclusions
This study revealed that the IL-2 -330 T/T genotype was more frequent in MS Egyptian patients and associated with increased plasma concentration of IL-2.
References
| [1] | Dimisianos N, Rodi M, Kalavrizioti D, Georgiou V, Papathanasopoulos P and Mouzaki A (2014). Cytokines as Biomarkers of Treatment Response to IFN𝛽 in Relapsing-Remitting Multiple Sclerosis. Multiple Sclerosis International, Article ID 436764, 8 pages. |
| [2] | Matesanz F, Fedetz M, Leyva L, Delgado C, Fernández O and Alcina A (2004). Effects of the multiple sclerosis associated -330promoter polymorphism in IL-2 allelic expression. Journal of Neuroimmunology, 148(2): 212-217. |
| [3] | Scarel-Caminaga RM, Trevilatto PC, Souza AP, Brito Jr RB and Line SR (2002). Frequencies of the −330 (T → G) IL-2 and −590 (T → C) IL-4 gene polymorphisms in a population from south-eastern Brazil, Blackwell Science Ltd, European Journal of Immunogenetics, 29: 293–296. |
| [4] | John S, Turner D, Donn R, Sinnott P, Worthington J, Ollier W, Hutchinson and Hajeer A (1998). Two novel biallelic polymorphisms in the IL-2 gene. Eur. J. Immunogenet, 25(1): 419–420. |
| [5] | Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. (2011). Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of Neurology, 69(2): 292-302. |
| [6] | Riccio P and Rossano R (2015). Nutrition Facts in Multiple Sclerosis. ASN Neuro, January-February: 1–20. |
| [7] | Ma X, Zhou J, Zhong Y, Jiang L, Mu P, Li Y, Singh N, Nagarkatti M and Nagarkatti P (2014). Expression, Regulation and Function of MicroRNAs in Multiple Sclerosis. International Journal of Medical Science, 11(8): 810-818. |
| [8] | Kikuchi S, Niino M, Fukazawa T, Yabe I and Tashiro K (2002). An assessment of the association between IL-2 gene polymorphisms and Japanese patients with multiple sclerosis. J Neurol Sci, 205(1): 47-50. |
| [9] | Shahbazi M, Roshandel D, Ebadi H, Fathi D, Zamani M, Boghaee M, Mohammad-hoseeeni M, Rshaidbaghan A, Bakhshandeh A and Shahbazi S (2010). High frequency of the IL-2 −330 T/HLA-DRB1*1501 haplotype in patients with multiple sclerosis. Clinical Immunology, 137: 134–138. |
| [10] | Thell K, Hellinger R, Emine Sahina E, Michenthaler P, Gold-Binder M, Haider T, Kuttkea M, Liutkeviciute Z, Göranssond U, Gründemanne C, Schabbauer G and Gruber C (2016). Oral activity of a nature-derived cyclic peptide for the treatment of multiple sclerosis. PNAS, 113(15): 3960–3965. |
| [11] | Petitto M, Streit J, Huang Z, Butfiloski E, and Schiffen-bauer J (2000). IL-2 gene deletion produces a robust reduction in susceptibility to experimental autoimmune encephalomyelitis in C57BL/6 mice. Neuroscience Lettters, 285(1): 66-70. |
| [12] | Sayad A, Allameh A, Sayad Ar, Noruzinia M, Mohseni Y (2013). Correlation of interleukin-2 and uric acid plasma concentration with multiple sclerosis in Iranian patients. Journal of biology and today’s world, 2(4): 72-75. |
| [13] | Martins T , Rose J, Jaskowski T, Wilson A, Husebye D, Seraj H and Hill H (2011). Analysis of Pro-inflammatory and Anti-Inflammatory Cytokine Serum Concentrations in Patients with Multiple Sclerosis by Using a Multiplexed Immunoassay. Am. J. Clin. Pathol., 136: 696-704. |
| [14] | Sayad A and Movafagh A (2014). The Association of −330 Interleukin-2 Gene Polymorphism with Its Plasma Concentration in Iranian Multiple Sclerosis Patients. Scientifica, ID 724653, 4 pages. |
| [15] | Sayad A, Allameh A, Sayad Ar, Noruzinia M and Sarzaeem A (2013). The influence of -330 IL-2 gene polymorphism on relapsing remitting and secondary progressive multiple sclerosis in Iranian patients. Neurology of Asia Neurology Asia, 18(1): 83–86. |
| [16] | Hoffmann S, Stanley E M, Darrin Cox E, Craighead M, DiMercurio B, Koziol D, Harlan D, Kirk A and Blair P (2001). Association of cytokine polymorphic inheritance and in vitro cytokine production in anti-CD3/CD28-stimulated peripheral blood lymphocytes. Transplantation, 72: 1444–1450. |




 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML







