-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2017; 7(2): 13-22
doi:10.5923/j.ajb.20170702.01

Evaluation of Interleukin 17 Level as a Prognostic Marker in Active Antiviral Treated Human Immunodeficiency Virus in Saudi Patients
Haifa M. Al-Nafea 1, Nouha M. Hamdy 2, 3, Nagwa M. A. Aref 4, 5
1Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Saud University, Riyadh, Kingdom of Saudi Arabia
2Clinical Pathology Department, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
3King Saud Medical City, Riyadh, Kingdom of Saudi Arabia
4Department of Botany and Microbiology, College of Science, King Saud University (KSU), Kingdom of Saudi Arabia
5Department of Microbiology, Ain Shams University, Cairo, Egypt
Correspondence to: Nagwa M. A. Aref , Department of Botany and Microbiology, College of Science, King Saud University (KSU), Kingdom of Saudi Arabia.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

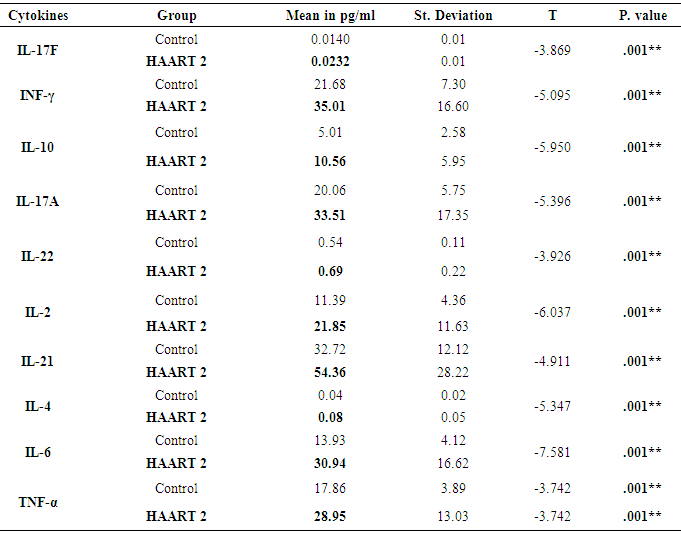
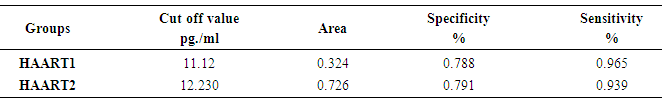
Interleukin-17 (IL-17) is a prototype member of new cytokine secreted by activated Helper (CD4+) and Suppressor (CD8+)T lymphocytes which involved in the proliferation, maturation, and chemotaxis of neutrophils. The expression level of IL-17 as a mediator of innate immunity needs to be considered compared to other proinflammatory cytokines in the Acquired Immunodeficiency Syndrom (AIDS) patients under Antiretroviral (ARV) therapy. IL-17 may play protective roles in host defense against HIV pathogenesis and promote induction of cytotoxic T-lymphocyte (CTL) responses. Moreover induced immune reconstitution in the gut mucosa. The experimental design was from 66 blood samples drawn from AIDS patients who were under medication with Highly Active Antiretroviral Therapy (HAART) for six months (HAART1) and followed up for another six months (HAART2) compared to 20 healthy individuals. The absolute counts (cells/µl) of lymphocytes subsets in whole blood were identified and determined by BD FACS Canto IITM flow cytometer. EMD Millipore’s MILLIPLEX MAP Human Th17 Magnetic Bead Kit utilized for the simultaneous quantification of Plasma levels of the following cytokines: IL-2, IL-4, IL-6, IL-10, IL-17A, IL-17F, IL-21, IL-22, interferon gamma (IFN-γ), and Tumor Necrosis Factor- alpha (TNF-α). The number of CD3+4+ T cells count was high significantly lower at P=0.001 for both groups of HIV/AIDS patients than control (960.41 cells/µl). The mean cell count for HAART1 was 567.22 cells/µl with the percent change of 41%. HARRT2 indicated 511.17 cells/µl with percent change of 46.8% with steady horizontal direction during treatment. A considerable increase improvement was noticeable during HAART2 treatment in the level of the following cytokines in pg/ml IL-17F, INF-γ, IL-10, IL-17A, IL-22, IL-2, IL-21 IL-4, IL-6, and TNF-α compared to control. It was evident for IL-17 A by Receiver operating characteristic (ROC) analysis which indicated 12.230pg/ml as a cutoff value of IL-17A during HAART2. The specificity and sensitivity of the test were 79.1% and 93.9% respectively, which necessitates using IL-17A as a progression marker during ARV therapy in HIV/AIDS patients. In HAART2, the percentage of IL-17 level elevated up to 67% compared to control. Also, IL-17A showed the cut-off of value was 12.230pg/ml with the specificity (79.1%) and sensitivity (93.9%). IL-17 plays protective roles in host defense against infectious diseases. It decreases the risk of infectious complications that allows effective therapies for treating inflammatory disorders. Accordingly, IL-17 recommended as a marker during one-year treatment with ARV therapy. It may rebuild the immune equilibrium in HIV/AIDS patients slowly.
Keywords: HIV/AIDS, HAART, CD4+Tcell, IL17
Cite this paper: Haifa M. Al-Nafea , Nouha M. Hamdy , Nagwa M. A. Aref , Evaluation of Interleukin 17 Level as a Prognostic Marker in Active Antiviral Treated Human Immunodeficiency Virus in Saudi Patients, American Journal of Biochemistry, Vol. 7 No. 2, 2017, pp. 13-22. doi: 10.5923/j.ajb.20170702.01.
Article Outline
1. Background
- One of the most pandemic diseases could be considered acquired immunodeficiency syndrome (AIDS) caused by the human immunodeficiency virus (HIV) [1]. AIDS caused a high degree of human suffering, as well as the immeasurable impact on demographics, cultures, economics, that were in the most societies around the globe [1]. The CD4+ T cells count the study predictor of subsequent disease progression and survival in several clinical trials [2]. Also, for determining and evaluating antiretroviral therapy, every 3–4 months for the need for initiation or discontinuation of prophylaxis for opportunistic infections (OIs) [3]. The increase in CD4+ T cell numbers, and prolong survival for AIDS patients could be fulfilled in highly active antiretroviral therapy (HAART) and can functionality control HIV replication [4]. The examining value of the CD4+ T cells counts in the management of a person with HIV infection considers aid to the clinician [5]. The pathogenesis of HIV-1 influenced the production of Cytokines, chemokines, and their receptors which were important factors that inhibit viral replication and affects HIV pathogenesis in vivo [6]. Interleukin-17 (IL-17) is a prototype member of new cytokine family with six species identified to date. IL-17 is characterized by secreted from activated CD4+ and CD8+ T lymphocytes. Moreover, its receptor distributed ubiquitously. IL-17 resembles most strikingly proinflammatory cytokine that is involved in the proliferation, maturation, and chemotaxis of neutrophils and can induce the expression of many mediators of inflammation. This connection was intriguing given that expression of IL-17 was finite to memory T cells when neutrophils were viewed primarily as mediators of innate immunity [7]. IL-17 induced tissue inflammation mainly by stimulating expression of several proinflammatory cytokines including IL-6, TNF-a, G-CSF, GM-CSF, and others. Previous studied proved that HAART therapy significantly increased IL-17 levels 6 and 12 months after treatment in AIDS patients. So the proinflammatory environment was upgraded. Accordingly, IL-17 stimulates and restore the balance of Th17 and Treg cell proliferation. HAART therapy could gradually restore the AIDS patients' immune imbalance [8].
2. Methods
- Subjects and study participantsOver a period of one year started from December 2014 sixty-six (13 females and 53 males) HIV-1/AIDS studied patients with an average age 20-50 years who chosen from HIV clinic according to RT-PCR test in King Saud Medical City in Riyadh as well as the “AIDS Diagnose and Treatment Guidelines.” Sixty-six patients were undertreated with HAART for six months (HAART1) and followed up for another six months (HAART2) in addition to twenty healthy control individuals (ten for each male and females). Real- time PCR results were less than 20 copies/ml in both groups. So there is no significant difference between HAART1 and HAART2 groups in viral load). All the subjects (controls and patients) given a brief introduction and the purpose of the study. Confidentiality of participating in the study for King Saud University was assured. The ethical approval was obtained and approved by Ministry of Health- King Fahad Medical City. The blood samples from each control person and AIDS patients kept in EDTA containers according to the methods of each test. Separated plasma was immediately stored at-80°C until further analysis.The CD4+ T cells subsets sorting as HIV disease Marker progressionThe absolute counts (cells/µl) of helper/inducer (CD3+CD4+ ) T lymphocytes, mature human T lymphocytes (CD3+), suppressor/cytotoxic (CD3+ CD8+ ) T lymphocytes, CD3+4+8+ (Double positives) cells CD16+ 56+ cells (NK lymphocyte) and CD19+ B cells (B- lymphocyte) in whole blood were identified and determined using the BD Multitest TM IMK kit (BD Biosciences, CA,USA) by following the manufacturer’s manual. The BD Multitest IMK kit and BD Trucount tubes designed for use on a flow cytometer equipped with special computer hardware and software. We use the BD FACS Canto IITM flow cytometer.Measurement of plasma IL-17 and other cytokines Plasma levels of cytokines were quantified using a multiplexed (Bio-Plex platform) immunometric assayed by EMD Millipore’s MILLIPLEX MAP Human Th17 Magnetic Bead Kit (Missouri, USA). It was for the simultaneous quantification of the following cytokines: IL-2, IL-4, IL-6, IL-10, IL-17A, IL-17F, IL-21, IL-22, IFN-γ, and TNF-α.Statistical analysesData presented as mean ±SD. T-test and correlations analyzed Spearman rank test analyzed differences between two groups. A p-value <0.05 was considered statistically significant. SPSS software (Version 17.0, USA) used for Statistical analyses.
3. Results
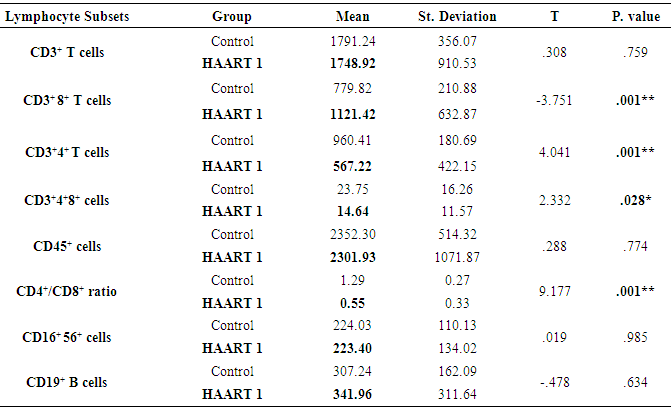
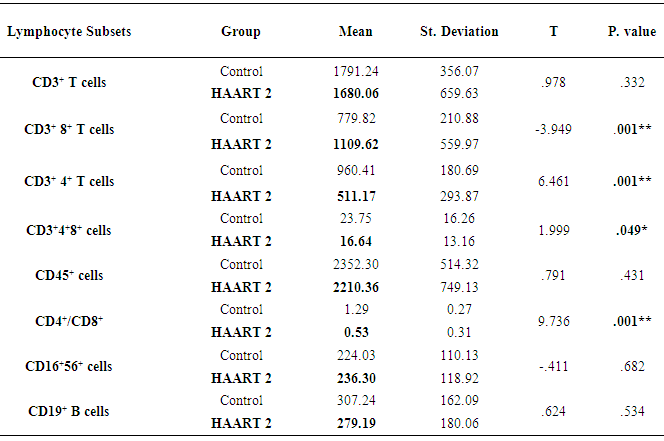
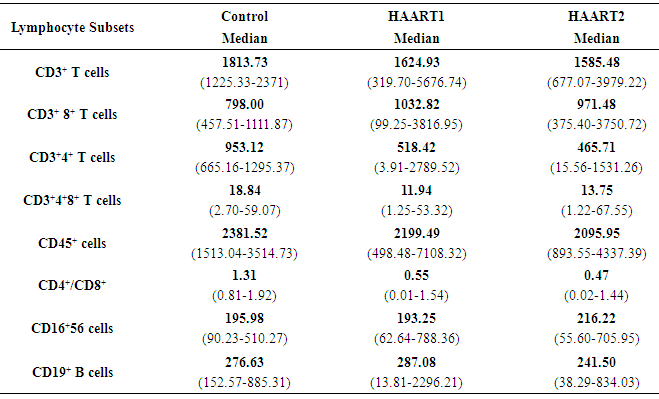
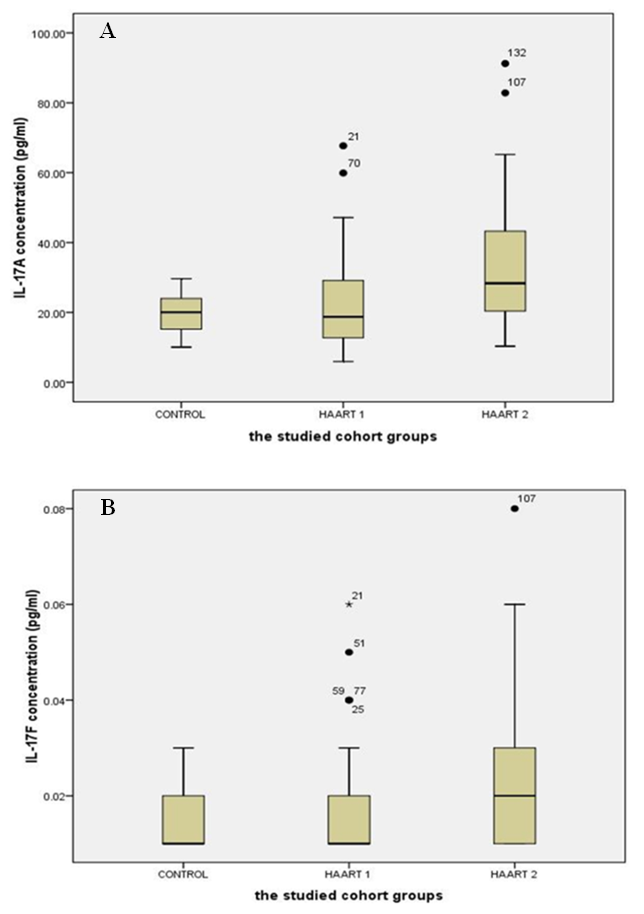
- The lymphocyte subsets count illustrated in Table 1. The counts were lower in HIV-1/AIDS patients (HAART1) compared to the uninfected controls except CD3+ CD8+ T cells count and CD19+ lymphocytes cell that elevated in patients. The number of CD3+4+ T cells and the CD4+/CD8+ ratio were significantly lower than controls after HARRT1 treatment (P≤0.01). Also, CD3+4+8+ T cell was lower than controls after HARRT1 treatment (P≤0.05). On the other hand, lymphocyte subsets were still lower in HIV/AIDS patients but without statistical differences. There are no statistical differences between control and HAART1 for CD3+ T cells, CD3+4+8+ cells, CD16+ 56+ cells and CD19+ B cells. Since the indication level value of these variables successively attained (0.634, 0.985, 0.774, 0.759), all of the values are greater than (0.05) and inconsiderable. Contrarily, the amount of CD3+ 8+ T cells indicated high negative significant value (P≤0.01) in AIDS patients compared to controls. Moreover, the number of CD19+ B cells was relatively higher in AIDS patients, but without statistical differences. For the HAART2 group in Table 2, the picture of lymphocyte subset was similar to HAART1 for CD3+8+ T cells, CD3+4+ T cells count, and CD4+/CD8+ ratio. The exception was the CD16+ 56+ cell count slightly increased in AIDS patients (mean = 236.30 cells/µl) compared to control (224.03 cells/µl), but this increasing was without statistical different. In contrast, the CD19+ cells count were lower in AIDS patients than control, but without statistical differences.The most cell count necessary as a progressive marker in AIDS is T-helper cell count. In cohort studies, the median of CD4 cell count were 953.12 cells/µl, 518.42 cells/µl, and 465.71 cells/µl respectively. Also, the maximum value in HAART2 (1531.26 cells/lµ) was less than in HAART1 (2789.52 cells/lµ) (Table 3); the T-helper cells count was high significantly lower in HIV/AIDS patients than control (P=0.001 for both groups). However, in Fig.1 this calculation was steady during treatment (HAART1 and HAART2) which statistically not significant differences between HIV/AIDS patients. The mean cell count for control was 960.41 cells/µl wherein HAART1 was 567.22 cells/µl so the percent change equal 41%. For HARRT2, the mean was 511.17 cells/µl so percent change equal 46.8%.
|
|
|
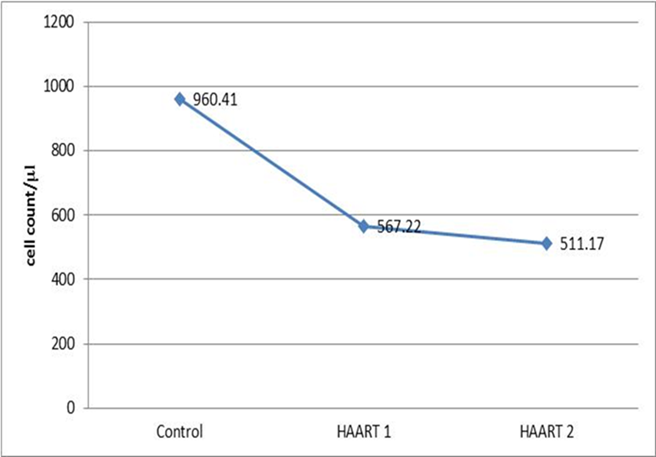 | Figure 1. The graph represents the steady means of the T-helper cell (cells/µl) in HAART1 and HAART2 groups |
|
|
|
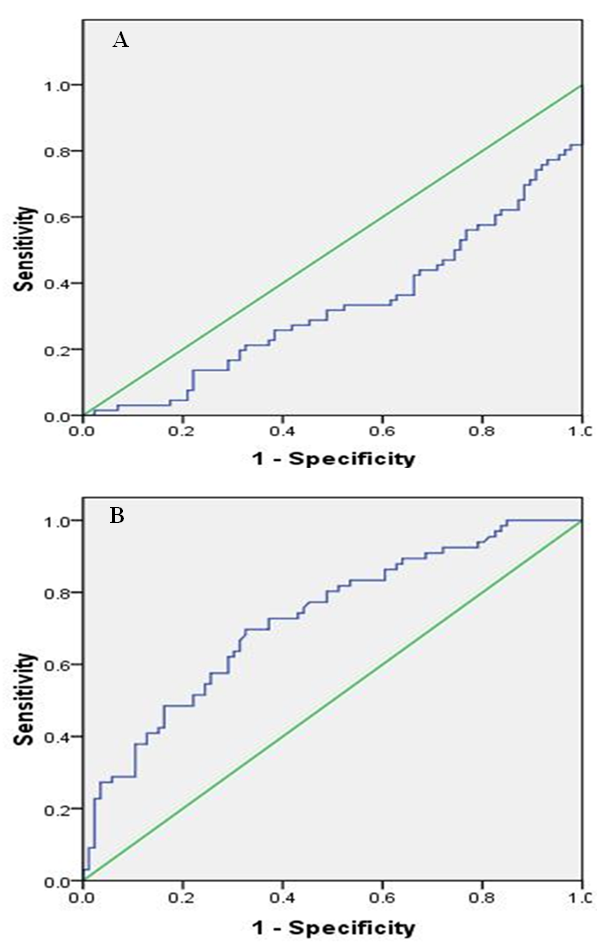 | Figure 3. AUROC curve for IL-17 in HAART1 and HAART2 groups. a HAART1. b HAART2 |
4. Discussion
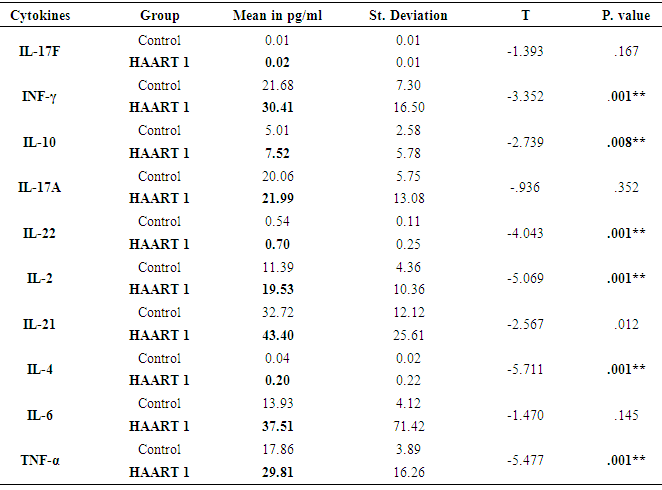
- Response to HAART highly complex is and often limited by the development of short- or long-term toxicities and the emergence of antiretroviral drug resistance [9]. Biomarkers of inflammation and immune activation moved toward HIV-negative levels within the first year after HAART-induced HIV suppression science several markers of T cell activation returned to levels present in HIV-negative. Residual immune activation, particularly monocyte/macrophage activation may represent a therapeutic target to improve the prognosis of HIV-infected individuals receiving HAART [10]. Antiretroviral therapy is a major factor, especially in low-income countries. [11] The results showed that 56% of patients had a successful virological and CD4+ T cells response [12, 13].In Saudi Arabia, we followed the same guideline of global HIV treatment that recommended the use of ART in the patients whose CD4+ T cells count be ≤ 350 [14]. Using correct ART results in rapid control of HIV and partial restoration of immune function, leading to prevention of the various complications that define AIDS [15] In the present study showed the same data concerning the count of CD4+T cells. The mean was 567.22 cells/µl with high significantly lower than controls which were 960.41 cells/µl after HARRT1 treatment (P≤ 0.01). CD4+ T cell for HAART2 was 511.17cells/µl.The levels of IFN-γ increased in at all the CD4+T cell categories of > 500 cell/µl, 200-500 cell/µl and < 200 cell/µl. In HIV-1 patients. IFN-γ contributes to the development; Maturation differentiated and function of NK and more other immune cells [16]. Similar increase level of IFN-γ occurred in Table 4, and five at the CD4+T cell categories of > 500 cell/µl in our study. This results reflected the non-significant differences between HAART1 and HAART2 for NK responses during medication at that category of CD4+T cell.Plasma levels of cytokines or chemokines related to immune activation might also be biomarkers of a higher risk of mortality and non-AIDS event [17]. Also, plasma levels of IL-6 were most significant biomarker cytokines dis-regulation associated with all-cause mortality in an individual with HIV-1 infection. Also, [18] confirmed our results concerning IL-10, which significantly elevated during acute HIV-1 infection, and was correlated directly with viral loads and associated with greater risk of CD4+ T cell loss.Concerning our results for IL-2 in both control and HAART1 and HAART2, significant elevation was noticed (19.35pg/ml and 21.85pg/ml) for the previous two treatment compare to (11.39 pg/ml) for the control one. We considered IL-2 as a cytokine that strengthens the immune response to CD4+ T cell proliferation during ARV therapy. IL-2 is a growth factor protein stimulate the proliferation of CD4+ T cells and B cells. Through the autocrine and paracrine actions of IL-2, CD4+ T cells have recognized antigen undergo extensive proliferation so that the number of antigen-specific CD4+ T cells is increased dramatically [19]. Regarding the advanced phase of infection by HIV-1, [20]. In opportunistic infections of some other studies, the cytokines can be markers of progression toward AIDS upon indicating a decrease of IL-2 and IFN-γ and concomitant increases in IL-4 and IL-10, which correlated with a decline in an antigen-specific immune response, [20]. In our, study IL-2 indicated constant levels in both HAART1 and HAART2 while IL-10 showed maximum significant for median values in HAART2. It revealed an improvement of the immune response to anti-inflammatory cytokines.IL-17 producing cells incorporated in the pathogenesis of various diseases such as allergies, autoimmune diseases, allograft transplantation and even malignancy. They may also have protective roles in host defense against infectious diseases and promote induction of cytotoxic T-lymphocyte (CTL) responses against cancer. Targeting of the IL-17 axisis under investigation for the treatment of inflammatory disorders [21].GM-CSF (granulocyte–macrophage colony-stimulating factor) are neutrophil-associated proteases that correlated with many cytokines, especially, IL-17, IL-8, IL-1b (interleukin-1b) and MIP-3a (macrophage inflammatory protein-3a). Analyses by Gene set enrichment implicated activated immune cells. It revealed strong linkages between mucosal cytokines, barrier function, proteases, and immune cell movement, and propose these as potential mechanisms that increase the risk of HIV acquisition [22].Th17 cells may play a role in HIV pathogenesis and antiretroviral therapy-induced immune reconstitution in the gut mucosa [23]. In the present study, it was apparent that percentage of IL-17 level elevated up to 67% in HAART2 compared to control. The long-term ARV treatment (HAART2) induced normalization of the capacity of T-cells to produced IL-17 as a marker for inducing balance in inflammatory disorder that was not clear (non-significant) in HAART1. The most maximum levels of plasma cytokines in HAART2 were IL-21(137.24 pg/ml), IL-33(131.27 pg/ml), IFN-γ (93.90 pg/ml) and IL-17A (91.23 pg/ml). The results of AUROC Support the use of IL-17A levels in clinical practice as a predictor of ARV treatment HIV/AIDS patients with cutoff (12.230 pg/ml) having specificity 79.1%and sensitivity 93.9%.The impact of the Th17 pathway is more profound than previously thought. The findings in preclinical animal models have supported therapeutic approaches targeting the IL-17A pathway. It is always kept in mind the limitations of targeting IL-17 in clinical therapy. The link between IL-17 targeting and neutropenia is important, as this may affect the host defenses against some pathogens [21].
5. Conclusions
- In our study, viral load in HAART1 and HAART2 decreased below the lower limit of detection with median values of ≤ 20 copies/ml. CD4 count indicated 41% change increase in HAART1 while it was 46.8% in HAART2 without any significant changes through one year. In HAART2, the percentage of IL-17 level elevated up to 67% compared to control. Also, IL-17A showed the cut-off of value was 12.230pg/ml with the specificity (79.1%) and sensitivity (93.9%). IL-17 plays protective roles in host defense against infectious diseases. It decreases the risk of infectious complications that allows effective therapies for treating inflammatory disorders. IL-17 recommended as a marker during treatment with ARV therapy to rebuild the immune equilibrium slowly in HIV/AIDS patients. The long-term ARV treatment in HAART2 induced normalization of T-cells capacity to produce IL-17 as a marker for inducing balance in inflammatory disorder. The present study recommends the potential use plasma cytokines concentrations during acute HIV-1 infection to predict subsequent disease progression. ARV therapies induce quantitative and qualitative alterations in the cytokine profile of persons that could be warrants attention which causes reductions in AIDS. The study helps clinicians who still require knowledge of ARV management (KSA). Immune-biomedical research like our study will be evolved accordingly in the future to decrease morbidity and mortality in HIV/AIDS patients.
Abbreviations
- HIV, human immunodeficiency virus; AIDS, acquired immunodeficiency syndrome; OIs, Opportunistic infections; HAART, highly active antiretroviral therapy; IL-17, Interleukin-17; CD3+, mature human T lymphocytes; CD3+CD4+, helper/inducer T lymphocytes; CD3+ CD8+, suppressor/cytotoxic T lymphocytes, CD3+4+8+, Double positives cells; CD16+ 56+ cells, NK lymphocyte; NK, natural killer and CD19+ B cells, B- lymphocyte. GM-CSF, granulocyte–macrophage colony-stimulating factor; IL-1b, interleukin-1b; MIP-3a, macrophage inflammatory protein-3a.; ARV, Antiretroviral; CTL, cytotoxic T-lymphocyte; ROC, Receiver operating characteristic; IFN-γ, interferon gamma; TNF-α, Tumor Necrosis Factor-alpha.
ACKNOWLEDGEMENTS
- Research Center of Female Scientific and Medical Colleges, Deanship of Scientific Research, KSU due to this research project supported by a grant by them. Acknowledge to the Research Committee, Epidemiology Unit, HIV Clinic, Immunology Department and Region Lab at King Saud Medical City in Riyadh.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML