-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2017; 7(1): 1-5
doi:10.5923/j.ajb.20170701.01

Antidepressant Activity of Ethanol Leaf Extract of Annona muricata L., in Sprague-Dawley Rats
Bikomo E. O., Ebuehi O. A. T., Magbagbeola O. A.
Department of Biochemistry, College of Medicine, University of Lagos, Lagos, Nigeria
Correspondence to: Ebuehi O. A. T., Department of Biochemistry, College of Medicine, University of Lagos, Lagos, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Annona muricata L. is a plant renowned for several therapeutic folklore uses, in many Caribbean communities where as revealed in literature the leaves, bark and roots are made into tea for calming effects as sedative, heart tonic and hypertensive medication. In Nigeria, these potential health benefits are untapped since A. muricata is classified as an under-utilized plant. The present study determined the antidepressant and behavioral properties of the Nigerian grown Annona muricata in Sprague-Dawley rats using the open field test and forced swim test. Methods: Rats were administered A. muricata leaf extract (50, 150 and 300mg/kg) alone, as well as in combination with imipramine or sertraline (10mg/kg) for 14days. Results: The extract was found to reduce the explorative tendencies of the rats in the open field test. In the forced swim test the extract caused a significant reduction in immobility time and increased swimming time. Combination of the extracts with imipramine or sertraline further decreased the explorative tendencies at 150mg/kg concentration and the immobility time at 150 and 300mg/kg. Conclusion: The results obtained proposed a sedative and antidepressant-like effect of ethanol extract of A. muricata, confirming the ethnomedicinal use of the ethanol leaf extract of A. muricata for the management of depression.
Keywords: Annona muricata, Antidepressant, Imipramine, Sertraline, Open field test, Forced swim test
Cite this paper: Bikomo E. O., Ebuehi O. A. T., Magbagbeola O. A., Antidepressant Activity of Ethanol Leaf Extract of Annona muricata L., in Sprague-Dawley Rats, American Journal of Biochemistry, Vol. 7 No. 1, 2017, pp. 1-5. doi: 10.5923/j.ajb.20170701.01.
Article Outline
1. Introduction
- Depression is an affective disorder which results in a state of low mood and aversion to activities that can have a negative effect on a person’s thought behavior, worldview and physical well-being [1]. Many psychiatric syndromes feature depressed mood as a main symptom, hence it has become the major global psychiatric problem [2]. About 350million people suffer from depression globally [3]. People suffering from depression struggle with loss of energy and motivation, which influence their ability to be productive, making depression the leading cause of disability worldwide [4]. According to WHO [5], depression is currently 4th among the 10 leading causes of the global burden of disease and it is predicted that by year 2020, it will be ranked 2nd. Unmanaged depression is a major risk for suicide [6]. Treatment is varied but psychotherapy and pharmaceutical drug treatment are most commonly used. Pharmaceutical drug treatment involves the use of antidepressant medications, which are based on neurotransmitters (naturally occurring endogenous neurochemicals) levels imbalance in the brain. Drugs can influence behavior/mood by altering neurotransmitters concentration in the brain hence the selection of antidepressant agent is based on the neurotransmitters that are thought to influence the symptoms of depression [7]. The tricyclic antidepressants (TCA), selective serotonin reuptake inhibitors (SSRI), nor-epinephrine reuptake inhibitors (NRI), dopamine reuptake inhibitors (DRI) and serotonin-nor-epinephrine reuptake inhibitors (SNRI) are the major classes of antidepressants used in pharmaceutical drug treatment. These drugs though effective, exhibit undesirable side effects hence new agents which will be divested of the distressing side effects are being solicited [8, 3]. Medicinal plants are used in complementary and alternative medicine for the management of mood disorders [9, 10]. There is a general shift towards natural products from medicinal plants sources which are perceived to have fewer (if any) of the side effects associated with synthetic orthodox drugs [11]. A review of literature revealed that A. muricata is widely employed in herbal medicine [12, 13]. It has been attributed with mood enhancing properties in folklore medicine while the leaf, bark and root are made into tea for calming effects as sedative, heart tonic and hypertensive medication in traditional medicine [12]. A. muricata is classified as an underutilized plant in Nigeria where it grows wildly and as such, the associated health benefits are untapped. The objective of the present study was to determine the antidepressant activity of the Nigerian grown A. muricata in Sprague-Dawley rats, using the forced swim test (FST) and open-field test (OFT).
2. Materials and Methods
2.1. Chemical and Reagents
- Sertraline hydrochloride used was a product of Ranbaxy Ltd, India, and imipramine hydrochloride was a product of Assos llac, Istanbul. (Sertraline and imipramine are antidepressant drugs). All other reagents used in the study were of analytical grade.
2.2. Plant Material
- Fresh leaves of A. muricata were collected from Igan-Otoko, Ogun State, South- West, Nigeria (Latitude 7.1455701 North, Longitude 3,361968700000034 East). This plant was identified and authenticated at the Department of Botany, University of Lagos, Lagos, by Dr. A. Kadiri and a voucher specimen was deposited in the Herbarium (LUH 5252) of the University.
2.3. Plant Extract Preparation
- A. muricata leaves collected were sorted out (by removing the dead and dried ones) and washed with clean water to remove dust. The cleaned leaves were air dried for three days and then transferred to the oven to dry at 40°C until crispy. The oven dried leaves were coarsely powered and packaged in air tight containers until used. The extract was prepared by maceration of six hundred grams (600g) of the coarsely powered leaves in 95% ethanol (5litres) at room temperature for 72h. The ethanol extract was filtered and evaporated under a rotary evaporator at controlled temperature (40-50°C), oven dried (40-55°C) and extract obtained was stored at 0°C until used.
2.4. Animal Handling and Treatment
- Seventy–eight female Sprague-Dawley rats weighing 175.64 ± 8.91g were obtained from the Laboratory Animal Centre, College of Medicine, University of Lagos, Nigeria. The rats were maintained under standard conditions (12h light/dark cycle and at 27 ± 2°C). They were fed with commercial rat chow (Pfizer feeds, Lagos, Nigeria) and were given water ad-libitum. They were allowed to acclimate for 14 days before used. The experimental protocols for this study conformed to the guide for care and use of laboratory animals by US National Institute of Health (NIH publication No 85-23 revised 1996). Administration of the extracts and drugs were between 9.00 and 10.30am daily. The rats were randomly assigned into thirteen (13) groups of six rats each. Control groups (1&2) were given distilled water and ethylene glycol, (The drugs sertraline and imipramine were dissolved in distilled water while the extract (being insoluble in water) was dissolved in 5% ethylene glycol hence there is a water and vehicle control groups). The rats in groups 3-5 were orally administered 50, 150 and 300mg/kg body weight of A. muricata leaf extract respectively. The rats in groups 6 and 7 were orally administered 20mg/kg body weight of sertraline or imipramine respectively, while animals in group 8-13 were administered A. muricata extract (50, 150 and 300mg/kg body weight) in combination with 10mg/kg body weight of sertraline or imipramine respectively. The drugs and extracts were given daily between 9.30 and 10.30h for 14 days. All the rats were weighed weekly and allowed feed and water ad-libitum throughout the period of the study. The neurobehavioral activity was evaluated using the open field test (OFT) and forced swim test (FST), one hour after the last treatment.
2.5. Open-Field Test (OFT)
- The ambulatory behavior was assessed in the OFT [14]. The rats were moved from their housing colony to the laboratory in their own cages and allowed to adapt to the laboratory conditions for about 1h. The open–field apparatus consisted of a wooden arena (64cm x 64cm and 40cm high). The floor of the wooden arena was divided into 16 equal squares marked by black lines. The rats were gently placed individually in the centre (marked) of the arena and allowed to explore freely. The number of squares crossed by the rat (locomotion frequency) and the number of rearings (i.e. the number of times the animal stood on the hind paws) were recorded during a test period of 5min. The floor of the arena was cleaned between each trial with 10% ethanol solution to eliminate possible bias caused by odors’ left by previous rats. The test was carried out at room temperature (27 ± 2°C) in a noise and light controlled room.
2.6. Forced Swim Test (FST)
- In the forced swim test [15, 16], the rats were forced to swim in a cylindrical transparent glass vessel (50cm high and 21cm diameter), that contained fresh water to a depth of 26cm at room temperature (27±2°C). The total duration of immobility was measured during a 5min test session carried out after 24h of a 10min pre-test session on the same rats. Rats were considered immobile when they ceased struggling and remained floating in water except small movements necessary to keep their heads above the water. The water was changed after each animal to avoid the influence of water temperature and substances left from the previous session.
2.7. Statistical Analysis
- The results were expressed as mean ± SEM. Data obtained from all the groups were analyzed using one-way analysis of variance (ANOVA), followed by Dunnet’s test to detect intergroup differences. The level of significance was determined at p< 0.05.
3. Results
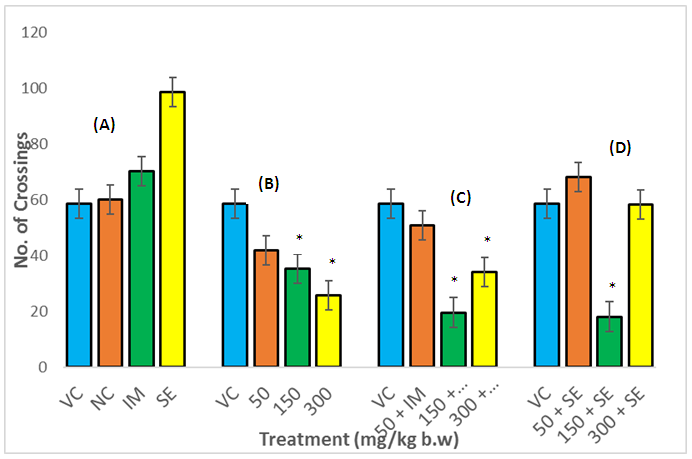
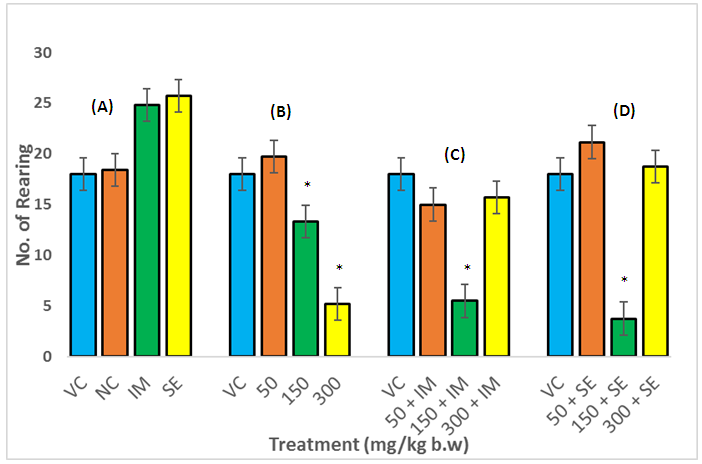
- In the OFT, the ethanol extracts (50,150 and 300mg/kg po) administered for 14 consecutive days to rats significantly decreased their locomotion tendencies (Fig 1 (B)) compared with the vehicle control group. Imipramine and sertraline administered rats exhibited a positive significant difference compared to the water and vehicle control group (Fig. 1 (A)). The locomotive tendencies of the rats were increased when the extract was combined with either imipramine or sertraline as shown in Figs. 1 (C) & (D), but at 150mg/kg body weight of extract imipramine (or sertraline) combination with the extract showed further decreased in locomotion tendencies.
4. Discussion
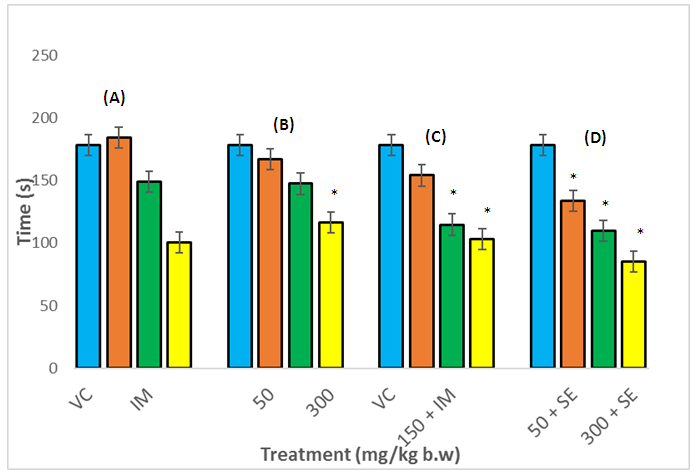
- A reduction in the explorative tendencies of the rats was observed in the OFT. This diminution in the locomotion after the administration of A. muricata leaf extract may be due to its sedative effect, confirming its ethno medicinal use as a sedative.The open-field test provides a way to assess environment exploration, general locomotion activity and provide an initial screening for anxiety-related behavior in rodents [17], while the forced swim test in rats is a pre-clinical test employed to evaluate drugs being screened for antidepressant activity [18]. Animals removed from their acclimatize cage and placed in a novel environment express their anxiety, fear and stress by showing decreased in ambulation and exploration as well as in normal rearing and grooming behaviors. Changes in these measurements are used to assess the sedative or stimulant effect of pharmacological agents [19]. These effects are subject to reversal by anxiolytics and certain antidepressants [20]. In this study, the ethanol extract of A. muricata caused a significant reduction in immobility time and increased swimming time, which demonstrated antidepressant-like effect. Drugs that selectively inhibit serotonin reuptake decrease immobility and increase swimming time [21]. Stimulant drugs can decrease immobility in the FST, resulting in false positive result which is distinguished from the anti-depressant-like reduction in immobility by assessment of the locomotors activity in the OFT. The extract significantly decreased locomotive activity alone and in combination with imipramine. The increase in active behavior (swimming) and decrease in immobility observed in the FST was not due to an increase in locomotors activity (as shown in the OFT). Therefore, the antidepressant-like effect of the extract is not related to a psycho stimulant effect.Immobility in the FST represent a state of hopelessness in the animal which correlate to negative mood when place in an inescapable place. The immobility time is decreased by various types of antidepressants.
5. Conclusions
- The results obtained in the present study suggested that ethanol leaf extract of A. muricata produced sedative and antidepressant-like effects in rats, when subjected to open field test and forced swim test. Therefore, the ethanol leaf extract of A. muricata may have potential therapeutic value for the management of depression.
ACKNOWLEDGEMENTS
- The authors are grateful to Mr. Chijioke A., of the Department of Pharmacology, College of Medicine, University of Lagos, Lagos, for providing technical assistance in the animal experimentation for this study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML