-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2016; 6(6): 149-152
doi:10.5923/j.ajb.20160606.03

Effect of Phase Composition on Partition Behaviour of Bioactive Phenolics Transfer in Aqueous Two-Phase Extraction System
Siti Hajar Adnan1, Zulkarnain Mohamed Idris2
1School of Bioprocess Engineering, Kompleks Pusat Pengajian Jejawi, Universiti Malaysia Perlis, Arau, Malaysia
2Department of Chemical Engineering Technology, Faculty of Engineering Technology, Universiti Malaysia Perlis, Arau, Malaysia
Correspondence to: Siti Hajar Adnan, School of Bioprocess Engineering, Kompleks Pusat Pengajian Jejawi, Universiti Malaysia Perlis, Arau, Malaysia.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The present study was to develop a cost-effective and eco-friendly separation and purification method of bioactive phenolics from Phanerochaete chrysosporium biomass using aqueous two-phase extraction system. The extraction system comprised of 80% (w/w) ethanol, 20% (w/w) di-potassium hydrogen phosphate, distilled water and solid biomass. The extraction was conducted at different phase composition range, which are 70 wt%, 50 wt% and 45wt% which paired with 27 wt%, 44 wt% and 50wt% concentrations from stock solutions of 80% (v/v) of ethanol and 40% (w/v) of K2HPO4, respectively. The highest partition coefficient (K) and recovery (R) of bioactive phenolics were found at 70 wt% of 80% (v/v) C2H5OH and 27wt% of 40% (w/v) K2HPO490 with values of 10.45 and 99.27%, respectively.
Keywords: Aqueous Two-Phase Extraction, Phenolics,Phanerochaete chrysosporium, Partition Behaviour
Cite this paper: Siti Hajar Adnan, Zulkarnain Mohamed Idris, Effect of Phase Composition on Partition Behaviour of Bioactive Phenolics Transfer in Aqueous Two-Phase Extraction System, American Journal of Biochemistry, Vol. 6 No. 6, 2016, pp. 149-152. doi: 10.5923/j.ajb.20160606.03.
Article Outline
1. Introduction
- Natural phenolic compounds are low molecular weight naturally occurring organic compounds which contains one or more phenolic group. They are naturally produced by plants and basidiomycete. Natural phenols also play many significant roles in human health as evident from their antifungal, antioxidant and anti-cancerous activities [1]. Phanerochaete chrysosporium is a filamentous basidiomycete white rot fungus that participates in the degradation process of complex woody materials. This fungus has great potential in many biotechnological applications including bioprotein production, the treatment of hazardous waste and the bioremediation of contaminated soils and biofuel production [2]. Owing to the increasing interest in new natural sources of antimicrobial compounds, this is the first study on Phanerochaete chrysosporium mycelia biomass as potential source of bioactive compounds.Extraction is the most important step in the recovery and purification of bioactive compounds from plants and basidiomycetes. Phenolic compounds are usually extracted from natural sources through solid-liquid extraction using organic solvents in heat-reflux systems [3]. However, this method has suffered low efficiency, time and solvent consuming, could lead to the degradation of bioactive phenolics and decrease the bioactivity of the extracts. Aqueous two-phase extraction is recognized as an effective, versatile, easy constituent recovery and reutilization, reduced settling times, and low viscosity. [4] It will achieve high product purity as well as high yield, while maintaining the biological activity of the molecule, which has been widely applied in the separation of proteins, enzymes, antibiotics and polyphenols [5].In the present study, the effect of phase composition on the partitioning of the bioactive phenolics from Phanerochaete chrysosporium mycelia biomass of the extracts were investigated in order to develop an efficient aqueous two-phase system for the extraction and purification of these compounds.
2. Materials and Methods
2.1. Chemicals and Reagents
- Folin-Ciocalteu reagent standards, and other solvents and reagent of analytical grade were purchased from Merck Chemicals (Darmstadt, Germany).
2.2. Fungal Strain and Preparation of Inoculum
- The white rot fungus Phanerochaete chrysosporium was obtained from the culture collection of School of Bioprocess Engineering, University Malaysia Perlis. Four square plugs (5 mm in length) of active mycelia were cut from the initial culture plate and transferred on new potato dextrose agar (PDA) plate. The plate was incubated at 30°C for 5 days. The inoculum was prepared by washing four PDA plates cultured with 100 ml of sterile water and their spore suspension were rubbed and poured into 250 ml Erlenmeyer flask.
2.3. Submerged Fermentation
- The fermentation was done at fixed media compositions. The media was supplemented with malt extract broth to enhance the strain’s growth. Fifty millilitres of sample were taken into 100-ml Erlenmeyer flasks and autoclaved at 121°C for 30 min. The sterile media was cooled to ambient temperature and inoculated with spore suspension of Phanerochaete chrysosporium. The fermentation was carried out at incubation time of 3 days, 4% (v/v) of inoculum size, inoculum age of 7 days, pH 6, agitation speed of 100 rpm, and fermentation temperature of 25°C. For reproducibility of results, all fermentations were carried out in triplicate.
2.4. Preparation of Mycelia Biomass Powder
- After fermentation, the mycelia biomass of Phanerochaete chrysosporium was collected by filtration using filter paper and rinsed with distilled water. The biomass was dried in oven at 60°C for 24 h and ground into powder by using mortar and pestle. The phase system was prepared in a 15ml graduated centrifuge tubes by weighing the appropriate amount use which is 80 wt. % of ethanol, 40 wt. % of potassium salts and 0.028g of sample powder. Distilled water was added to the system to obtain final mass of 14g. The phase system was mixed evenly by gentle agitation, after that each phase system was centrifuged at 3000 g for 10minutes to induce phase separation. The phase diagram used was developed using cloud point method. The extraction was conducted at different phase composition range, which are 70 wt%, 50 wt% and 45wt% which paired with 27 wt%, 44 wt% and 50 wt% concentrations from stock solutions of 80% (v/v) of ethanol and 40% (w/v) of K2HPO4, respectively for 2 hours at pH 7 and temperature 25°C.The partition coefficient (K) and the percentage of recovery, R (%) of bioactive phenolic were calculated as follows:
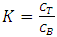 | (1) |
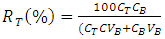 | (2) |
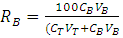 | (3) |
2.5. Determination of Total Phenolic Content
- The total phenolic content was determined based on the suggested method with modification [2], using the Folin-Ciocalteu reagent with gallic acid as a standard. In 15 ml test tube, 2.37 ml of distilled water, 0.03 ml of sample extract or blank and 0.15 ml of Folin-Ciocalteu reagent were added and vortexed. After 1 min, 0.45 ml of 20% saturated sodium carbonate (Na2CO3) was added, and then the mixture was vortexed and allowed to stand at 40°C for 30 min. The absorbance was taken at 750 nm. The total phenolic content was expressed as mg of gallic acid equivalent per liter (GAE mg/l). All measurements were measured in triplicate.
3. Results and Discussions
3.1. Partition Behaviour of Bioactive Phenolics in Aqueous Two-Phase Extraction System
- The extraction of bioactive phenolics was conducted different phase composition range, which 70 wt%, 45 wt% and 25wt% which paired with 27 wt%, 44 wt% and 50 wt% concentrations from stock solutions of 80% (v/v) of ethanol and 40% (w/v) K2HPO4, respectively for 2 hours at pH 7 to investigate their effect on the separation of bioactive phenolics from Phanerochaete chrysosporium biomass using aqueous two-phase extraction system. The optimum extraction system comprised of 80% (w/w) ethanol, 40% (w/w) di-potassium hydrogen phosphate and distilled water was selected from the phase diagram of ethanol with K2HPO4 at 25°C shown in Figure 1.
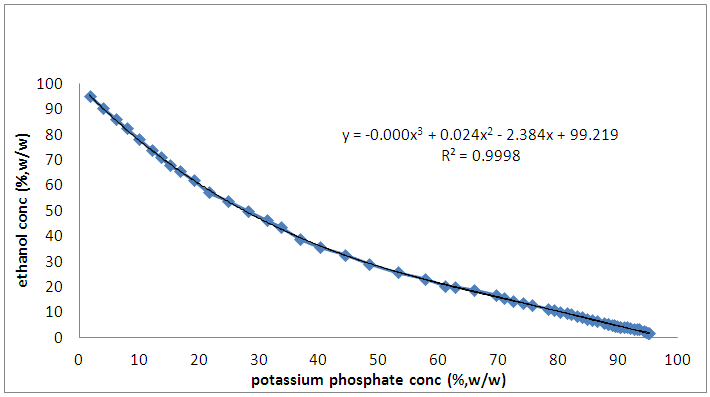 | Figure 1. Phase Diagram of Ethanol + K2HPO4 + H2O at 298.15 K |
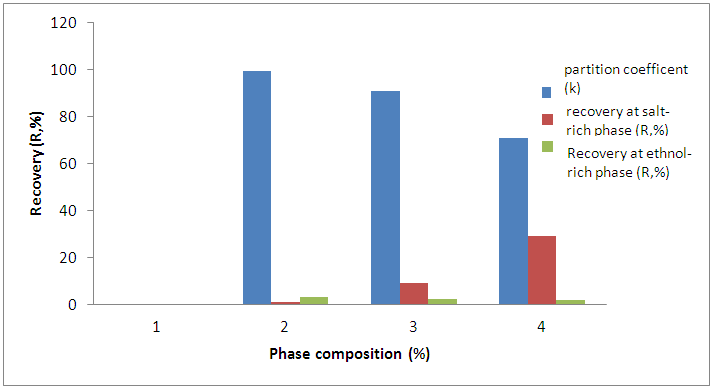 | Figure 2. Effect of extraction phase composition on the partition coefficient (K) and recovery of bioactive phenolic in ethanol/K2HPO4 based system |
4. Conclusions
- This study demonstrated that the aqueous two-phase extraction system consisted of 80% (w/w) ethanol, 40% (w/w) di-potassium hydrogen phosphate and distilled water was successfully separated and purified bioactive phenolics from Phanerochaete chrysosporium biomass. The highest partition coefficient (K) and recovery (R) of bioactive phenolics were found at phase composition with values of 10.41 and 99.27%, respectively. For further study, the thermodynamics analysis and the identity of the phenolic compound will be investigated.
ACKNOWLEDGEMENTS
- The authors are grateful to the Ministry of Education, Malaysia for financing this research under a research grant (RAGS 9003-00431), Research, Management and Innovation Centre, School of Bioprocess Engineering and Faculty of Engineering Technology, Universiti Malaysia Perlis for supporting and providing the necessary facilities.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML