-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2016; 6(5): 113-121
doi:10.5923/j.ajb.20160605.01

Ameliorative Effects of Quercetin and Naringenin on Diethylnitrosamine/2-acetyl aminoflourene-Induced Nephrotoxicity in Male Wistar Rats
Adel Abdel-Moneim A. , Osama M. Ahmed , Hanaa I. Fahim , Mohamed Y. Zaky
Physiology Division, Zoology Department, Faculty of Science, Beni-Suef University, Beni-Suef, Egypt
Correspondence to: Adel Abdel-Moneim A. , Physiology Division, Zoology Department, Faculty of Science, Beni-Suef University, Beni-Suef, Egypt.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Nephrotoxicity is one of the most widespread kidney tribulations. Quercetin and naringenin are natural flavonoids highly enriched in fruits, and vegetables which have a bioactive effect on human health. The current study was designed to evaluate the preventive effects of quercetin and naringenin on Diethylnitrosamine (DEN)/2- Acetylaminofluorene (2AAF)-induced nephrotoxicity in rats. The Wistar rats used in this study were allocated into 4 groups. The 1st group was kept as a normal group while the other 3 groups were intraperitoneally administered DEN (150 mg/kg b.w./week for two weeks) dissolved in 0.9% saline at the beginning of experiment. One week after the last injection of DEN, 2-AAF was orally administrated at dose 20 mg/kg b.w. in 1% tween 80 four days per week for three weeks. One of the three DEN/2AAF-administered groups was kept as control and the others were orally treated with quercetin and naringenin at dose 10 mg/kg b.w. every other day for 20 weeks. DEN/2AAF administration induced kidney injury evidenced by histological alterations as well as significant increases of serum creatinine, urea and uric acid concentrations. There was also a significant increase in the levels of renal lipid peroxidation and nitric oxide. Moreover, renal glutathione content and activities of superoxide dismutase, catalase, glutathione peroxidase and glutathione-s-transferase were significantly declined as a result DEN/2AAF administration. The treatments of DEN/2AAF-administered rats with the two flavonoids quercetin and naringenin successfully amended kidney histological perturbations and improved serum markers related to kidney function in association with alleviation of the deteriorated kidney oxidative stress and anti-oxidant defense system. In conclusion, quercetin and naringenin successfully attenuate the DEN/2AAF-induced nephrotoxicity and this effect may be mediated via suppression of the oxidative stress and stimulation of the antioxidant defense system.
Keywords: Quercetin, Naringenin, Nephrotoxicity, Diethylnitrosamine, Acetylaminofluorene, Oxidative stress
Cite this paper: Adel Abdel-Moneim A. , Osama M. Ahmed , Hanaa I. Fahim , Mohamed Y. Zaky , Ameliorative Effects of Quercetin and Naringenin on Diethylnitrosamine/2-acetyl aminoflourene-Induced Nephrotoxicity in Male Wistar Rats, American Journal of Biochemistry, Vol. 6 No. 5, 2016, pp. 113-121. doi: 10.5923/j.ajb.20160605.01.
Article Outline
1. Introduction
- The kidney is the target of many xenobiotic toxicants, including environmental chemicals. An environmental chemical has the potential to adversely affect human health through the disruption of kidney functions [1]. An exposure to environmental pollutants increased risks for kidney disease [2]. Mixture of physiologic and biochemical proceedings donate to the propensity of the kidney to several distinct classes of nephrotoxicity [3]. When kidney is evaluated with other organs, the kidney is uniquely susceptible to chemical toxicity, partially because of its unduly high blood flow, or due to its intricacy both physiologically and anatomically [4]. Nephrons toxicity may be consequent to direct cytotoxic damage to kidney structures by environmental toxicants, immunologic processes, indirect toxicity due to alterations in renal hemodynamics, or the production of endogenous nephrotoxic substances [5].Diethylnitrosamine (DEN), a well-known potent carcinogenic agent, is produced from the metabolism of some drugs and also present in tobacco, cheese and wide variety of foods [6]. DEN-induced renal damage was reported to occur through stimulation of oxidative stress and abolishment of antioxidant defense system [7]. In the same way, DEN was suggested to induce generation of free oxygen species and eventually resulting in oxidative stress and cellular injury which play an important role in the pathogenesis of kidney diseases [8]. Hence, the use of antioxidants could offer ameliorative effects against drug-induced renal damage.Flavonoids were considered one of the most important groups of secondary metabolites and bioactive compounds in plants and good sources of natural antioxidants in human diets [9]. Flavonoids have been suggested to exhibit a powerful antioxidant activity due to their ability to reduce free radical formation and scavenge free radicals, together with the up-regulation of antioxidant defenses [9]. Several studies have estimated the anti-oxidative, tissue protective and anti-tumor effects of flavonoids [10, 11]. Quercetin, one of the most natural abundant flavonoids found in fruits and vegetables such as apples and onions, has been shown to have very potent antioxidant [12, 13], and cytoprotective effects by preventing endothelial apoptosis caused by oxidants [14]. In rats, quercetin exhibited a protective effect against cisplatin-induced oxidative damage in kidneys [15]. Naringenin, another natural flavonoid highly enriched in citrus fruit, tomatoes and cocoa, has been reported to have a bioactive effect on human health including anti-tumor [16], anti-inflammatory [17, 18], and antioxidant activities [19]. Previous studies demonstrated that naringenin significantly ameliorated oxidative and inflammatory tissue injuries in different experimental models [20, 21]. Therefore, this study was designed to assess the preventive effects of quercetin and naringenin on DEN/2AAF-induced kidney toxicity in male Wistar rats.
2. Material and Methods
2.1. Experimental Animals
- Adult male Wistar albino rats, weighing 100-120 g and aging 8-9 weeks, were used in the present study. The animals were obtained from Helwan Station for Experimental Animals, Egyptian Organization for Biological Products and Vaccines (VACSERA), Helwan, Cairo, Egypt. They were kept under observation for two weeks before the onset of the experiment to exclude any intercurrent infections. The animals were housed in good aerated polypropylene cages in the Zoology Department Animal House in Faculty of science, Beni-Suef University, Egypt at normal temperature (20-25°C) and normal daily lighting cycle (10-12 hr/day), and given enough food (balanced standard diet) and water ad libitum. All animal procedures are in accordance with the general guidelines of animal care and the recommendations of the Experimental Animal Ethics Committee of Faculty of Science, Beni-Suef University, Egypt. All efforts were done to reduce the number and suffering of animals.
2.2. Chemicals
- Diethylnitrosamine and, 2- acetyl aminoflourine, quercetin and naringenin, were purchased from Sigma Chemicals Co., St. Louis, MO, USA, stored at 2-4°C. All other chemicals used for the investigation were of analytical grade.
2.3. Induction of Nephrotoxicity
- Nephrotoxicity was induced by intraperitoneal injection of DEN (150 mg/kg b.w./week for two weeks) dissolved in 0.9% saline. One week after the last injection of DEN, 2-AAF (20 mg/kg b w) [22] in 1% tween 80 was administrated orally by gavage four days per week for three weeks, then rats were sacrificed after 20 weeks from the beginning of the experiment.
2.4. Preparation of Dose of Quercetin and Naringenin
- Querctin and naringenin each at dose level of 10 mg/kg b. w. [23, 24] was dissolved in 5 ml of 1% carboxymethylcellulose (CMC) (1% w/v) and was administered to rats by oral gavage every other day for 20 weeks from the beginning of the experiment.
2.5. Experimental Design
- The experiment was performed on forty adult male Wister rats which were randomly distributed into 4 groups, each of ten animals. The 1st group was kept as a normal group, while the other 3 groups were administered DEN, followed by 2-AAF as previously described. One of three DEN/2AAF-administered groups were kept as control while the two others were orally given quercetin and naringenin every other day for 20 weeks.
2.6. Blood and Kidney Sampling
- By the end of the experiment, animals were sacrificed under mild anesthesia. Blood samples were collected from jugular veins, left to coagulate and centrifuged at 3000 rpm for 15 min to separate the serum. Kidney samples were immediately excised and perfused with ice-cold saline. Frozen kidney samples were homogenized in chilled saline (10% wt/vol) by using Telfon homogenizer (Glas-Col, Terre Haute, USA) and the homogenates were centrifuged at 3000 rpm for 10 min. The homogenate supernatants were collected and kept in deep freezer at –30°C until used for the determination of oxidative stress parameters and antioxidant defense markers.
2.7. Biochemical Assays
- Serum creatinine, urea and uric acid concentrations were assayed using reagent kits purchased from Biosystems (Spain), following the methods of Fabiny and Ertingshausen [25], Tabacco et al. [26] and Fossati et al. [27], respectively. The supernatants were used for estimation of lipid peroxidation (LPO) [28], Nitric oxide [29] using chemical reagents prepared in laboratory. Reduced glutathione (GSH) content [30], and activities of kidney antioxidant enzymes including glutathione peroxidase (GPx) [31], superoxide dismutase (SOD) [32], catalase (CAT) [33], and glutathione-S-transferase (GST) [34] were also determined using chemical reagents prepared in laboratory.
2.8. Histopathological Study
- After sacrifice, decapitation and dissection, kidney from each rat was rapidly excised and then perfused in saline solution. Pieces from the kidney of rats of different groups were taken and fixed in 10% neutral buffered formalin for twenty four hours. Washing was done in tap water and serial dilutions of ethyl alcohol were used for dehydration. Specimens were cleared in xylene and embedded in paraffin at 56°C in hot air oven for twenty four hours. Paraffin wax tissue blocks were prepared, then sectioning at 4 µm thickness by slide microtome was performed. The obtained tissue sections were mounted on glass slides, deparaffinized and stained with hematoxylin and eosin (H&E) according to the method of Banchroft et al. [35].
2.9. Statistical Analysis
- Results were expressed as mean ± standard error (SE). The data were analyzed using SPSS version 20 software [36] by Duncan’s method for post-hoc analysis to compare different groups at p-value, P<0.05.
3. Results
3.1. Effect on Serum Creatinine, Urea and Uric Acid Concentrations
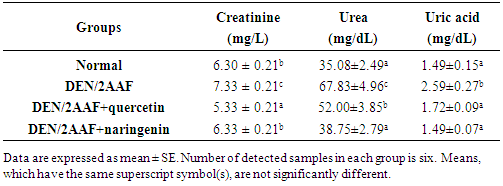
- The rats-administered DEN/2AAF exhibited a significant (P < 0.05) increase in serum creatinine, urea and uric acid concentrations as compared to the normal control rats. The treatment of DEN/2AAF-administered rats with quercetin and naringenin induced a significant (P < 0.05) improvement in the elevated serum creatinine, urea and uric acid concentrations as compared to DEN/2AAF-administered control group (Table 1).
|
3.2. Effect on Kidney Oxidative Stress and Antioxidant Defense System
3.2.1. Effect on Kidney LPO and NO Level
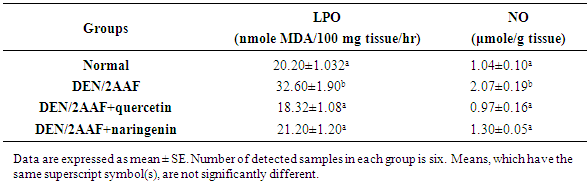
- The administration of DEN/2AAF to normal rats produced a significant (P < 0.05) increase in the LPO and NO level. However, the treatment of DEN/2AAF- administered rats with quercetin and naringenin significantly (P < 0.05) prevented this elevation in LPO and NO level (Table 2).
|
3.2.2. Effect on Kidney GSH Content and Its Metabolizing Enzymes, GPx and GST Activities
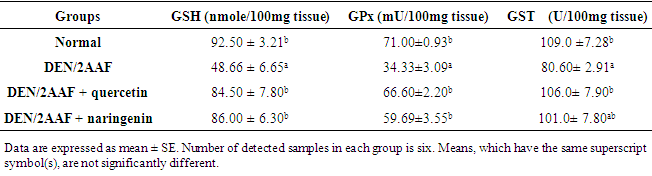
- DEN/2AAF-administration produced a significant (P<0.05) decrease in GSH content as well as GPx and GST activities stores as compared to the normal control group. The administration of quercetin to DEN/2AAF-administered rats significantly (P<0.05) prevented the decrease in GSH content and GPx, and GST activities. On the other hand, the administration of naringenin to DEN/2AAF-administered rats significantly (P < 0.05) improved the depleted GSH content as well as GPx activity, however, its effect on GST activity was non-significant (p > 0.05) (Table 3).
|
3.2.3. Effect on Kidney SOD and CAT Activities
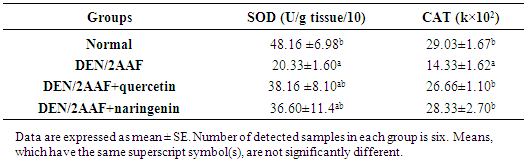
- The kidney SOD and CAT activities were significantly (P<0.05) decreased in rats administered DEN/2AAF as compared to normal group. The treatment of DEN/2AAF-administered rats with quercetin and naringenin produced a non-significant (P < 0.05) amelioration of the decreased SOD activity, however, quercetin and naringenin administration to DEN/2AAF- rats produce a significant (P < 0.05) ameriloation in the CAT activity (Table 4).
|
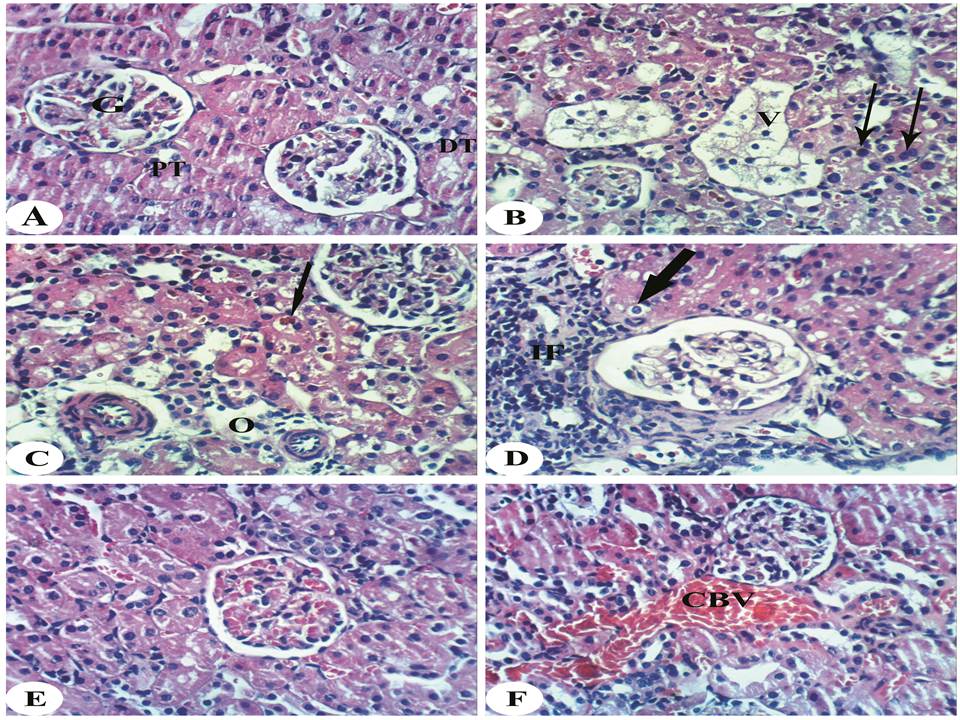
3.2.4. Effect on Kidney Histological Changes
- Histopatholgical examination of the kidney sections of normal rats revealed normal histological structure of renal parenchyma, glomerulus, proximal tubule and distal tubules (Figure A). On the other hand, DEN/2AAF-administration induced histpathological changes and several lesions that include karyomegaly (long arrows), sever vacuolar changes in renal tubule (Figure B). Also, DEN/2AAF-aministered group showed intraepithelial accumulation of brown pigments and perivascular oedema (Figure C), focal periglomerular mononuclear inflammatory cells aggregation and vacuolation of epithelial lining renal tubules (Figure D). The treatment of DEN/2AAF-administered rats with quercetin improved the histological architecture and integrity resulting in nearly normal renal tubules (Figure E). Also, DEN/2AAF-administered rats treated with naringenin showed congestion in the blood vessels and nearly normal renal tubules (Figure F). In comparison with DEN/2AAF-administered control rats, the kidney histological integrity and architecture as a result of treatment of DEN/2AAF-administered rats were markedly improved.
4. Discussion
- The kidney is a biochemically active and essential organ in the human body and part of the urinary system carrying out many essential functions like clearance of metabolic waste products, control of fluid volume status, maintenance of electrolyte and acid-base balance, and endocrine function [37]. It removes the metabolic waste and possesses the common xenobiotic metabolizing enzymes, mainly localized in proximal tubular cells [38]. The present study showed that the administration of DEN/2AAF has induced renal injury and this was evident by the increased serum concentration of markers related to the kidney function like creatinine, urea and uric acid. It has been reported that serum creatinine concentration relates to glomerular function and its rise is an indicator of renal failure [39, 40]. Urea is a by-product of protein metabolism and is used as a marker in acute kidney injury for retention and elimination of uremic solutes [41]. Also, Serum uric acid was proposed as a potential risk factor for new onset of kidney disease [42, 43]. These findings are in agreement with other studies [7, 44-45]. Recently, it was found that DEN administration induced nephrotoxicity in rats marked by a significant elevation of the levels of serum creatinine and urea [46].On the other hand, the DEN/2AAF-administered groups treated with quercetin and naringenin markedly decreased serum levels of creatinine, urea and uric acid suggesting nephrooprotective effects of querctin and naringenin. These finding are in accordance with the results described previously that showed quercetin modulate nephrotoxicity by reducing levels of kidney markers [47, 48]. Our study is also in agreement with Badarya et al. [49] and Vitaglione et al. [50] who showed that naringenin treatment significantly protected nephrotoxicity in rat and mice. Many investigators attributed this damage to exacerbated production of reactive oxygen species (ROS) and oxidative stress. ROS have the ability to cause oxidative damage in DNA, proteins and lipids [51]. The kidney is susceptible to injury caused by ROS because of the plenty of long chain polyunsaturated fatty acids found in the composition of renal lipids [52]. DEN has been suggested to cause the generation of ROS resulting in oxidative stress, alteration of the antioxidant defense system in tissues and cellular injury [53, 54]. In addition, some ROS interact with various tissue compounds leading to dysfunction and injury to the kidney, liver and other organs [55].In the present study, the DEN/2AAF administration deleteriously increased the kidney MDA and NO production. MDA, an indicator of LPO, has long been used as ultimate biomarker of oxidative damage and the increase of MDA reflects the enhancement of LPO [56]. NO can be both a scavenger and a producer of free oxygen radicals since it reacts with superoxide anions to form peroxynitrite which becomes a damage-producing radical [57]. In the current study, levels of LPO and NO in kidney were elevated in response to the administration of DEN/2AAF. The kidney injury may be attributed to ROS which induce mesangial cells contraction, altering the filtration surface area and modifying the ultrafiltration coefficient factors that decrease the glomerular filtration rate [58]. It was found that DEN is degraded and contact with renal tubular epithelium and is converted to active electrophilic species following α or β hydroxylation, resulting in the formation of unstable hydroxyalkyl compounds that converted to alkyl carbonium ions bind to DNA forming adducts and generate superoxide radicals through LPO [59, 60]. Further, this may be due to an enhanced generation of superoxide radicals (O2.-) and hydrogen peroxide radicals that accelerated peroxidation of native membrane lipids [55].On the same line, NO interacts with superoxide to generate the potent cytotoxic agent, peroxynitrite [61, 62]. The major effect of peroxynitrite is the nitration of cellular proteins leading to nitrosative stress and tissue injury [63]. Hence, the kidney injury is probably due to the deleterious effect of DEN itself and/or its metabolites which includes ethylcarbonium ions, NO and ROS [64]. Our results are in agreement with previous studies [65] that was found the level of MDA was significantly elevated in DEN administration to rats in comparison to normal control. Also, our results in accordance with Bishayee et al. [64] who stated that DEN also induced iNOS gene expression and generates NO radicals which react non enzymatically with O2- forming peroxynitrite (ONOO- a reactive nitrogen species).In the current study, the DEN/2AAF administration caused a marked deterioration in the kidney antioxidant defense system manifested by depletion of kidney glutathione level and suppression of activities of antioxidant enzymes including GPx, GST, SOD and CAT. In this situation, it worth mentioning that for the purpose of preventing cellular damage induced by ROS, the anti-oxidant defense system may scavenge ROS that play an important role in the initiation of LPO [64]. This defense system operates through enzymatic (including SOD, GPx, GST and CAT), and non-enzymatic components (mainly GSH) [66, 67]. SOD is the primary step of defense mechanism in the antioxidant system against the oxidative stress, as it dismutates the highly toxic superoxide anions (O2-) to O2 and H2O2. Gpx and CAT can scavange H2O2 and convert it into harmless byproducts, thereby providing protection against ROS [68, 69]. GPx also has a high potency in scavenging reactive free radicals in response to oxidative stress and detoxifies peroxides and hydroperoxides that lead to the oxidation of GSH [70]. Furthermore, GST catalyzes the conjugation of the thiol functional groups of GSH to electrophilic xenobiotics, leading to elimination or conversion of xenobiotic-GSH conjugate [71]. GSH is the most important non-enzyme antioxidant in mammalian cells [72]. It is said to be involved in many cellular processes including the detoxification of endogenous and exogenous compounds and efficiently protects cells against deleterious effects of oxidative stress by scavenging free radicals, removing H2O2, and suppressing LPO [73]. GSH is a potent antioxidant which protects the cellular constituents against the damage induced by the free radicals [74] through the formation of S-conjugates with products of LPO [75]. It was reported that GSH decline leads to lowered cellular defense against free radical induced cellular injury resulting in cell death [76]. Thus, these antioxidant enzymes together with glutathione are highly effective in inhibiting various ROS-mediated injuries and could protect the kidney from DEN-induced nephrotoxicity that occurred in the present investigation.The striking decease in antioxidants (SOD, CAT, GSH, GPx and GST) in DEN/2AAF-exposed rats, in the present investigation, elicits strong evidence for the involvement of oxidative damage in DEN/2AAF-induced nephrotoxicity. This may be due to ROS produced from metabolism of DEN that cause decreases the activities of renal antioxidant enzymes. Our results in agreement with Shaheen et al. [77] who reported that DEN decreased renal GSH content as well as GST and SOD activities and with Mahmoud et al. [7] and Ahmed et al. [46] who found that renal GSH content and activities of SOD, CAT, GPx and GST were significantly declined in the DEN-administered rats. In the present study, it was found that the treatment with quercetin and naringenin reduced the kidney LPO and NO level and counteracted the formation of free radicals induced by DEN/2AAF-mediated nephrotoxicity. Moreover, quercetin and naringenin treatments successfully resulted in an improvement of the lowered GSH content, and SOD, CAT, GPx and GST activities in the kidney of DEN/2AAF-intoxicated rats. This could be due to the ability of these antioxidant substances to transfer electrons free radicals, catalayse and activate antioxidant enzymes [78] and this proves that quercetin and naringenin administration overcomes the oxidative stress by their antioxidant properties.Quercetin significantly attenuated the LPO and NO production in the renal tissue probably because of its antioxidant capacity to scavenge oxygen free radical in the kidney tissue cells of rats. In addition to its free radicals-scavenging capability, quercetin enhanced the antioxidants enzymes SOD, CAT, GPx and GST activities and the GSH recovery in the kidney of DEN/2AAF-intoxicated rats reflecting its antioxidant activities. Many studies demonstrsted that quercetin has abroad range of pharmacological activities such as antioxidant and anti-inflammatory [79, 80]. Our results are in accordance with Almaghrabi, [81] who stated that quercetin had the ability to overcome oxidative stress through the reduction of free radical levels and elevating the antioxidant enzymes proving the renoprotective effects of qurecetin against oxidative stress induced by cisplatin. Also, naringenin ameliorated the renal damage that occurred due to DEN/2AAF-by reducing LPO and NO level and elevated SOD, CAT, GPx and GST activities and increased production of reduced form of glutathione in the kidney. Many studies reported that naringenin exerts marked antioxidant activity, scavenges ROS, suppresses LPO and maintains the antioxidant defense mechanisms [82, 83]. Our results are in agreement with the previous studies which stated that naringenin inhibits iNOS activity and therefore prevents nitrosative tissue stress [84, 85]. Recently, it was concluded that naringenin, through its antioxidant effects, may represent a therapeutic option to protect against gentamicin nephrotoxicity [86].In addition, renal injury induced by DEN/2AAF was confirmed by the observed histpathological alternations that include severe karyomegaly, sever vacuolar changes in renal tubule, intraepithelial accumulation of brown pigments and perivascular oedema, focal periglomerular mononuclear inflammatory cells aggregation and vacuolation of epithelial lining renal tubules. Our results are in agreement with Mahmoud et al. [7] and Ahmed et al. [87] who stated that renal injury induced by DEN confirmed by the observed histological alterations, including adenoma, dysplastic renal tubules with karyomegalic nuclei, atrophy of glomerular tuft, and inflammatory cells infiltration. Recently, it was reported that DEN induced renal damage which confirmed by histological changes that include focal fibrosis, necrosis and focal inflammatory cells infiltration in between the tubule [46]. As well, DEN induced injury and lesions in other body organs like liver [88]. The treatment of DEN/2AAF-administered rats with quercetin and naringenin improved kidney architecture and integrity since the kidney tissues showed nearly normal histological structures in these animals. However, the presence of congested intetubular blood vessels in kidney section of DEN/2AAF-administered rats treated with naringenin may be attributed to the increased blood flow and/or diltation of these blood vessels. These histological results are concomitant with the biochemical results which indicated improvements of the serum markers related to kidney function and antioxidant defense system as a result of treatments of DEN/2AAF-administered rats treated with quercetin and naringenin.In conclusion, the possible ameliorative and preventive effects of quercetin and naringenin on DEN/2AAF-induced nephrotoxicity may be attributed, at least in part, to suppression of oxidative stress and enhancement of the antioxidant defense system on the basis of oxidant-antioxidant system. Thus, these treatments may act as antioxidant preventive agents. However, further clinical studies are required to assess the safety and efficacy of these agents in human beings.
ACKNOWLEDGEMENTS
- We would like to thank Prof. Dr. Kawkab Abdel Aziz Ahmed, Professor of Histopathology, Faculty of Veterinary Medicine, Cairo University, Egypt and Prof. Dr. Rasha Rashad Ahmed, Professor of Molecular Cell Biology, Faculty of Science, Beni-Suef University, Egypt for their help in examinations of histological sections of detection of kidney lesions.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML