-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2016; 6(4): 100-111
doi:10.5923/j.ajb.20160604.03

The Anti-inflammatory Activity and the Antioxidant Effect of Some Natural Ethanolic Plant Extracts at the Molecular Level in Rat Liver Lysosomes In-vitro
Nermien Z. Ahmed
National Organization for Drug Control & Research (NODCAR), Molecular Drug Evaluation Department, Giza, Egypt
Correspondence to: Nermien Z. Ahmed, National Organization for Drug Control & Research (NODCAR), Molecular Drug Evaluation Department, Giza, Egypt.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This study was to evaluate the effect of Aloe Vera; Carica Papaya(leaves), Zingiber officinale (rhizomes), Solanum melongena (Fruits), Asparagus racemosus (Aerial part) by (25, 50, 100 µg/ml) of each compared with Rutin as standard on DPPH(2,2-diphenyl-1-picrylhydrazyl) radical assay in-vitro. Liver lysosomes were isolated from rat by ultra-cooling centrifuge, and the lysosomal fractions were incubated for 30 min with Aloe Vera, Zingiber officinale by (1, 5, and 10 mg/ml) to determine the activity of the released enzymes(acid phosphatase; β-galactosidase; β-N-acetylglucosaminidase), also, the labializing and stabilizing effects on the membrane permeability. In addition, the effect of the combination between Aloe and Zingiberby the three doses was investigated. The results revealed that the release rate of the three lysosomal enzymes appeared to be significantly decreased (P<0.05) as compared to control under the effect of the extracts and their mixtures, different percentage values of inhibition were observed. A low dose exerted highly inhibition, while a high dose revealed a less stabilizing effect on membrane permeability and this stabilizing effect was dose-dependent. It was concluded that the protective effect of each flavonoid was varied according to dose and enzyme type. The most potent inhibitory effect was observed for Zingiber officinale followed by Aloe Vera, then mixtures.
Keywords: DPPH, Aloe vera, Zingiber officinale, Lysosomal enzymes, Rutin, Fe++/Ascorbate
Cite this paper: Nermien Z. Ahmed, The Anti-inflammatory Activity and the Antioxidant Effect of Some Natural Ethanolic Plant Extracts at the Molecular Level in Rat Liver Lysosomes In-vitro, American Journal of Biochemistry, Vol. 6 No. 4, 2016, pp. 100-111. doi: 10.5923/j.ajb.20160604.03.
Article Outline
1. Introduction
- The plant kingdom offers a wide range of natural antioxidants. In the group of secondary plant metabolites, polyphenols, such as phenolic acids and flavonoids are strong antioxidants and are commonly found in a variety of fruits, vegetables, herbs, and seeds [1].Aloe Vera is a unique plant which is a rich source of many chemical compounds and plays an important role in the international market. Chemistry of the plant revealed the presence of more than 200 different biologically active substances including: Vitamins, minerals, enzymes, phenolic compounds, lignin, saponins, sterols and amino acids [2]. Biological activities of Aloe Vera have been established by large number of studies because of its demand, it is cultivated in large quantities in many parts of the world, and it has multiple constituents possessing potential biological activities [3]. The Aloe Vera plants have been used worldwide due to its medicinal properties as: Anti-inflammatory; wound healing; antiviral and antitumor and helps in immune system and burn. Aloe Vera has enjoyed a long history as a herbal remedy and is most popular herbal plant. Major value added products from Aloe are gel and juice. Plants belonging to genus Aloe particularly Aloe Vera (A. barbadensis) has been known for their medicinal properties for many centuries [4], Aloe Vera is used as anti-septic, germicidal, blood purifier and in chronic ulcers to stimulate healing. These Aloe species are currently listed in the pharmacopoeia of many countries in form of pain Aloe extract and powder. There are many reports showing the anti-inflammatory effect of Aloe Vera gel [5, 6], Aloe contains the enzymes carboxypeptidase which has been shown to decrease inflammation and swelling and beneficial effects of plant extracts on skin, there are some evidences that show Aloe Vera extract may be useful in the treatment of diabetes [7]. Aloe gel has few side effects the Handbook of Medicinal herbs [8] has given Aloe the lowest ranking for toxicity. Studies by [9] shown that the aqueous and the ethanol extracts of Aloe Vera powder have anti-mutagenic and anti-leukemic activities. Antioxidants effects have been studied in many studies by [9, 10]. A study used an assay system for free radicals to confirm the antioxidant action of Aloe Vera extract [10].Zingiber officinale the rhizome of ginger is one of the most widely used food species of the ginger family [11], recent evidence revealed the potential of ginger for treatment of diabetes mellitus, data from in-vitro and in-vivo and clinical trials has demonstrated the antihyperglycaemic effect of ginger. The mechanisms underlying these actions are associated with insulin release and action and improved carbohydrate and lipid metabolism [12]. The most active ingredients in ginger are the pungent principles gengerols, and shogaol, ginger has shown protective effects on diabetic liver, kidney, eye, and neural system complications. The pharmacokinetics, bioavailability, and the safety issues of ginger are also discussed [13]. Ginger is used as anti-inflammatory, antioxidant and a cholesterol- lowering herb [14]. Ginger contains a number of pungent constituents and active ingredients, steam distillation of powdered ginger products ginger oil, which contains a high proportion of sesquiterepene hydrocarbons, predominantly zingiberene [11]. The major strong compounds in ginger from studies of the lipophilic rhizome extracts have yielded potentially active gingerols, which can be converted to shogaols, zingerone and paradol. The compound 6-gingerol appears to be responsible for its characteristic taste. Zingerone and shogaols are found small amounts in fresh ginger and in larger amounts in dried or extracted products [15]. The analyzed chemical composition of aqueous extracts of ginger root (Zingiber officinale) was polyphenols, vitamin C, B, β-carotene, flavonoids and tannis [11, 16]. While the HPLC analysis of Zingiber officinale ethanolic extract were shogaol and gingerol, moreover, based on recent observations that 6-shogaol may have more potent bioactivity than 6-gingerol [11, 12, 17]. Several studies documented antioxidant effect of Zingiber officinale extracts in rats fed a high fat diet, intoxicated rats by paracetamole, radiation and arsenic, where ginger supplementation provide significant, raising tissue concentrations of superoxide dismutase, catalase, and reducing glutathione [18-20], respectively. Moreover, the hypolipdemic effect of Zingiber officinale extracts have been investigated in high fat feed diet rats by [21, 22] documented by lowering blood level of cholesterol, triglycerides, while HDL was not significant change when compared with high fat diet fed [11]. Aqueous extract of Zingiber officinale rhizomes were studied to evaluate their anti-diabetic effects on protein glycation and on the diffusion of glucose in-vitro in the study of [23-25]. Zingiber officinale “ginger” rhizome is one of the classic examples of an herb used for not only culinary preparation but also for unique therapeutic significance owing to its antioxidant, antimicrobial, anti-inflammatory and chemo protective potential [26-32]. Although several studies have mentioned anti-diabetic activity of Zingiber officinale [31, 33-36], Zingiber officinale can lower the blood glucose, improve activities of mitochondrial enzymes and could be used as a nephro-protective supplement particularly to reverse diabetic complications [35, 36]. The potential effects of Zingiber officinale in terms of protein glycation and glucose diffusion inhibition were evaluated. It was found that Zingiber officinale rhizomes with anti-diabetic/hyperglycemic properties might provide a viable approach, either food based or pharmacological in the treatment of diabetic complications 9250. The rhizome of the ginger is generally used as a spice in food. Gingerols and their corresponding dehydration products shogaols were considered as the active principles of ginger and these constituents are responsible for strong antioxidant activity [37, 38]. Ginger posses various pharmacological activity including hypoglycemia; anticancer; anticardic; antirenal; hepatoprotective and antioxidant [39]. Ginger has many antioxidant compounds; these compounds may either mitigate or prevent generation of free radicals in toxic conditions. The active ingredients of ginger include gingerols, shagogals, phytochemicals and other compounds show antioxidant activity in various models [40]. It has varied pharmacological activities including antioxidant, anti-inflammatory, anticancer [41], and analgesic [42, 43].Eggplant (Solanum melongena L.) is an important and widely consumed vegetable crop of India grown, the constitutive defenses of plants include structural barriers such as the plant cell wall, as well as inhibitory compounds including phenolics [44-46]. However, there have been no reports on induction of resistance in eggplant against R. solanacearum by elicitors [44, 47, 48].Aqueous extract of Asparagus racemosus was evaluated for lead detoxification from the hepatic tissue by the oral administration route in mice. The toxic effects of lead were studied simultaneously on hepatic biochemical and also on histopathological parameters; the hepatic system showed hepatocyte pycnosis, vaculation, blood congestion and high lymphocytic infiltration around the control vein, results suggested that beneficial effect of aqueous extract may be probably due to its antioxidant properties [49]. Asparagus racemosus is recommended for prevention and treatment of various human ailments. The decoction of root has been used in blood diseases diarrhea, dysentery, cough, bronchitis and general debility [50-52]. Reports indicated that the pharmacological activities of extract include antinuclear [53], anti-tissue [54], antioxidant [55], and anti-bacterial activities [56]. It was investigated that, mice consumed the given amount of lead nitrate showed a significant lower body weight and liver weight, it may be due to the interruption in absorption and metabolism with Asparagus racemosus on growth performance of body and organ (liver) weight, this study was similar to the observation of [49) who recorded body weight gain in experimental model after Asparagus racemosus administration.Carica papaya L. (Papaya) is belongs to caricaceae family, it has been used empirically as food or as medication for kidney stones, hypertension, urinary tract disorders, abdominal pain during menstruation, analgesic, dysentery, diarrhea, fever [57]. The anti-inflammatory activity of an ethanolic extract of carica papaya was investigated in rats using carrageenan induced paw edema, the ulcerogenic activity of the extract was also investigated, and the extract also produced slight mucosal irritation at high dose. The study of [58] establishes the anti-inflammatory activity of carica papaya leaves, many parts of the plant are employed in the treatment of several ailments for example the seed is used for expelling worms; the seeds and the roots are used as abortifacient agent; the leaves (especially fallen ones) are used variously for the treatment of fever, diabetes, and inflammation [59, 60], and several other studies [58]. Powdered leaves of Carica papaya L. were extracted with ethanol; partitioned in chloroform and distilled water, the extracts and the fractions were tested for antibacterial activity against clinical isolates of Escherichia coli and pseudomonas species using disc diffusion and micro broth dilution technique, the extracts and fractions were further subjected to phytochemical tests for the presence of secondary metabolites using standard procedures [61]. The extract of C. papaya can inhibit sub-chronic inflammation in which various types of cellular migration are (e.g. fibroblast) involved [62]. The juice of C. papaya has lowering the blood pressure in renal [63].Anti-inflammatory activity of the three flavonoids: Rutin, quercetin and hesperidin were investigated in rats using the model of acute and chronic inflammation, interaperitoneal administration of the three flavonoids given daily at doses equivalent to 80mg/kg, inhibited both acute and chronic phases of this experimental model of inflammation. Rutin was the most active in the chronic phase, the antihyperglycemic and the antioxidant effect of Rutin, apolyphenolic flavonoid, in normal and streptozotocin-induced diabetic Wister rats [64], diabetes as induced in rats by an intraperitoneal injection of streptozotocin, Rutin was orally administered to normal and diabetic rats for 45 days. Fasting plasma glucose, glycosylated haemoglobin, thiobarbituric acid reactive substances and lipid hydroperoxides was significantly increased, whereas insulin, c-peptide, total haemoglobin, protein levels, non-enzymatic antioxidants (glutathione, vitamin C and vitamin E) were decreased significantly in diabetic rats. The result showed that Rutin exhibits antihyperglycemic and antioxidant activities in streptozotocin-induced diabetic rats. Also, it was found that the metabolic and pharmacological properties of Rutin underlying the Rutin-mediated amelioration of the rat colitis. Apparent partition coefficients of Rutin and its aglycone quercetin were compared. The biochemical/chemical stability of Rutin was examined in the contents of various segments of gastrointestinal tracts of rats [65]. Inflammatory effect was determined in the colitis rats after oral administration of Rutin or rectal administration of quercetin. In human colon epithelial cells, the effect of quercetin on tumor necrosis factor (α-TNF)-induced nuclear factor Kappa B (NF Kappa B) activation was examined. Rutin acted as a quercetin deliver to the large intestine and its anti-inflammatory action in TNBS-induced colitis rats may be through quercetin mediated inhibition of TNF-α-induced NF kappa B activation [65].In many pathological conditions, changes in the state of lysosomes take place, the loss of the stability of lysosomal membrane has been observed in the leakage of enzymes from lysosomes [66-68]. The direct effects of the test compounds on the lysosomal membrane was to determine the relative stabilizing or labializing effects within the limits of antioxidant concentration level and the exposure period of times [69]. Lysosomes have a central role in cellular homeostasis as sites for digestion of foreign materials and for degradation of intracellular components undergoing autolytic processing [70]. Autophagy provides cells with oxidizable substrate when nutrients become scarce but also that it can provide protection against aging and a number of pathologies such as cancer, neuro-degeneration, cardiac disease, diabetes, and infections [71]. It was supported that the intracellular release of the lysosomal enzymes proceeds cellular death by initiating the cellular injury process ultimately causing tissue necrosis. The compounds which antagonize the effect of labilizers and prevent or reduce the release of the lysosomal enzymes are designated stabilizers [72, 73]. Lysosomes are membrane –bonded structures containing hydrolytic enzyme capable of degrading most of the cellular constituents, play an important role in secretion and transport processes. Leakage of lysosomal enzymes accounts for many tissue and target organopathies [74], lysosomes are found in all animal cells and in disease-fighting cells. The major lysosomal enzymes according to their importance as liver lysosomal markers are: Acid phosphatase (ACP), β-galactosidase (β-GAL), β-N-acetyl glucosaminidase and β-glucuronidase (β-NAG) [75].The purpose of the present study was to investigate the antioxidant activity of five natural ethanolic plant extracts as: Aloe Vera (leaves); Zingiber officinale (rhizome); Solanum melongena (fruits); Asparagus racemosus (aerial part), and Carica papaya (leaves) on DPPH (2,2-diphenyl-1-picrylhydrazyl) radical assay in-vitro. Also, the anti-inflammatory effects of Aloe Vera (leaves); Zingiber officinale (rhizome) and their mixtures on the release rates of the three lysosomal acid hydrolases “ACP, β-GAL, β-NAG” in rat in-vitro were performed.
2. Materials and Methods
- v PlantsThe families; Latin name; Arabic name; English name; part used [76], and source of plant samples are listed in Table 1. All plant samples were authenticated via plant herbarium of Orman Garden, Ministry of Agriculture.
|
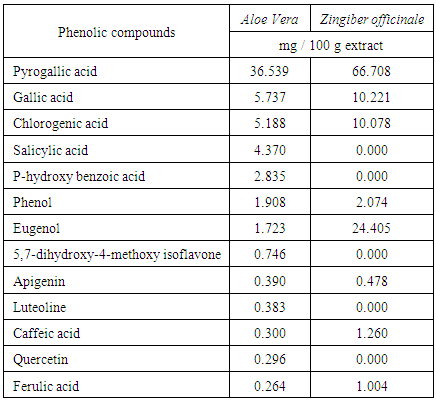
 v 2,2-Diphenyl-1-picrylhydrazyl assay (DPPH)The free radical scavenging effect of the five ethanolic natural plant extracts was assessed by the discoloration of a methanolic solution of DPPH according to [78-79].v Preparation of lysosomal fractionTwenty male albino rats weighing about 200-270g were used. After decapitation and bleeding, the liver was perfused in situ with 0.25 M ice-cold sucrose medium via portal vein at a rate of approximately 15 ml/minute according to the method of [80]. The tissue was cutting into small pieces and dispersed in 0.25 M sucrose buffer pH (7.4) placed in CAT (R18) homogenizer. After homogenization, the volume was adjusted to 6.0 ml sucrose buffer 0.25 M contains 1.0g wet tissue of liver.v Enzyme substrates1. p-nitrophenyl phosphate (Sodium salt) was used for acid phosphatase (EC 3.1.3.2) “ACP”.2. p-nitrophenyl-ß-D-galactopyranoside was used for ß-galactosidase (EC 3.2.1.23)”β-GAL”.3. p-nitrophenyl-2-acetamido-2-deoxy-ß-D-glucopyranoside was used for N-acetyl-ß-glucosaminidase (EC 3.2.1.30)”β-NAG”.All these substrates were purchased from Sigma Chemical Co. U.S.A.v Isolation of lysosomal fractionThe liver homogenate was then centrifuged at 2500 r.p.m. in a Beckman refrigerating ultracentrifuge (model J2-21) for 15 minutes. The pellet was washed under the same conditions, and then the first supernatant and the wash were combined together. The whole lysosomal fraction was prepared by centrifuging the last supernatant at 14,000 r.p.m. for 15 minutes. The pellet was washed and resuspended in the 0.25 M. sucrose buffer and this step was repeated three times for isolating pure lysosomal fraction. After washing and purification, the pellet was resuspended in the 0.25M sucrose buffer medium to give 1.0g liver weight per 1.25 ml sucrose buffer [80].v Incubation of lysosomes with the antioxidant compoundsIncubation mixtures consisted of 1.0 ml of lysosomal fraction and 1.0 ml of antioxidant solution, the total volume was completed to 3.0ml by the addition of sucrose buffer solution. The tubes were incubated in a shaking water bath at 37°C/30min., Tubes of each antioxidant concentration were removed and centrifuged at 19000 r.p.m./15 min. The resulting supernatant was subjected to enzyme assay to determine the activity of released enzymes [81].v Methods of enzyme assay For determination the total enzymatic activity, some culture tubes containing 1ml antioxidant compounds+1ml lysosomal fraction + 1ml TritonX-100 (0.1%) were exposure to thawing and freezing for three times, then centrifuged at 19000r.p.m/15min. The resulting supernatant was also subjected to each enzyme assay for determination the total enzymatic activities of each by lysosomal enzyme. The activities were measured spectrophotometerically according to the method of [82] with slight modifications described by [83].v Determination of individual phenolic compounds by HPLCDetermination of phenolic compounds was performed according to the method outlined by [84, 85], Identification of individual phenolic compounds by using an Agilent HPLC series 1090 (Agilent, Waldron, Germany) apparatus. HPLC method was performed for the photochemical screening and identification of active ingredients (major and minor components with bioactive).v Statistical analysis of the results All values are mean ± S. E. obtained from eight animals. For statistical analysis, one way ANOVA [86] with Duncan's variance (SPSS 10) was used to compare groups. In all the cases a difference was considered significant when ρ was < 0.05.
v 2,2-Diphenyl-1-picrylhydrazyl assay (DPPH)The free radical scavenging effect of the five ethanolic natural plant extracts was assessed by the discoloration of a methanolic solution of DPPH according to [78-79].v Preparation of lysosomal fractionTwenty male albino rats weighing about 200-270g were used. After decapitation and bleeding, the liver was perfused in situ with 0.25 M ice-cold sucrose medium via portal vein at a rate of approximately 15 ml/minute according to the method of [80]. The tissue was cutting into small pieces and dispersed in 0.25 M sucrose buffer pH (7.4) placed in CAT (R18) homogenizer. After homogenization, the volume was adjusted to 6.0 ml sucrose buffer 0.25 M contains 1.0g wet tissue of liver.v Enzyme substrates1. p-nitrophenyl phosphate (Sodium salt) was used for acid phosphatase (EC 3.1.3.2) “ACP”.2. p-nitrophenyl-ß-D-galactopyranoside was used for ß-galactosidase (EC 3.2.1.23)”β-GAL”.3. p-nitrophenyl-2-acetamido-2-deoxy-ß-D-glucopyranoside was used for N-acetyl-ß-glucosaminidase (EC 3.2.1.30)”β-NAG”.All these substrates were purchased from Sigma Chemical Co. U.S.A.v Isolation of lysosomal fractionThe liver homogenate was then centrifuged at 2500 r.p.m. in a Beckman refrigerating ultracentrifuge (model J2-21) for 15 minutes. The pellet was washed under the same conditions, and then the first supernatant and the wash were combined together. The whole lysosomal fraction was prepared by centrifuging the last supernatant at 14,000 r.p.m. for 15 minutes. The pellet was washed and resuspended in the 0.25 M. sucrose buffer and this step was repeated three times for isolating pure lysosomal fraction. After washing and purification, the pellet was resuspended in the 0.25M sucrose buffer medium to give 1.0g liver weight per 1.25 ml sucrose buffer [80].v Incubation of lysosomes with the antioxidant compoundsIncubation mixtures consisted of 1.0 ml of lysosomal fraction and 1.0 ml of antioxidant solution, the total volume was completed to 3.0ml by the addition of sucrose buffer solution. The tubes were incubated in a shaking water bath at 37°C/30min., Tubes of each antioxidant concentration were removed and centrifuged at 19000 r.p.m./15 min. The resulting supernatant was subjected to enzyme assay to determine the activity of released enzymes [81].v Methods of enzyme assay For determination the total enzymatic activity, some culture tubes containing 1ml antioxidant compounds+1ml lysosomal fraction + 1ml TritonX-100 (0.1%) were exposure to thawing and freezing for three times, then centrifuged at 19000r.p.m/15min. The resulting supernatant was also subjected to each enzyme assay for determination the total enzymatic activities of each by lysosomal enzyme. The activities were measured spectrophotometerically according to the method of [82] with slight modifications described by [83].v Determination of individual phenolic compounds by HPLCDetermination of phenolic compounds was performed according to the method outlined by [84, 85], Identification of individual phenolic compounds by using an Agilent HPLC series 1090 (Agilent, Waldron, Germany) apparatus. HPLC method was performed for the photochemical screening and identification of active ingredients (major and minor components with bioactive).v Statistical analysis of the results All values are mean ± S. E. obtained from eight animals. For statistical analysis, one way ANOVA [86] with Duncan's variance (SPSS 10) was used to compare groups. In all the cases a difference was considered significant when ρ was < 0.05.3. Results and Discussions
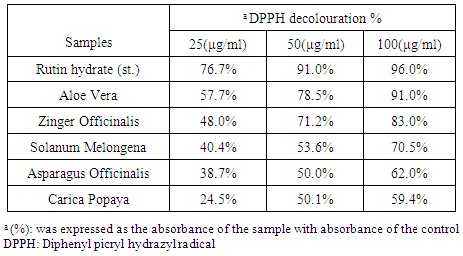
- 1) DPPH radical-scavenging activityThe effect of antioxidant activity on DPPH radical scavenging is thought to be due to their hydrogen donating ability: DPPH• + AH → DPPH− H + A• . DPPH is a stable free radical and accepts an electron or hydrogen radical to become a stable diamagnetic molecule, the reduction capability of DPPH radicals was determined by the decrease in its absorbance induced by antioxidants, it is visually noticeable as a discoloration from purple to yellow colour [87].The three concentrations of each ethanolic extract were used (25, 50, and 100 µg/ml) in scavenging test, the antioxidant activity of Rutin as standard antioxidant at high concentration (100µg/ml) appeared to be the highest value of inhibition by (96.0%), followed by the extracts: Aloe Vera (leaves; 91.0%), Zingiber officinale (rhizome; 83.0%), Solanum melongena (fruits; 70.5%), Asparagus racemosus (aerial parts; 62.0%) and Carica papaya (leaves; 59.4%), and the decrease could be directly attributed to the increase of the phenolic contents of each plant. The close correlation between antioxidant activity and phenolic content obtained from various natural sources has been already demonstrated [88, 89]. It was also reported that the solvent used in extraction may be important in antioxidant activity of the extract depending on phenolic content (phenolic and flavonoid contents of an endophytic xylaria species higher in methanol than in hexane extracts [88, 90] (Table 2 and Fig. 1).
|
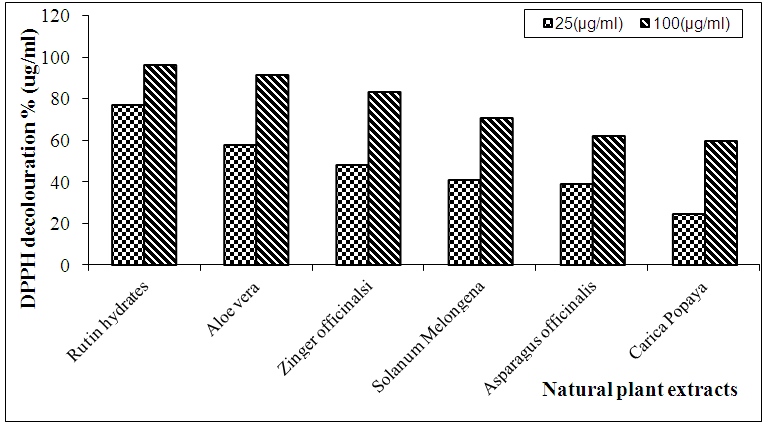 | Figure 1. Free radical scavenging activity of the natural plant extracts |
|
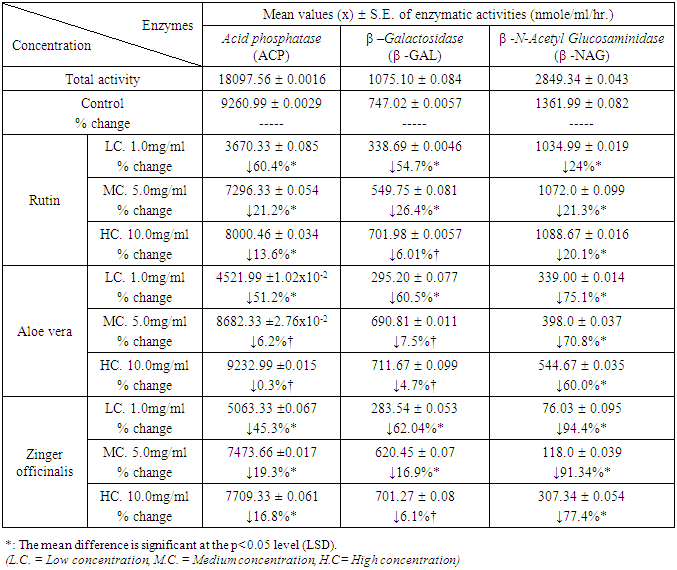
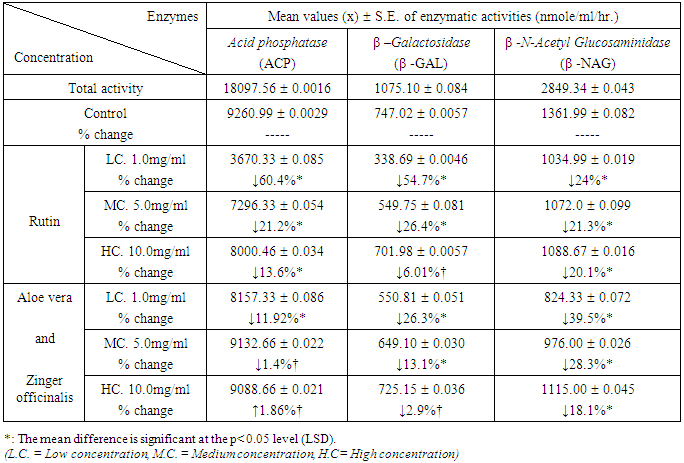
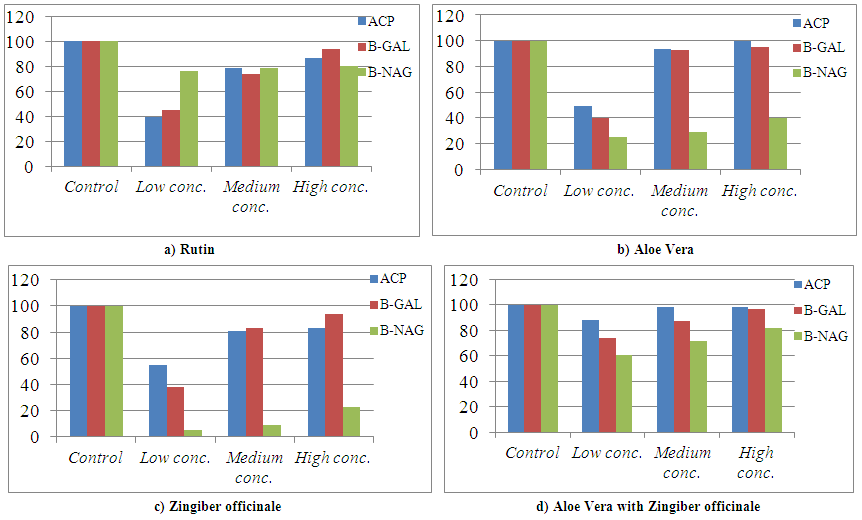 | Figure 2. Relative Activity effects of Aloe Vera, Zingiber officinale, and their mixtures by three concentrations and Rutin on extralysosomal relative enzymatic activity of rat liver in-vitro |
|
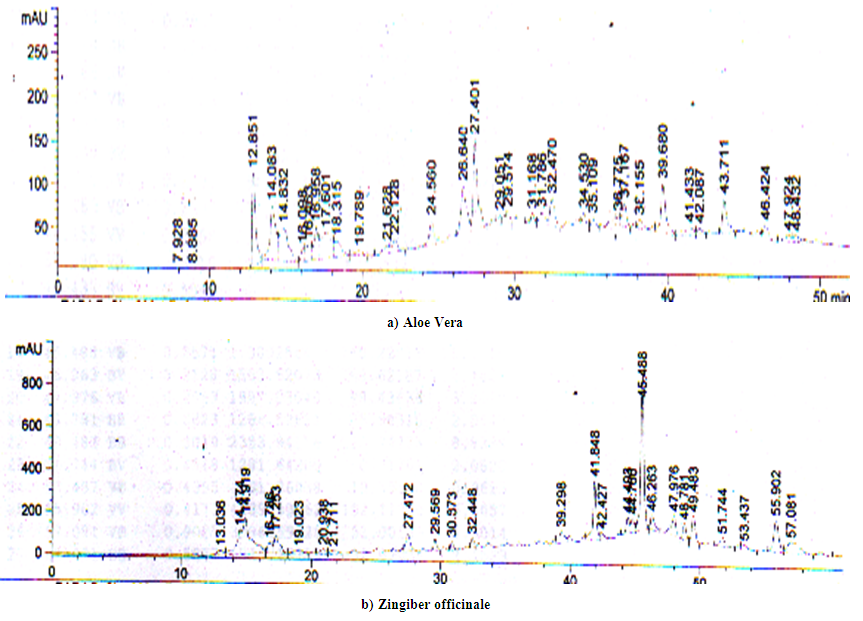 | Figure 3. HPLC chromatogram of Aloe Vera (leaves) (a) and Zingiber officinale (rhizome) (b) |
4. Conclusions
- It was concluded that all the plant extracts under investigation show a highly antioxidant effect and potent anti-inflammatory activities on the lysosomal acid hydrolases such as: ACP; β-GAL and β-NAG in rat liver in-vitro which have a stabilizing effect on membrane permeability and also, its ability to scavenge free radical to protect the cell membrane. Also, it was concluded that Aloe Vera and Zingiber officinale are recommended as a spice in food plants and due to their medicinal properties as: Anti-inflammatory; antioxidant and so on…., because they posses various pharmacological activities.
ACKNOWLEDGMENTS
- Deepest gratitude and many thanks to Prof. Dr. / Zakaria Ahmed Teleb, Dept. of Molecular Drug Evaluation, National Organization for Drug Control and Research, for his help in the research work for his valuable discussions and presentation.
References
| [1] | Slusarczyk, S., Hajnos, M., Skalicka-Wozniak, K., and Matkowski, A. (2009) Antioxidant activity of polyphenols from Lycopus lucidus Turcz. Food Chem 113: 134–138. |
| [2] | Chandan, B.K., Saxena, A.K., Shukla, S., Sharma, N., Gupta, D.K., Suri, K.A., Suri, J., Bhadauria, M., and Singh, B. (2007) Hepatoprotective potential of Aloe barbadensis Mill. Against carbon tetrachloride induced hepatotoxicity. J Ethnopharmacol 111: 560-566. |
| [3] | Femenia, A., Sanchez, E.S., Simal, S., and Rosello, C. (1999) Compositional features of polysaccharides from Aloe Vera (Aloe barbadensis Miller) plant tissues. Carbohydr Polymn 39: 109-117. |
| [4] | Antherton P (1998) Aloe Vera revisited. Br J Phytother 4: 76-83. |
| [5] | Hutter, J.A., Salmon, M., Stavinoha, W.B., Satsangi, N., Williams, R.F., Streeper, R.T. (1996) Anti-inflammatory C-glucosyl chromone from Aloe barbadensis. J Nat Prod. 59: 541-543. |
| [6] | Vazquez, B., Avila, G., Segura, D., Escalante, B. (1996) Anti-inflammatory activity of extracts from Aloe Vera gel. J Ethnopharmacol 55: 69-75. |
| [7] | Boudreau, M.D. and Beland, F.A. (2006) an evaluation of the biological and toxicological of Aloe Barbadensis (Miller), Aloe Vera. J Environ Sci Health C 24: 103-154. |
| [8] | Ernst, E. (2000) Mthodological considerations in testing the efficacy of complentary / alternative treatments (CATs). Int J Alt Comp Med. 16: 8-10. |
| [9] | Lee, K.Y., Weintraub, S.T., and Yu, B.P. (2000) Isolation and identification of a phenolic antioxidant from Aloe Vera baebadesis. Free Radic Biol Med 28: 261-265. |
| [10] | Hu, Y., Xu, J., and Hu, Q. (2003) Evaluation of antioxidant potential of Aloe Vera (Aloe barbadensis miller) extracts. J Agric Food Chem 51: 7788-7791. |
| [11] | Abdullah, G.K., Mohamed El-Sayed, B., Naser, A.S., Osama, A., Shaikh, O., Eslam, A.H. (2013) Pathological comparative studies on aqueous and ethanolic extracts of Zingiber officinale on antioxidants and hypolipidemic effects in rats. Life Sci J 10(2): 2393-2403. |
| [12] | Kim, S.O., Chun, K.S., Kundu, J.K., and Surth, Y.J. (2004) Inhibitory effects of [6]-gingerol on PMA-induced COX-2 expression and activation of NF-kB and p38 MAPK in mouse skin. J Biofac 21(1-4): 27-31 |
| [13] | L,i Y., Tran, V.H., Duke, C.C., Roufogalis, B.D. (2012) Preventive and protective properties of Zingiber officinale (ginger) in diabetes mellitus, diabetic complications and associated lipid and other metabolic disorders: A Brief Rev., Hindawi Publishing corporation, Evidence-based complementary and alternative medicine. ID: 516870, 10 pages, Doi: 10.1155/2012/516870. |
| [14] | Grant, K.L. and Lutz, B. (2000) Ginger. Am J Health Syst Pharm 57:945-947. |
| [15] | Govindarajan, V.S. (1982) Ginger – chemistry, technology, and quality evaluation: part 2. Crit Rev Food Sci Nutr 17:189-258. 24. |
| [16] | Shirin, A.P.R. and Jamuna, P. (2010) Chemical composition and antioxidant properties of ginger root (Zingiber officinale). J of Med Plants Res 4(24): 2674-2679. |
| [17] | Bak, M.J., Ok, S., Jun, M., and Jeong, W.S. (2012) 6-shogaol-rich extract from ginger up-regulates the antioxidant defense systems in cells and mice. Mole 17(7): 8037-55. |
| [18] | Asmah, H., Siti, B.B., Rafidah, A.P.M., Noraida, A.M., Nor, Y.Y., Khairana, H., Zariyantey, A.H., and Jamaludin, M. (2010) Role of oxidative stress in the protective effects of Zingiber zerumbet smith ethyl-acetate extract against paracetamol-induced hepatotoxicity in Sprague-dawley rats. Austr J of Bas and App Sci 5(8): 1519-1525. |
| [19] | Debrup, C., Avinaba, M., Sourav, S., Avijit, P., Samrat, G., and Anisur, R.K. (2012) Gingerol Isolated from ginger attenuates sodium arsenite induced oxidative stress and plays a corrective role in improving insulin signaling in mice. Toxicol. Lett. 210: 34–43. |
| [20] | Ghada, M.N., Atef, M.M., El-hag, M.A. (2009) Radio protective Effect of Dietary (Zingiber officinale Rosc.) Against Fast Neutron-induced Oxidative Stress in Rate. World App Sci J 6 (4): 494-498. |
| [21] | Atta, A.H., Elkoly, T.A., Mouneir, S.M., Gehan, K., Al-wabel, N.A., Shaimaa, Z. (2010) Hepatoprotective effect of methanol extracts of Zingiber officinale and cichorium intybus. Ind J of Pharm Sci 72(5):564-70. |
| [22] | Srinivas, N., Satyanarayana, S., and Basil, D.R. (2009) Protective effects of ethanolic extract of Zingiber officinale Rhizome on the development of metabolic syndrome in High-Fat Diet-Fed Rats. J compilation Nordic Pharmacol Soci Basic & Clin Pharmaco & Toxicol 104: 366–373. |
| [23] | Chen, L., Magloano, D.J., and Zimmet, P.Z. (2011) The worldwide epidemiology of type 2 diabetes mellitus-present and future perspectives. Nat Rev Endocrinol 8(4): 228-236. |
| [24] | Hussain, F., Arif, M., and Sheikh, M.A. (2011) Prevalence of diabetic retinopathy in Faisalabad, Pakistan: A population based study. Turk J Med Sci 41(4): 735-742. |
| [25] | Naila, A.S., Fatma, H., Tahira, L., and Munir, A.S. (2012) Determination of in-vitro antidiabetic effects of Zingiber officinale roscoe. Brazilian J of Pharmaceu Sci 48(4): 601-607. |
| [26] | Al-Suhaimi, E.A., Al-Riziza, N.A., Al-Essa, R.A. (2011) Physiological and therapeutically roles of ginger and turmeric on endocrine functions. Am J Chinese Med 39(2): 215- 231. |
| [27] | Baliga, M.S., Haniadka, R., Pereira, M.M., D’Souza, J.J., Pallaty, P.L., Bhat, H.P., and Popuri, S. (2011) Update on the chemo preventive effects of ginger and its phytochemicals. Crit Rev Food Sci Nutr 51(6): 499-523. |
| [28] | Butt, M.S. and Sultan, M.T. (2011) Ginger and its health claims: molecular aspects. Crit Rev Food Sci Nutr 5(51): 383-393. |
| [29] | EL-Ghorab, A.H., Nauman, M., Anjum, F.M., Hussain, S., and Nadeem, M.A. (2010) Comparative study on chemical composition and antioxidant activity of ginger (Zingiber officinale) and cumin (Cuminum cyminum). J Agric Food Chem 58(14): 8231-8237. |
| [30] | Ghasemzadeh, A., Jaafar, H.Z.E., and Rahmat, A. (2010) Antioxidant activities, total phenolics and flavonoids content in two varieties of Malaysian young ginger (Zingiber officinale Roscoe). Molec 15(6): 4324- 4333. |
| [31] | Rani, M.P., Padmakumari, K.P., Sankarikutty, B., Cherian, O.L., Nisha, V.M., and Raghu, K.G. (2011) Inhibitory potential of ginger extracts against enzymes linked to type 2 diabetes, inflammation and induced oxidative stress. Int J Food Sci Nutr 62(2): 106-110. |
| [32] | Rehman, R., Akram, M., Akhtar, N., Jabeen, Q., Saeed, T., Shah, S.M.A., Ahmed, K., Shaheen, G., and Asif, H.M. (2011) Zingiber officinale Roscoe (pharmacological activity). J Med Plant Res 5(3): 344-348. |
| [33] | Madkor, H.R., Mansour, S.W., and Ramadan, G. (2011) Modulator effects of garlic, ginger, turmeric and their mixture on hyperglycaemia, dyslipidaemia and oxidative stress in streptozotocin-nicotinamide diabetic rats. Br J Nutr 105(8): 1210-1217. |
| [34] | Ogbera, A.O., Dada, O., Adeleye, F., Jewo, P.I. (2010) Complementary and alternative medicine use in diabetes mellitus. West Afr J Med 29(3):158-162. |
| [35] | Ramudu, S.K., Korivi, M., Kesireddy, N., Lee, L.C., Cheng, I.S., Kuo, C.H., and Kesireddy, S.R. (2011) Nephro-protective effects of a ginger extract on cytosolic and mitochondrial enzymes against streptozotocin (STZ)- induced diabetic complications in rats. Chinese J Physiol 54(2): 79-86. |
| [36] | Saraswat, M., Suryanarayana, P., Reddy, P.Y., Patil, M.A., Balakrishna, N., Reddy, G.B. (2010) Antiglycating potential of Zingiber officinalis and delay of diabetic cataract in rats. Mol Vis 16(165-166):1525- 1537. |
| [37] | Gupta, R.K., Chawla, P., Tripathi, M., Shuklaand, A.K. (2014) Synergistic antioxidant activity of tea with ginger, black pepper and tulsi ISSN-0975-1491. Intern J of Pharm and Pharmaceut Sci 6(5): 477-479. |
| [38] | Stoilova, I., Krastanov, A., Stoyanova, A., Denev, P., Gargova, S. (2007) Antioxidant activity of a ginger extract (Zingiber officinale). Food Chem 102:764–77. |
| [39] | Nicoll, R. and Henein, M.Y. (2009) Ginger (Zingiber officinale roscoe): A hot remedy for cardiovascular disease. Int J Cardiol 131: 408-409. |
| [40] | Dugasani, S., Pichika, M.R., Nadarajah, V.D., Balijepalli, M.K., Tandra, S., and Korlakunta, J.N. (2010) Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]- gingerol, [10]-gingerol and [6]-shogaol. J Ethnopharmacol 127: 515–520. |
| [41] | Kim, J.K., Kim, Y., Na, K.M., Surh, Y.J., Kim, T.Y. (2007) [6]-gingerol prevents UVB-induced ROS production and COX-2 expression in-vitro and in-vivo. J Free Radic Res 41(5): 603-614. |
| [42] | Subbaiah, G.V., Naliniprasad, R., Himabindu, J., Anila, M., Devi, I., Nagaraju, P., Chandrakala, P., Mohan, K.R., Shanmugam, C.H., Ramakrishna, S., Ravi, B.S., and Reddy, K.S. (2013) Screening of potential efficacy of dietary ginger on ethanol induced oxidative stress in rat cardiac tissue: A study on changes in basic metabolic profiles. ISSN: 0976-7126. Intern J of Pharm and Life Sci 4(5): 2649-2655. |
| [43] | Young, H.V., Luo, Y.L., Cheng, H.Y., Hsiech, W.C., Liao, J., and Peng, W.C. (2005) Analgesic and anti-inflammatory activities of [6]-gingerol. J Ethnopharmacol., 96: 207. |
| [44] | Mandal, S. (2010) Induction of phenolics, lignin and key defense enzymes in eggplant (Solanum melongena L.) roots in response to elicitors. African J of Biotechno 9(47): 8038-8047. Doi: 10.5897/AJB 10.984. |
| [45] | Nürnberger, T., Brunner, F., Kemmerling, B., and Piater, L. (2004) Innate immunity in plants and animals: striking similarities and obvious differences. Immunol. Rev. 198: 249-266. |
| [46] | Strange, R.N. and Scott, P.R. (2005) Plant disease: A threat to global food security. Annu Rev Phytophathol 43: 83-116. |
| [47] | Desender, S., Andrivon, D., and Val, F. (2007) Activation of defense reactions in Solanaceae: where is the specificity? Cell Microbiol. 9:21-30. |
| [48] | Mandal, S. and Mitra, A. (2007) Reinforcement of cell wall in roots of Lycopersicon esculentum through induction of phenolic compounds and lignin by elicitors. Physiol. Mol. Plant Pathol. 71: 201-209. |
| [49] | Sharma, V., Verma, R., and Sharma, S. (2012) Preliminary evaluation of the hepatic protection by pharmacological properties of the aqueous extract of Asparagus racemosus in lead loaded Swiss albino mice. Inern J of Pharm and Pharmaceut Sci 4(1): 55-62. |
| [50] | Deokar, A.B., Rajagaon, D.S., Manav, V. (1998) 125 Medicinal plants grown at foundation. 20-21. |
| [51] | Dey, A.C. (1980) Indian Medicinal Plants used in Ayurvedic preparations. 136-137. |
| [52] | Goyal, R.K., Singh, J., and Lal, H. (2003) Asparagus racemosus- An update. Indian J Med Sci 57: 408-414. |
| [53] | Sairam, K., Priyambada, S., Aryya, N.C., and Goel, R.K. (2005) Gastro-duodenal ulcer protective activity of Asparagus racemosus: an experimental, biochemical and histological study. J Ethanopharmacol. 86: 1-10. |
| [54] | Mandal, S.C., Kumar, C.K.A., Mohana-Lakshmi, S., Sinha, S., Murugesan, T., Saha, B.P., and Pal, M. (2000b) Antitussie effect of Asparagus racemosus root against sufur dioxide-induced cough in mice. Fitoterapia 71: 686-689. |
| [55] | Kamat, J.P., Boloor, K.K., Devasagayam, T.P., and Venkatachalam, S.R. (2000) Antioxidant properties of Asparagus racemosus against damage induced by gamma-radiation in rat liver mitochondria. J Ethanopharmacol 71: 425-35. |
| [56] | Mandal, S.C., Nandy, A., Pal, M., and Saha, B.P. (2000a) Evaluation of antibacterial activity of Asparagus racemosus Wild root. Phytother Res 14: 118-119. |
| [57] | Hasimun, P., Suwendar, G., and Ernasaria, I. (2014) Analgesic activity of Papaya (Carica papaya L.) leaves extract. Procedia Chem 13: 147-149. Doi: 10.1016/j. proche.2014.12.019. |
| [58] | Owoyele, B.V., Adebukola, O.M., Funmilayo, A.A., Soladoye, A.O. (2008) Anti-inflammatory activities of ethanolic extract of Carica papaya leaves. Inflammopharmacol 16(4):168-173, Doi: 10.1007/s10787-008-7008-0. |
| [59] | Gill, L.S. (1992) Ethnomedical uses of Plants in Nigeria. Union Press Benin Nigeria. |
| [60] | Mikhal′chik, E.V., Ivanova, A.V., and Anurov, M.V. (2004) wound healing effect of papaya-based preparation in experimental thermal trauma. Bull Exp Biol Med 137: 560-562. |
| [61] | Yusha, U.M., Onuorah, F.C., and Murtala, Y. (2009) In-vitro sensitivity pattern of some urinary tract isolated to Carica papaya leaves extract. Bayero J of Pure and App Sci 2(2): 75-78. |
| [62] | Olajide, O.A., Makinde, J.M., and Okpako, D.T. (2003) Evaluation of the anti-inflammatory property of the extract of Combretum micranthum. G. Don (Combretaceae). Inflammopharmacol 11: 293-298. |
| [63] | Eno, A.E., Owo, O.I., and Itam, E.H. (2000) Blood pressure depression by the fruit juice of Carica papaya (L.) in renal and DOCA induced hypertension in the rat. Phytotherapy Res 14: 235–239. |
| [64] | Kamalakannan, N. and Prince, P.S. (2006) Anti-hyperglycaemic and antioxidant effect of Rutin, a polyphenolic flavonoid, in streptozotocin – induced diabetic Wister rats. Basic and Clin Pharmacol Toxicol 98(1): 97-103. |
| [65] | Kim, H., Knog, H., Choi, B., Yang, Y., Kim, Y., Lim, M., Neckers, L., and Jung, Y. (2005) Metabolic and Pharmacological properties of rutin, dietary quercetin glycoside for treatment of inflammation bowel disease. Pharm Res 22(9): 1499-1509. |
| [66] | Fouad, A.A., Nermien, Z. Ahmed, and Dalia, A. Hashim (2012) Biochemical Studies of some natural antioxidants on diabetic rats. Advances in Food Sci 34(1): 6-13. |
| [67] | Nermien, Z.A. (2011) Anti-inflammatory effect of Some Natural Flavonoids on the Hepatic Lysosomal Enzymes in Rats. New York Sci J 4(8):6-14. |
| [68] | Stvolinskaya, N.S., Goncharenko, T.M., Krylova, O., Nikulina, S.E., Poliakova, E.D., and Korovkin, B.F. (1992) "The effect if insulin on the changes in acid phosphatase activity and cAMP level the primary monolayer culture of hepatocytes from new born rats during anoxia" Vorp Med Khim 36(4): 60-62. |
| [69] | Nermien, Z.A. (2003) Biochemical Studies of Some Antioxidant compounds on Subcellular Level, M.Sc. thesis in Biochemistry, Faculty of Agriculture, Cairo University. |
| [70] | De Duve, C. (1968) Lysosomes revisited. Eur J Biochem 137(3): 391-397. |
| [71] | Meijer, A.J. and Codogno, P. (2009) Autophagy: Regulation and role in disease. Crit Rev Clin Lab Sci. "PMID: 19552522" 24. |
| [72] | Abdel Gawad, S.M., El-Sayed, A.S., Teleb, Z.A., and Zeinab, Y.A. (2005) Drug – induced hepatotoxicity: Study the effect of sulphonylurea on the labilization of four marker lysosomal enzymes in rat liver in-vitro. J Drug Res 25(1-2). |
| [73] | De Duve, C. (1968) Lysosomes as targets for drugs. In: Campbell PN (ed) The interaction of drugs and subcelluar components in animal cells. pp. 155-169. |
| [74] | Subashini, R., Gnanapragasam, A., Senthilkumar, S., Yogeeta, S.K., and Devaki, T. (2007) Protective efficacy of Nardostachys jatamansi (Rhizomes) on mitochondrial respiration and lysosomal hydrolases during doxorubicin induced myocardial injury in rats. J of Health Sci 53(1): 67-76. |
| [75] | Sheeler, P. and Bianchi, D. (1987) In: "Cell and Molecular Biology". 3rd ed., Cpat.19, p. 467 – 469. John Wiley and Sons, Inc., U.S.A. |
| [76] | Bedevian, A.K. (1936) Illustrated pyglottic dictionary of plant names In: W. Lawrence Balls (ed.) pp 148-524. |
| [77] | Lee, J.C., Kim, H.R., Kim, J., and Jang, Y.S. (2002) Antioxidant property of an Ethanol extract of the stem of Opuntia Ficusindica var. Saboten. J Agric and Food Chem 50: 6490-6496. |
| [78] | Astudillo, L., Schmeda-Hischmann, G., Herrea, J.P., and Cortes, M. (2000) Proimate composition and biological activity of Chilean Prosopis species. J Sci Food Agri 80: 567-573. |
| [79] | Verzelloni, E., Tagliazucchi, D., and Conte, A. (2007) Relation between the antioxidant properties and the phenolic and flavonoid content in traditional balsam vinegar. Food Chem. 105: 564-571. |
| [80] | Tanaka, K. and Iizuka, Y. (1968) Suppression of enzyme release from isolated rat liver lysosomes by non–steroidal anti-inflammatory drugs. Biochem. Pharmacol 17: 2033 – 2032. |
| [81] | Robi,n C.R. and William, B.W. (1978) The temperature-dependence of the loss of latency of lysosomal enzymes. Biochem J .172: 163-173. |
| [82] | Van Hoof, F. and Hers, H.G. (1968) The abnormalities of lysosomal enzymes in mucopolysaccharides. European. J Biochem 7: 34-44. |
| [83] | Younan, E.A. and Rosleff, F. (1974) Changes of lysosomal enzymatic activities in human skin fibroblasts at various passages. J Drug Res Egypt 6(3): 137-139. |
| [84] | Dimitrios, K.P., Constantin, E. and Harrala, C. (2000) Achemometric comparison of three taxa of Scabiosa L.S.1. Plant Biosys 134(1): 67-70. |
| [85] | Fernandez de Simon, B., Perez-Jlzarbe, J., Heranadez, T.C., and Estrella, I. (1990) HPLC study of the efficiency of extraction phenolic compounds. Chromatographia 30: 35-37. |
| [86] | Steal R.J. and Torry, J.W. (1980) Principles and procedures of statistics. A biochemical approach, 2nd MC. Graw Hill Inc., London. |
| [87] | Siddhuraju, P. (2007) Antioxidant activity of polyphenolic compounds extracted from defatted raw and dry heated tamarindus indica seed coat. Lebensmittel-Wissenschaft and Technology 40: 982-990. |
| [88] | Liu, X., Dong, M., Chen, X., Jiang, M., Lv, X., and Yan, G. (2007) Antioxidant activity and phenolics of an endophytic xylaria sp. from Giunko biloba. Food Chem 105: 548-554. |
| [89] | Ma, X. and Gang, D.R. (2006) Metabolic profiling of in-vivo micropropagated and conventionally greenhouse grown ginger Zingiber officinale. J Phytochem 67(20): 2239-2255. |
| [90] | Sabina, E.P., Pragasam, S.J., Kumar, S., and Rasool, M. (2011) [6]-gingerol, an active ingredient of ginger, protects acetaminophen-induced hepatotoxicity in mice. J of Chinese Integ Med 9(11): 1264-1269. Doi: 10.3736/jcim20111116. |
| [91] | Baydar, N.G., Ӧzkan, G., and Yasser, S. (2007) Evaluation of the antiradical and antioxidant potential of Grape extracts. Food Control 18:1131-1136. |
| [92] | Siddhuraju, P. and Becker, K. (2007) The antioxidant and free radical scavenging activities of processed cowpea (Vigna unguiculata) (L.) seeds extracts. J Food Chem 101: 10-19. |
| [93] | Graziani, G., D′Argenio, G., and Tuccillo, C. (2005) Apple polyphenol extracts prevent damage to human gastric epithelial cells in-vitro and to rat gastric mucosa in-vivo. Gut 54: 193-200. |
| [94] | Zayachkivska, O.S., Konturek, S.J., and Drozdowicz, D. (2005) Gastro-protective effects of flavonoids in plant extracts. J Physiol. Pharmacol. 56(suppl.-1): 219-231. |
| [95] | Cowan, M.M. (1999) Plant products as antimicrobial agents. Clinical Microbiol Rev 12(4): 564-582. |
| [96] | Singh, B. and Bhat, T.K. (2003) Potential therapeutic applications of some antinutritional plant secondary metabolites. J Agric Food Chem 51: 5579-5597. |
| [97] | Lin, S.B., Wu, L.C., Huang, S.L., Hsu, H.L., Hsieh, S.H., Chi, C.W., and Au, L.C. (2000) In-vitro and in-vivo suppression of growth of rat liver epithelial tumor cells by antisense oligonucleotide against protein kinase C alpha. J Hepatol 33(4): 601-608. |
| [98] | Cany, J., Avril, A., Pichard, V., Aubert, D., Ferry, N., and Conchon, S. (2007) A transgenic mouse with β-galactosidase as a fetal liver self-antigen for immunotherapy studies. J. Hepatol 9: 20-24. |
| [99] | Teleb, Z.A., Abdel Gawad, S.M., Buthaina, S.S., and Madkour, M.K. (1990) Sub-cellular studies of Nephrotoxicity evoked by short chronic oral piroxicam medication in adult male albino rats. J Egypt Soc Toxicol 5: 29-36. |
| [100] | Guardia, T., Rotelli, A.E., Juarez, A.O., and Pelzer, L.E. (2001) Anti-inflammatory properties of plant flavonoids, effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco 56(9): 683-387. |
| [101] | Caruso, J.A., Mathieu, P.A., Joiakim, A., Zhang, H., and Reiners, J.J. (2006) Aryl hydrocarbon receptor modulation of tumor necrosis factor-alpha-induced apoptosis and lysosomal disruption in a hepatoma model that is caspase-8-independent. J Biol Chem 281(16): 10954-10967. |
| [102] | Honsi, T.G. and Strenersen, J. (2000) Activity and localization of the lysosomal marker enzymes acid phosphatase, β-N- acetyl glucosaminidase, and β-galactosidase in the earthworms Eisenia fetida and E. veneta comparative Biochemistry and physiology part B: Biochem And Mol Biol 125(3): 429-437. |
| [103] | Teleb, Z.A., Abd El-Gawad, S.M., El-Allawy, R.M., Abd El-Galil, F.M., and El-Sayed, A.S. (1998) Protective effect of two antihepatotoxicity agents: Silymarin and α-Tocopherol against the subcellular toxicity induced by the hypolipidemic agent etofibrate. J Drug Res Egypt 22:1 – 2. |
| [104] | European Pharmacopoeia (2010) Published by the direct-torate for the quality of medicine of council of Euro. Printed in Germany by Druckerei C.H. Beck, (EDQM). |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML