-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2016; 6(4): 91-96
doi:10.5923/j.ajb.20160604.01

Purification and Characterization of Highly Active Glucoamylase and Pre-Clinical Study of General Toxicology of Fungus Aspergillus oryzae UzMU K-14
K. T. Normurodova1, G. A. Muratov1, L. I. Alimdjanova2, B. A. Niyazmetov1
1Department of Biotechnology, Faculty of Biology, National University of Uzbekistan named after М.Ulugbek, Uzbekistan
2Tashkent Pharmaceutical Institute, Tashkent, Uzbekistan
Correspondence to: B. A. Niyazmetov, Department of Biotechnology, Faculty of Biology, National University of Uzbekistan named after М.Ulugbek, Uzbekistan.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

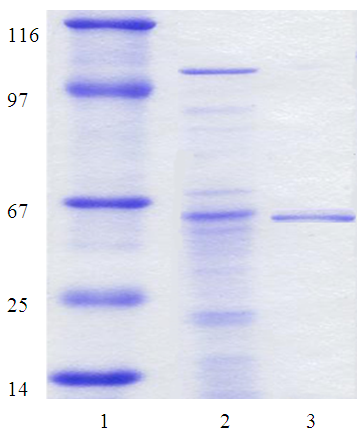
Highly activeand thermostable amylolytic enzymes are currently being investigated to improve industrial processes of starch degradation. In this study, we developed obtaining of highly activity fungi strain Aspergillus oryzae UzMU K-14 producers of thermostability glucoamylase and studied some cultural-morphological properties got strain, the method fungi is received allowing get in laboratory condition, purification andcharacterization of a thermostable glucoamylase from Aspergillus oryzae UzMU K-14. A highly active extracellular glucoamylase (exo-1,4-a-D-glucanohydrolase, E.C.3.2.1.3) from the culture supernatant of a thermophilic fungus Aspergillus oryzae UzMU K-14was purified by gel-filtration, gel electrophoresis (SDS PAGE) homogeneity by using ammonium sulfate fraction. Enzyme was purified until homogeneous electrophoretically by gel-filtration over HW-55 TSK-gel with specific activities of 198.5 U/mg for glucoamylase.SDS-PAGE of the purified enzyme showed a single protein band of molecular weight 56 kDa. The glucoamylase exhibited optimum catalytic activity at pH 6.0 and 65-70°C. It was thermostable at 50°C and 60°C, and retained 80% activity after 30 min at 60-65°C. The half-life of the enzyme at 85°C was 20 min. Different metal ions showed different effects on the glucoamylase activity. Ca2, Mg2, Na, and K enhanced the enzyme activity, whereas Fe2, Ag, and Hg2 cause obvious inhibition. Acute toxicity of Aspergillus oryzae UzMU K-14 culture liquid on rats with oral and dermal introduction, as well as cumulative and local irritant effect (subacute toxicity) in mice is studied. It was determined that the strain of fungus is non-toxic. These properties make it applicable to other biotechnological purposes and to improve industrial processes of starch degradation.
Keywords: Aspergillus oryzae, Microorganism, Fungi, Degradation, Protein, Characterization, Purification, Cultural-Morphological properties, Toxicology, Glucoamylase, Ion-Exchange, Gel-Filtration
Cite this paper: K. T. Normurodova, G. A. Muratov, L. I. Alimdjanova, B. A. Niyazmetov, Purification and Characterization of Highly Active Glucoamylase and Pre-Clinical Study of General Toxicology of Fungus Aspergillus oryzae UzMU K-14, American Journal of Biochemistry, Vol. 6 No. 4, 2016, pp. 91-96. doi: 10.5923/j.ajb.20160604.01.
Article Outline
1. Introduction
- Glucoamylase is an exo-hydrolase, exo-acting enzyme that removes the β-D-glucose units from the non-reducing ends of starch and related oligo- and poly-saccharides, amylose, amylopectin and glycogen by hydrolyzing α-1, 4 linkages in a consecutive manner, and producing D glucose as the sole product. It also hydrolyzes α-1, 6 and the rare α-1, 3 linkages although at a much slower rate [1]. Glucoamylases are an important group of enzymes in starch processing. They are second to protease in worldwide distribution and sales among industrial enzymes. Glucoamylases find many applications in industry. This enzyme is used in dextrose production, in the baking industry, in the brewing of low-calorie beer and in whole grain hydrolysis for the alcohol industry [2]. The primary commercial application of glucoamylases is the production of glucose syrups from starch [3]. The primary commercial application of glucoamylases is the production of glucose syrups from starch. A number of glucoamylases from cultures of various microorganisms, including Aspergillus, Rhizopus, and Saccharomyces species have been screened for potential industrial application. In recent years glucoamylases from thermophilic fungi, which are expected to be thermostable, have aroused increasing attention among researchers. Various methods for isolating highly purified and homogeneous forms a fungal glucoamylase have been reported [4-6].In the world practice as producers of commercial preparations of highly active and highly specific glucoamylase most widely used strain of Aspergillus fungi [7]. Here, we describe the purification and biochemical characterization of thermostability glucoamylase from Aspergillus oryzae UzMU K-14. The strain Aspergillus oryzae UzMU K-14 differed from the previously studied strains glucoamylase activity and in certain cultural sinnatures [8-9]. Earlier we obtained by screening high-yield strain of fungus Aspergillus oryzae UzMU K-14 producing glucoamylase [10]. The aim of this work is to study the general toxicology of fungus Aspergillus oryzae UzMU K-14. The objective of study included: 1. Determination of acute toxicity by oral and dermal introduction. 2. The study of cumulative effects (subacute toxicity) in mice. 3. Definition of locally irritating.
2. Materials and Methods
2.1. Grown Culture
- We used mesophilic spore-forming Aspergillus sp. fungus UzMU K-14, isolated from the rhizosphere of rice grown in the Kashkadarya region and preserved laboratory at number № UzMU K-14 in the culture collection of microorganisms of the National University of Uzbekistan named after М.Ulugbek, starter culture used for the production of alcohol by local grows optimally at 45-50°C and secretes a significant amount of glucoamylase at pH 6.0 into the culture media. For the production of the enzyme, actively growing fungal mycelium was an innoculation (0.25%) of the nutrient medium was transferred to optimized fermentation medium and grown for 120 h at 45-50°C on a rocker rotating at 150 rpm, to choose the optimal liquid medium for fermentation 250 ml Erlenmeyer flasks with 50 ml liquid medium containing the following (g/L): maize flour, 30.0; wheat bran, 5.0; yeast extract, 3.0; (NH4)2HPO4, 5.0; MgSO4, 3.0; CaCL2, 0.5, KCL, 0.5. The culture fluid was filtered and centrifuged at 8000 rpm for 20 min, 4°C, and the supernatant was used as a crude enzyme extract preparation.
2.2. Enzyme Assay
- Determination of glucoamylase activity was carried out according to GOST - 20264.4-89. One unit of glucoamylase activity adopted by the ability of the enzyme to catalyze the hydrolysis of soluble starch at 50°C and pH 6.0 for 1 min releasing 1 micromol of glucose.
2.3. Determination of Protein
- Protein concentration was determined by the Lowry method. As protein standard bovine serum albumin was used. In the fractions obtained after chromatography, the protein was determined spectrophotometrically by absorbance at 280 nm using a spectrophotometer SF-26 “Lomo” [11].
2.4. Pre-Clinical Study of General Toxicology
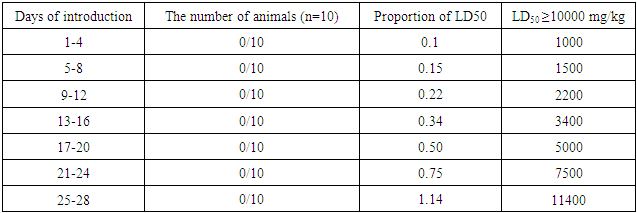
- Fungus Aspergillus oryzae UzMU K-14 was transferred from the National University of Uzbekistan named after M. Ulugbek to the laboratory of Pharmacology IBOC (Institute of Bio-Organic Chemistry) to conduct pre-clinical studies of general toxicology.The general action and “acute” toxicity of Aspergillus oryzae UzMU K-14 was determined on 36 white outbred mice and on 12 white rats of both sexes weighing 25 ± 1.5 and 150 ± 10 g after single, oral introduction. To define "acute" toxicity method of Litchfield and Wilcoxon [12] was used, and dermal application method of Noakes and Sanderson at seven animals per group [12] was used. The determination of Aspergillus oryzae UzMU K-14 cumulation was conducted by method of Lim et al. in 10 mice and eye mucosa of 10 rabbits [13]. On the local irritant effect of Aspergillus oryzae UzMU K-14 was judged by the impact of the fungus on the skin of 20 rats [14].
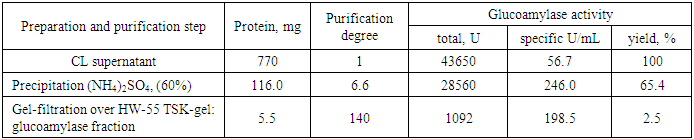
2.5. Isolation, Purification and Physic-Chemical Characterization of the Enzyme
- Fungal cells were separated from cultural liquid (CL) by centrifugation at 8000 rpm for 20 min. CL was precipitated using of ammonium sulphate 60%. Fractional salting out of enzyme protein with ammonium sulfate was carried out at 4°C for 24 hours. The precipitate was collected by centrifugation the solution at 8000 rpm/min for 20 min. Desalting was performed with the enzyme solution by dialysis first against a 0.05 M phosphate buffer (pH 6.0) and then against distilled water for 24 hours. The precipitate obtained after 60% saturation of ammonium sulfate was dissolved in 3 ml of 0.05 M phosphate buffer and used for further purification of glucoamylase.Gel-filtration of the active protein fraction after precipitation of CL by (NH4)2SO4 was performed on a column (20×600 mm) packed with HW-55 TSK-gel (Toyopearl, Japan) equilibrated with potassium phosphate buffer (0.05 M) containing KCl (0.1 M) at pH 6.0 with elution rate 48 mL/h and fraction volumes 1.5 mL.The homogeneity of amylolytic enzymes was monitored at all purification stages by electrophoresis in PAAG (12%) with SDS using the Laemmli method [15].
2.6. Optimum Temperature and Thermostability
- The optimum temperature of the enzyme was established by incubating the enzyme reaction mixture with substrate for 10 min at 20-90°C in phosphate buffer (0.05 M) at pH 6.0. Thermostability was investigated by incubating the enzyme at 40, 50, 60, 70, 80, and 90°C in the same buffer without substrate.
2.7. pH Optimum and pH Stability
- The optimum pH and pH stability of the studied enzyme toward soluble starch was determined in phosphatebuffer (0.05 M) at pH 3-10 and 50°C. Enzyme without substrate at different pH values of phosphate buffer (0.05 M) was incubated for 30 min after which the activity toward soluble starch was determined.
2.8. Effects of Various Metal Ions on the Enzyme Activity
- The effects of various metal ions on enzyme activity were determined in phosphate buffer (0.05 M) at pH 6.0 and metalion concentrations of 1 mM.
3. Results and Discussion
- The methods screening has been obtained for production of high activity strain Aspergillus sp. UzMU K-14, which produces thermostability glycoamylase, which allows obtaining glycoamylase with activity up to 170.6 U/mlL in laboratory scale. Aspergillus sp. fungus UzMU K-14, which is isolated from the sources of the local region of the Republic of Uzbekistan, isolated from the rhizosphere soil of wheat Kashkadarya region starter culture used for the production of alcohol by local grows optimally at 45-50°C and secretes a significant amount of glycoamylase at pH 6.0 into the culture media. By studying the morphology and physiology of Aspergillus sp. UzMU K-14 fungi it this determined that it belongs to Aspergillus oryzae. It this also determined the conidial heads of Aspergillus sp. UzMU K-14 develops more tightly and length of gifs consist about 120-400 х 20-40 mkm, and colony haslight color (Fig. 1).
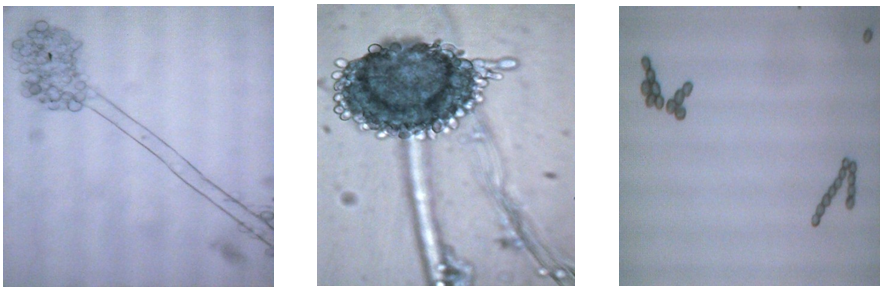 | Figure 1. Aspergillus sp. UzMU K-14 strains of development |
|
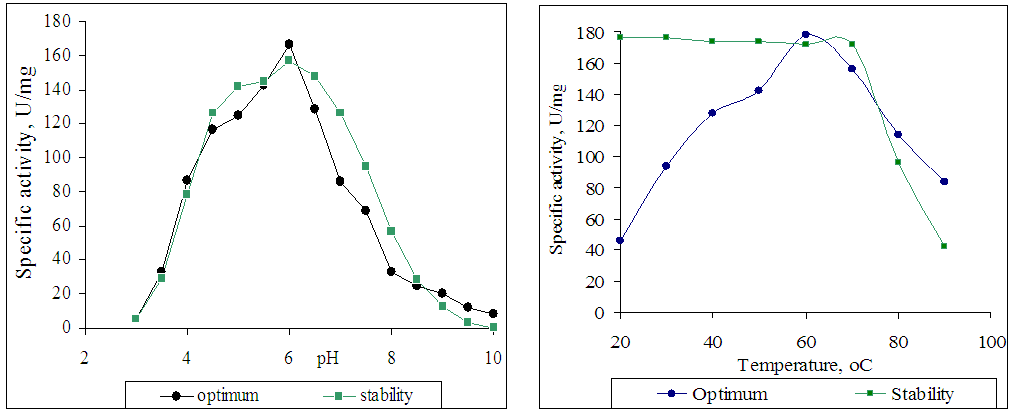 | Figure 3&4. Effect of pH and of temperature on activity and stability of purified glucoamylase from Aspergillus oryzae UzMU K-14 |
|
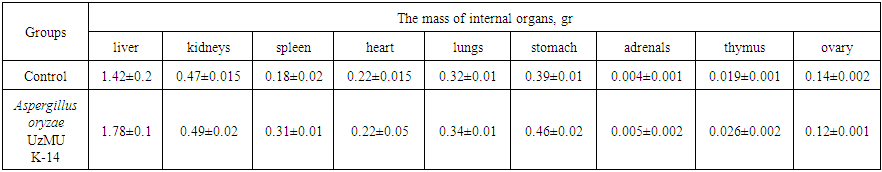 | Table 3. Averages mass of internal organs of mice after oral introduction of Aspergillus oryzae UzMU K-14 (M±m; n = 10; P <0.05) |
4. Conclusions
- Thus, a purified enzyme preparation of glucoamylase from Aspergillus oryzae UzMU K-14 was prepared by sequential use of precipitation by ((NH4)2SO4, and chromatography over HW-55 TSK-gel and the results allow us to conclude that the Aspergillus oryzae UzMU K-14 is not an irritant to the skin and conjunctiva of the animal’s eyes.
ACKNOWLEDGMENTS
- This work was supported by the Department of Biotechnology, Faculty of Biology, National University of Uzbekistan named after М.Ulugbek and Tashkent pharmaceutical institute.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML