-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2016; 6(3): 82-90
doi:10.5923/j.ajb.20160603.03

Rosmarinic Acid Attenuates the Hepatotoxicity Induced by Ethanol in Rats
Mahgoub M. Ahmed
Molecular Drug Evaluation Department, National Organization for Drug Control and Research (NODCAR), Giza, Egypt
Correspondence to: Mahgoub M. Ahmed, Molecular Drug Evaluation Department, National Organization for Drug Control and Research (NODCAR), Giza, Egypt.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Rosmarinic acid (RA), a natural polyphenolic compound, shows some biological activities including antioxidant and anti-inflammatory activity. The objective of the present study is to investigate the protective effect of orally administered rosmarinic acid (RA) on ethanol (EtOH)-induced hepatotoxicity in rat liver. Forty adult male albino rats were divided into four groups as control, EtOH (3 g/kg), RA (10 mg/kg) and RA+EtOH groups. RA was given one hour prior to EtOH by oral gavage for 28 days. The results showed that administration of EtOH caused a significant decrease (P<0.05) in serum total protein (TP) whereas the serum aminotransferase activities (ALT, AST) and lipid peroxidation (LPO) were increased following EtOH treatment. RA treatment significantly (P<0.05) ameliorated the previous parameters. Protein carbonyl (PC) and LPO levels were significantly (P<0.05) elevated in EtOH treated rat liver showing oxidative organ damage. Furthermore, catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GR), glutathione-S-transferase (GST) and reduced glutathione (GSH) were significantly (P<0.05) decreased in EtOH group. Moreover, cytochrome P450 2E1 (CYTP2E1) activity and gene expression were significantly enhanced in rat liver treated with EtOH. In EtOH group, gene expression ratios and contents of tumor necrosis factor-α (TNF-α) and interleukin-1beta (IL-1β) were significantly increased. However, RA pretreatment significantly inhibited all these adverse effects. The hepatoprotective activity of RA could be attributed to the suppression oxidative stress (PC and LPO), the down-regulation of TNF-α, IL-1β and CYP2E1 gene expressions and the improvement of CAT, GPx, GST and GSH in the liver tissues. In conclusion, oral administration of RA had a hepatoprotective role against EtOH-induce oxidative stress and inflammation in rat liver. However, the mechanism underlying the effects of RA requires further investigation and comparative human studies are also needed.
Keywords: Ethanol, RA, GSH, CYP2E1, IL-1β and TNF-α, Hepatoprotective, Rats, Antioxidant enzymes
Cite this paper: Mahgoub M. Ahmed, Rosmarinic Acid Attenuates the Hepatotoxicity Induced by Ethanol in Rats, American Journal of Biochemistry, Vol. 6 No. 3, 2016, pp. 82-90. doi: 10.5923/j.ajb.20160603.03.
1. Introduction
- Ethanol is hepatotoxic through redox changes produced by the NADH generated in its oxidation via the alcohol dehydrogenase pathway, which in turn affects the metabolism of lipids, carbohydrates, proteins and purines [1]. It is well known that heavy consumption of alcohol is associated with liver damage. Biochemical signs of hepatotoxicity and oxidative damage were displayed in experimental animals exposed to two-carbon alcohol. These signs suggested a possible role of free radicals in causing some of the toxic effects of alcohol [2]. About 80% of ingested alcohol is metabolized to the cytotoxic acetaldehyde by the enzyme alcohol dehydrogenase in the liver. It is well-known aldehyde oxidase or xanthine oxidase oxidizes acetaldehyde to acetate, giving rise to reactive oxygen species (ROS) via cytochrome P450 2E1 (CYP 2E1) [3]. Production of superoxide anions and hydroxyl radicals were increased after ethanol consumption. These radicals react rapidly with biological materials, causing oxidative damage in living organisms [4]. Excessive alcohol consumption enhances hepatic oxidative stress due to increased generation of reactive oxygen species and reduced antioxidant capacity [5]. ROS from xenobiotics, including ethanol, cause oxidative DNA damage through single-strand breaks [6].Oxidative stress increases lipid peroxidation, which can cause membranes damage of cells and organelles leading to the release of reactive aldehydes with potent pro-inflammatory and pro-fibrotic properties [7]. Development of new substances that have hepatoprotective effects against ethanol-induced liver toxicity is a prevailing strategy for preventing the progression of alcoholic liver impairment [8]. Natural products are one of the best ways to minimize ethanol hepatotoxicity.Rosmarinic acid (RA) is an ester of caffeic acid present in many medicinal of the plant including basil, sage and rosemary. Rosmarinic acid (RA) is a water-soluble polyphenolic compound [9]. Various biological activities are attributed to RA, including antioxidant [10], anti-inflammatory [11], anti-depressive [12] and anticancer activities [13]. In addition, RA has Protective effects against gamma irradiation [14].
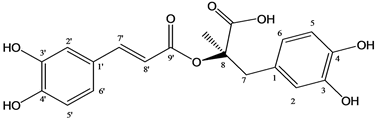 | Figure 1. Chemical structure of the rosmarinic acid |
2. Materials and Methods
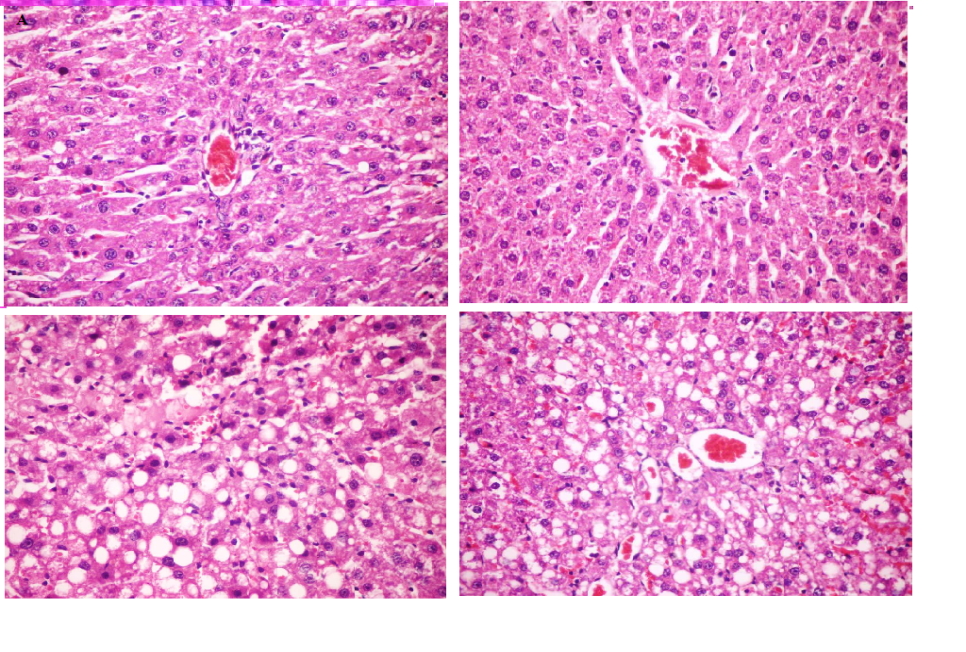
- Chemicals and reagentsRosmarinic acid, glutathione (GSH), thiobarbituric acid (TBA) 2,2-dinitrophenyl hydrazine (DNPH) and guanidine hydrochloride were procured from Sigma Chemical Co. (St. Louis, MO, USA). Griess reagents were purchased from Merk-Schuchardt Chemical Company (Hohenbrunn, Germany), with purity of 99%. All other chemicals were of analytical grades.AnimalsMale albino rats (160±20g) were obtained from the laboratory stock colony of National Organization for Drug Control and Research (NODCAR), Egypt. The animals were kept under normal environmental conditions for one week before the initiation of the experiment. They were allowed free access to water and were fed on a basal diet. They were kept individually in stainless steel cages at air condition 20-22 °C and a relative humidity of about 55%.Experimental designThe rats were allotted in four groups of ten rats each and the treatment was given as follows:1- Control Group received 0.5 ml saline orally by gavage once a day, for 28 days.2- RA Group, received 10 mg/kg in normal saline, orally once a day, for 28 days [15].3- Ethanol Group received 3 g/kg in normal saline, orally by gavage once a day, for 28 days [16].4- RA+Ethanol Group received 3 g/kg ethanol one hour after treatment of RA (10 mg/kg), orally by gavage once a day, for 28 days.Blood samples were collected and kept at room temperature for 1h, then centrifuged at 3000 rpm/30min and the separated serum was used for estimation of AST and ALT activities [17] and TP content [18] using Reactivos GPL Kits, Barcelona, España. LPO contents were determined according to Ohkawa et al. [19]. Preparation of liver homogenatesRats were sacrificed by cervical dislocation to obtain livers then washed with cooled saline (0.9 %). Livers were homogenized in ice-cold 1.15% KCl with a Potter-Elvehjem glass homogenizer to prepare 10 % w/v homogenate. The homogenates were centrifuged at 10,000×g for 20 min at 4C (Cooling centrifuge, Sigma-3K30, Germany) to obtain the supernatants.Assessment of oxidative stress status in the liver tissuesLevels of protein carbonyls (PC) were determined according to Levine et al. [20]. Lipid peroxidation (LPO) was determined as described by Ohkawa et al. [19].Determination of antioxidant enzyme activities and GSH content in the liver tissues The activity of CAT was determined by the method of Sinha [21]. CAT activity is expressed as µmol of H2O2 utilized per minute per mg protein. Glutathione reductase (GR) activity was assayed using the method of Carlberg and Mannervik [22] and the values are expressed as µmol of NADPH oxidized per minute per mg protein. Glutathione peroxidase (GPx) activity was measured by the method of Rotruck et al. [23]. GPx activity is expressed as µmol of GSH utilized per minute per mg protein. GSH levels were assayed in tissue homogenates according to the method of Ellman [24]. The activity of GST was estimated in the liver tissues using the method of Habig et al. [25].Assessment of pro-inflammatory markers in the liver tissuesLevels of IL-1β and TNF-α in rat liver were quantified using ELISA kits (Glory Science Co., Ltd, USA) according to the manufacturer’s instructions and guidelines. Isolation of liver microsomes Microsomes were isolated using the procedure described by Chang and Waxman [26]. Measurement of CYP2E1 activity CYP2E1 activity was measured in liver microsomal fraction as described by Chang et al. [27]. Determination of protein content Protein content in the homogenate and microsomal fractions was estimated by the method of Lowry et al. [28].Quantitative real time PCR (qRT-PCR)TNF-α, IL-1β and CYP2E1 relative gene expressions in the liver tissues were detected by quantitative real time PCR (qRT-PCR):-RNA Isolation and Reverse TranscriptionBriefly, the heart tissues (100 mg) were homogenized in 1 ml ice-cold TRIzol reagent 1 ml ice-cold TRIzol reagent following the manufacturer's instruction. The RNA quality was verified using spectrophotometric and agarose gel electrophoresis. cDNA was synthesized from 2 µg total RNA using RevertAidTM first strand cDNA synthesis kit (Ferments life science, Thermo Scientific, USA) by incubating at 37 °C for l h with reverse transcriptase with random hexanucleotides according to the manufacturer's instructions. -Quantitative Real Time PCRqRT-PCR was performed using the Real-Time PCR Systems (Step One instrument, Applied Biosystems, Foster City, CA, USA). Each 10 µl reaction contained 5 µl SYBR Green Master Mix (Applied Biosystems), 0.3 µl gene-specific forward and reverse primers (10 µM), 2.5 µl cDNA and 1.9 µl nuclease-free water. The relative expression of the studied genes was calculated using the comparative threshold cycle method. Histopathological analysis Liver sections were fixed in 10% formalin solution, dehydrated with a sequence of ethanol solutions and embedded in paraffin. Fixed tissues were sliced at a thickness of 5–6 µm and stained with hematoxylin and eosin. Statistical analysisThe values were expressed as the mean±SE for the 10 rats in each group. Differences between groups were assessed by one-way analysis of variance (ANOVA) using the statistical package for social sciences (SPSS) software package for Windows (version 13.0). Post hoc testing was performed for intergroup comparisons using the least significant difference (LSD) test. A value corresponding to P<0.05 was considered statistically significant.
3. Results
- Effects of RA on body weight, liver weight, AST, ALT, TP and LPO There were no significant differences in weight gain among all groups (Table 1). Liver weight was significantly increased (P<0.05) in the ethanol-only group (Table 1) versus to control group. The activities of ALT and AST and LPO level in serum were significantly increased in the EtOH-treated group when compared with those of the control group (P<0.05). RA treatment decreased their levels significantly (P<0.05) compared with EtOH-treated group (Table 1). Serum TP level was significantly decreased in the EtOH-treated group com-pared with those of the control group. RA treatment significantly (P<0.05) improved TP level in rats treated with EtOH when compared to the EtOH group (Table 1).
|
4. Discussion
- In the present study, we evaluated the protective effect of RA against ethanol-induced hepatotoxicity. The liver exposure a variety of hepatotoxins, such as excessive alcohol intake, heavy metals and organic and inorganic solvents resulting in excessive generation of free radicals which cause hepatotoxic lesions including acute hepatitis, cirrhosis, portal fibrosis and hepatic carcinoma [29].In our study, administration of ethanol caused significant increase in AST and ALT activities and decreased total protein. The elevation of AST and ALT activities due to hepatocytes damage caused by the ethanol where the leakage of cell membrane participated in the accumulation of these enzymes into the plasma [30]. RA reduced the level of these enzyme markers, which could be attributed to its antioxidant activity [31]. RA reduces liver injury induced by D-galactosamine and LPS in mouse, due to scavenging of superoxide molecules [32]. The polyphenolic compounds such as RA are potent scavengers of superoxide, hydroxyl, peroxyl and peroxy-nitrite radicals, they chelate redox-active metals and they can protect cell membranes against oxidative attack [33].The importance of oxidative stress in the development of alcoholic liver disease has long been appreciated. When the antioxidant defense systems impaired, the oxidative stress may produce lipid peroxidation, protein carbonyl formation and antioxidant enzymes inactivation [34]. In the present work, the levels of PC and LPO in ethanol treated rats increased, whereas GSH content and GST activity decreased. These results are in agreement with other studies demonstrated that ethanol significantly increased LPO level and decreased GSH content in the liver and kidney of ethanol treated rats [35]. Ethanol administration induces hepatic oxidative stress due to increased generation of reactive oxygen species (ROS) and/or reduced antioxidant capacity [36]. ROS increase lipid, protein and DNA peroxidation results in hepatocyte injury [37]. Free radicals can also lead to the formation of protein/protein cross-linkages, oxidation of protein back-bone resulting in protein fragmentation and modification of amino acid side chains, which includes oxidation of sulphydryl moieties and formation of protein carbonyls [38]. Plant antioxidants are generally known to possess phenolic moiety, which can easily donate electrons to reactive free radicals because of the resonance stability of phenoxy radical and thus retard radical chain reactions. RA possesses strong antioxidant property due to its intramolecular hydrogen bond effect after H-abstraction [39]. Thus, the potent antioxidant properties of RA may have contributed to its hepatoprotective effect against EtOH. CAT is responsible for the detoxification of H2O2 to water. GPx are another group of enzymes capable of reducing hydroperoxides, including lipid hydroperoxides, using glutathione (GSH) as substrate. The glutathione disulphide (GSSG), oxidized form of GSH, is reduced by glutathione reductase (GR). Our results showed that CAT, GPx and GR activities were significantly decreased in rats treated with EtOH alone. This could be because initiation of oxidation process results in the elevation of ROS. These results are in agreement with Panda et al. [40] who found that ethanol-depleted levels of GSH, SOD, CAT, GPx and GR. Supplementation with RA enhanced the activities of CAT, GPx and GR in the liver tissues. This might be because RA acts as an antioxidant thereby sparing the activities of CAT, GPx and GR, endogenous antioxidants. It has been showed that RA produced significant increased in the activity of SOD, CAT and GPx [41]. The current results showed that EtOH enhanced the activity of phase I enzyme, CYP2E1 and decreased the activity of the phase II enzyme, GST. In addition, GSH was decreased after EtOH treatment. RA supplementation significantly reduced the activity of phase I and increased the activity of phase II enzyme and GSH content as well. Phase I metabolizing enzymes are primarily the microsomal mixed function oxidases and the polar groups of chemicals become substrates for the conjugation by phase II enzymes.The phase II metabolic enzymes are related to conjugation and formation of glucuronides, glutathione conjugates and sulphates. GST activity was decreased in EtOH treated rats, which could be most likely due to the decreased availability of GSH, whereas supplementation with RA increased the activity of GST near normal. GSH reacts with numerous activated carcinogens via catalysis by glutathione-S-transferases [42] to make them more excretable and less toxic. GSH reacts with superoxide radicals, hydroxyl radicals and singlet oxygen, followed by formation of oxidized glutathione (GSSG) and other disulphides [43]. RA restored GSH level in EtOH treated animals and protected GSH from depletion.Cytochrome P450 (CYP450), a phase I drug-metabolizing enzyme, is one of the major enzymes involved in the activation of carcinogens [44]. CYP2E1 is a key member of CYP450 superfamily and plays an important role in metabolizing low-molecular hydro-phobic chemicals [45]. Our results demonstrated that CYP2E1 activity was elevated by ethanol and this effect is in agreement with Lieber [46, 47]. CYP2E1 can oxidize ethanol and generate reactive products from ethanol oxidation, for example acetaldehyde and the 1-hydroxyethyl radical. Ethanol oxidation can also generate ROS such as the superoxide anion radical and H2O2 [48]. Therefore, the induction of CYP2E1 may be partly responsible for the toxicity of ethanol. Administration of RA with ethanol diminished CYP2E1 activity in liver tissues. Sharmila and Manoharan [49] showed that oral administration of RA reduced CYP 450 and CYT b5 (phase I), whereas increased GST (phase II) in mice liver. In addition, Domitrović et al. [50] revealed that RA administration reduced cytochrome P450 2E1 (CYP2E1) in rats treated with cisplatin. Rosmarinic acid inhibited some metabolic enzymes including GST isoenzymes [51]. On the contrary, it has been shown that RA had no modification of xenobiotic metabolizing enzymes (XME) [52]. Phenolic acids can modulate carcinogen metabolism, particularly those in which CYP2E1 is involved [53]. RA prevents EtOH induced activation of CYP2E1 as well as the conversion of chemicals into carcinogens. Our results suggest that RA treatment might enhance the detoxification mechanism by modulating the phase I and phase II enzymes.IL-1β and TNF-α are known to be important cytokines linked to hepatocyte damage induced by chronic alcohol consumption [54]. Our study exerted that ethanol increased IL-1β and TNF-α levels in liver rats after four weeks in comparison with the control group. These results are in agreement with Yang et al. [55] who showed that the levels of inflammatory factors such as TNF-α and IL-6 were significantly increased by ethanol. In this study, administration of RA with ethanol protected the liver TNF-α and IL-1β levels. Therefore, RA treatment is helping in attenuating inflammation caused by ethanol. Rosmarinic acid reduced pro-inflammatory proteins, decreased neutrophil infiltration and less lung parenchymal and interstitial edema in animals exposure to diesel exhaust [56]. Genistein, a polyphenolic blocked the increase in mRNA of IL-1β, IL-6 and TNF-α produced by lipopolysaccharide-stimulated monocytes [57].
5. Conclusions
- The present study showed that the RA had potent hepatoprotective effects against ethanol-induced hepato-toxicity in rats. RA inhibited the hepatic damage accompanied by decreased activity of serum liver enzymes. Treatment with RA resulted in restoration of GSH, CAT, GR, GPx and GST, the antioxidant defense system, which was impaired by ethanol exposure and suppressed LPO and PC. Furthermore, the protective effect of RA demonstrated by the significant reduction of TNF-α, IL-1β and CYP2E1 in liver tissues. Therefore, RA is a potential candidate for the prevention of ethanol-induced liver damage in rats.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML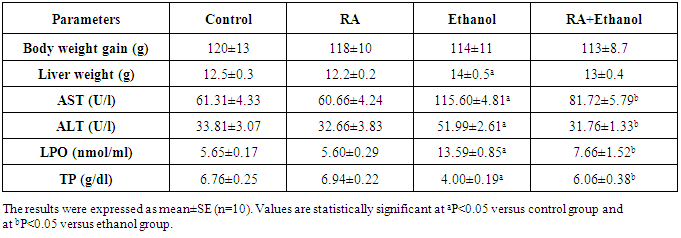
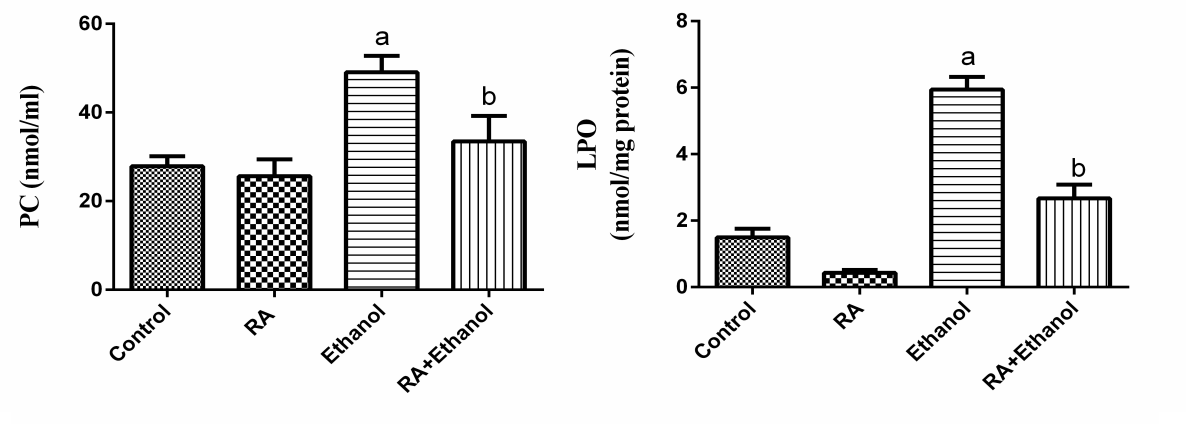
 versus control group and at
versus control group and at  versus ethanol group
versus ethanol group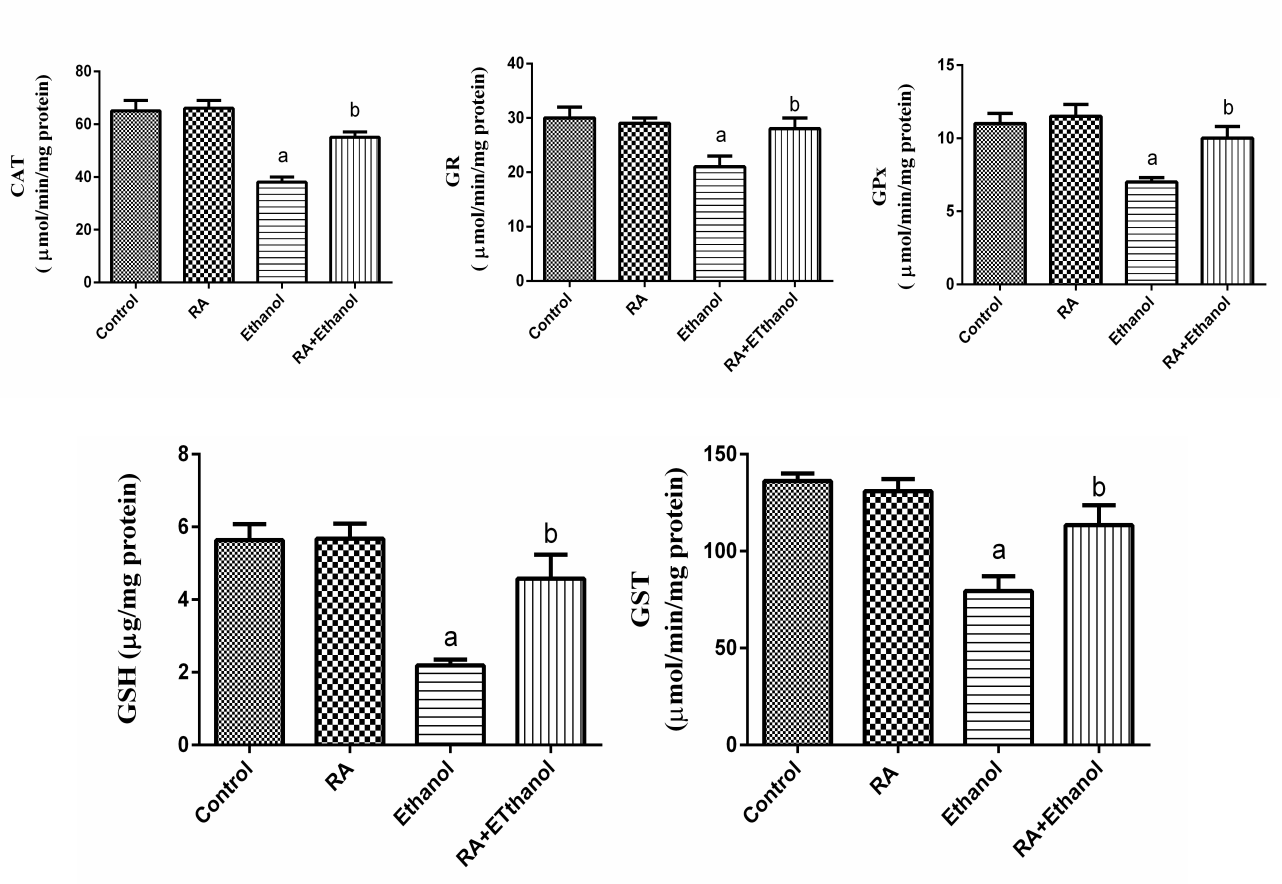
 versus control group and at
versus control group and at  versus ethanol group
versus ethanol group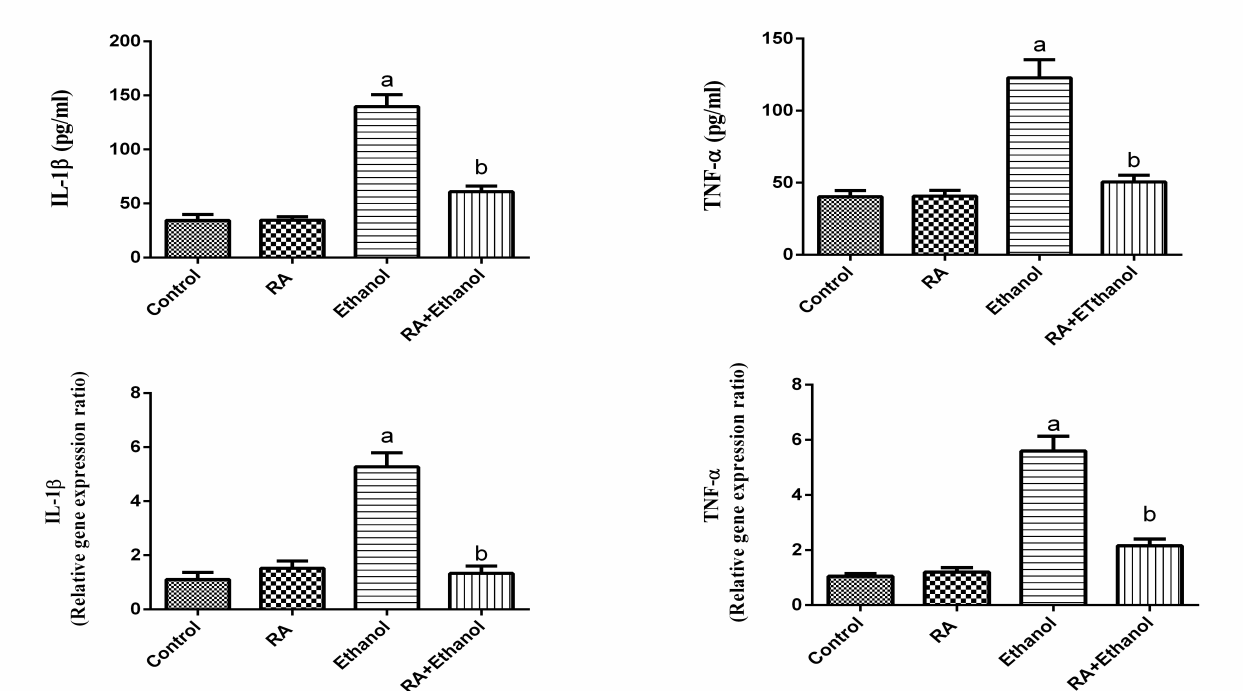
 versus control group and at
versus control group and at  versus ethanol group
versus ethanol group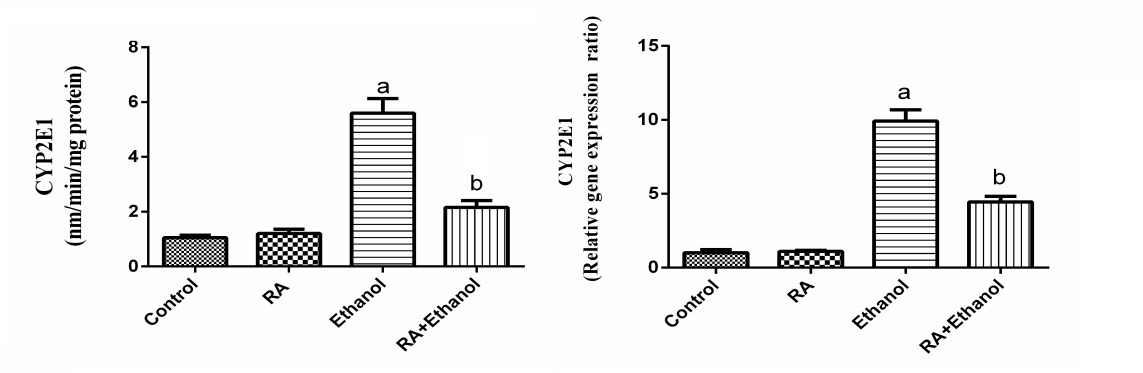
 versus control group and at
versus control group and at  versus ethanol group
versus ethanol group