-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2016; 6(3): 72-81
doi:10.5923/j.ajb.20160603.02

Detection and Isolation of Novel Lectin Gene from Piliostigma thonningii Seeds
Oluchi Ekwutosi Nwosu, Hajiya Mairo Inuwa, Elewechi Onyike, Dingwoke John Emeka Francis
Department of Biochemistry, Ahmadu Bello University, Zaria, Nigeria
Correspondence to: Dingwoke John Emeka Francis, Department of Biochemistry, Ahmadu Bello University, Zaria, Nigeria.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Novel Lectin gene was isolated from the seeds of an indigenous plant Piliostigma thonningii (PTL) using Zymo Research Plant/Seed Kit. Agglutination reaction for confirmation of the lectin was performed using chicken erythrocytes. Galactose-specific lectin coding protein was detected by optimized Polymerase Chain Reaction using a primer pair LEC5 (Forward) and LEC6 (Reverse). The lectin was partially purified under a working condition of phosphate-buffered saline (PBS, pH 7.0) followed by ammonium sulfate precipitation and gel filtration on Sephadex G-75 column. Electrophoresis of the purified protein under denaturing conditions was performed on 12% Acrylamide gel using a tris-glycine buffer, pH 8.3. The crude protein of the plant seeds and the partially purified lectin were tested for antimicrobial activity against certain microorganisms. The PTL DNA was isolated using Zymo Research Plant/Seed Kit. The result revealed a 195bp product, confirming the presence of lectin gene in PTL seeds. The purified protein had an estimated molecular weight of 21kDa. The result revealed no antimicrobial activity against Staphylococcus aureus,Escherichia coli,Salmonella typhi, Bacillus subitilis and a fungi, Candida albicans. The novelty of this study presents lectin gene in an indigenous PTL and the use of conventional hemagglutination and Polymerase Chain Reaction, as sensitive and specific detection methods.
Keywords: Lectin gene detection, Piliostigma thonningii, Lectin, Piliostigma thonningii DNA
Cite this paper: Oluchi Ekwutosi Nwosu, Hajiya Mairo Inuwa, Elewechi Onyike, Dingwoke John Emeka Francis, Detection and Isolation of Novel Lectin Gene from Piliostigma thonningii Seeds, American Journal of Biochemistry, Vol. 6 No. 3, 2016, pp. 72-81. doi: 10.5923/j.ajb.20160603.02.
Article Outline
1. Introduction
- Lectins are carbohydrate-binding proteins that specifically recognize diverse sugar structures and mediate a variety of biological processes [1]. They are proteins, other than antibodies and enzymes, that bind specifically and reversibly to carbohydrates, resulting in cell agglutination or precipitation of polysaccharides and glycoconjugates. Lectins are ubiquitous in nature, found in all kinds of organisms, from virus to humans [2]. Hundreds of different lectins have been isolated from plants. About 1-10% of the total soluble protein of legume seeds is composed of lectins [3]. Generally, lectins are classified into five groups on the basis of their affinity for (i) Glucose/Mannose; (ii) Galactose and N-acetyl-D-galactosamine; (iii) N-acetylglucosamine; (iv) L-fucose, and (v) Sialic acid [4]. Based on evolutionary and structural relatedness, [5], placed the lectins into seven families: legume lectins, Chitin binding lectins, Type-2-ribosome-inactivating proteins (RIPs), monocot mannose binding lectins, Amaranthins, Cucurbittaceae phloem lectins and jacalin related lectins. Among all these the legume lectins are extensively studied and are present in several hundreds of species of legumes. However, almost all well-characterized Leguminosae lectins are from the Papilinoidae subfamily, and only few lectins from the Caesalpiniacea species have been reported, such as Bauhinia purpurea, Griffonia simplicifolia and Bauhinia variegata [6]. Piliostigma thonningii is a leguminous plant of sub-family Caesalpiniacea, comprising of trees, shrubs or very rarely scramblers. Different parts of P. thoningii such as the root and twig have been described as useful medicinal plant parts. They are used for the treatment of dysentery, fever, respiratory ailments, snake bites, hookworm and skin diseases [7]. Although lectins are associated with toxicity, their importance in cancer therapeutics, immunology, and antibacterial property cannot be neglected. Scientific research has shown that few of the peculiar characteristics of plants (anti-bacterial properties, cytotoxicity) are because of the presence of lectins and the understanding of these characteristics at molecular level could only be gained through investigation of their associated lectins [4]. Non availability of lectin protein militate its efficient use as a research tool. Although the preparation of plant lectin is in principle, relatively easy because powerful affinity chromatography can be included in the purification scheme, many lectin of interest cannot be prepared on a large scale for practical reasons [5]. In some cases the starting material is not available in sufficient quantities. For instance, the preparation of large quantities of the orchid lectin (which exhibits a very interesting specificity and has unique biological activities) is virtually excluded because these species are protected. In other cases, the lectin concentration in the plant materials is so low that the cost for the preparation of the purified proteins impedes their commercialization, as in the lectin from onions or leek [5]. Even when the lectin concentration in a given plant tissue is considerably high, the preparation of the pure protein may be very cumbersome because of the presence of large quantities of polysaccharides, phenolic compounds or interfering substances. The complex and costly procedure required for purification of these lectins makes them unattractive for large scale isolation. For instance, problems caused by high level of pectic substance and anthocyanin in elderberry fruits make a cost effective purification of the fruit-specific lectin virtually impossible [5]. Many food plants (especially legumes and cereals) contain lectins which are believed or suspected to have negative effect on food quality and safety. Only few lectins from the Caesalpiniacea species have been reported, such as Bauhinia purpurea [8], Griffonia simplicifolia and Bauhinia variegata [6]. Several plant lectins cannot be classified because of lack of sequence information. Novel lectin families will not be discovered if more sequence data is not made available.Lectin binds glycoconjugates regardless of their origin, hence an important tool in a wide variety of studies, including cell-cell recognition and cell activation [9], [10]. These potential uses have motivated the continued search for new lectins and for seeds to be prioritized for screening. Molecular cloning and analysis of plant lectin genes as well as the expression of lectins in heterologous systems are new developments offering interesting perspectives not only for a more efficient use of lectins but also for a novel application in other fields.This Study presents the detection and isolation of novel lectin gene in the DNA of Piliostigma thonningii seeds, using polymerase chain reaction and established antimicrobial activity of the purified lectin protein.
2. Literature Review
- Lectins are carbohydrate-binding proteins that specifically recognise diverse sugar structures and mediate a variety of biological processes [1]. They are proteins, other than antibodies and enzymes that bind specifically and reversibly to carbohydrates, resulting in cell agglutination or precipitation of polysaccharides and glycoconjugates. Lectins are ubiquitous in nature, found in all kinds of organisms, from virus to humans [2]. Hundreds of different lectins have been isolated from plants. About 1-10% of the total soluble protein of legume seeds is composed of lectins [3]. They are the natural partners of the various oligosaccharides that nature uses to "tag" cells via glycoproteins and glycolipids. Lectins play a role in important processes like infection, host defence, fertilization, cancer, protein transport and embryogenesis [11]. They are also useful in Biology, Histology and Medicine, as an important tool that aids the purification of glycoproteins or the determination of blood groups [12]. Lectin-carbohydrate recognition is one of the three fundamental biological recognition mechanisms, the two others being protein-nucleic acid recognition and protein-protein recognition. The term “hemagglutinins” or “phytoagglutinins” came into existence with the discovery of proteins possessing the ability to agglutinate erythrocytes. The first hemagglutinin capable of showing this property was reported in the castor bean extract [5]. In the 1940s, it was found that some of the hemagglutinins selectively agglutinated blood cells of a particular human blood group, therefore the novel term “lectin” was proposed by Boyd and Sharpleigh [13] for the class of carbohydrate-binding proteins, which selectively agglutinate erythrocytes. Toxic and therapeutics relevance of lectins is known since ages. Some lectins are resistant to the gut enzymes and do not break easily, they might bind to the wall of the gut and cause damage to the gut lining. This could be related to diseases such as colitis, Crohn’s disease, and Coeliac-Sprue. Years of research has shown that after the interaction of lectin with the intestine, it is edocyted and cause many disturbances in the systemic levels [4]. It was observed that the kidney bean lectin fed rats had thymus atrophy and it is speculated that this atrophy developed may be because of the unusual proliferation of bacteria in the gut, as the immunological system of the rats may have been depressed due to toxic effects of lectins. The ingestion of kidney bean lectin also disturbs the intermediary metabolism, leading to loss of weight, inadequate development and eventually death of the experimented rats [4]. Other diseases associated with lectins are insulin dependent diabetes, rheumatoid arthritis, IgA nephropathy and peptic ulcers. Lectins sensitivity could occur due to the failure of a certain type of barrier protection in the body, namely Secretory Immunoglobulin A (sIgA) barrier protection. It could be argued that lectins are specific to certain carbohydrates and when they attach to their specific carbohydrate substrate, they may damage the cell membrane and may damage the cell [4].Despite these toxic characteristics, lectins possess several important features as they are used in various biological fields. They are very useful in cell identification and separation, detection, isolation, and structural studies of glycoproteins, investigation of carbohydrates on cells and subcellular organelles [4]. Lectins are important in the mapping of neuronal pathways, mitogenic stimulation of lymphocytes, purging of bone marrow for transplantation and studies of glycoprotein biosynthesis [14]. Certain lectins are also used as carriers for the delivery of chemotherapeutic agents; they are also used in investigating cell surface receptors in various bacteria, protozoa and fungi. Lectins can also be used in determining bacterial cell wall components and bacteriophage receptors. Lectins are generally monoclonal proteins and they possess a spectrum of specificities and molecular weights, due to this, they are classified as substantial tools for diagnostic microbiology application [15]. One of the advantages of applying lectins in clinical microbiology is that cellular or surface receptor sites can be partially analyzed by hapten inhibition studies [15]. Unlike the production of antisera, which requires pre-treatment of microorganisms for antigen preparation followed by injection of the microorganism and glyconjugate into animals, such as rabbits leading to the absorption of antisera to eliminate nonspecific antibody reactions, lectins are simple to use, when they are conjugated to a histochemical label such as fluorescein, peroxidase, or colloidal gold, lectins may be used as histochemical probes to identify and localize specific carbohydrate residues in microorganisms by light or electron microscopy, as well as by blotting methods [15]. The cell binding property of lectins elicit a wide range of biological phenomena for instance, lectins have been used to fractionate animal cells, including B and T lymphocytes and also illustrates changes in cell surface architecture following virus infection or parasite infection [4]. They are very important and versatile tools and are applied as probes for fluorescence and electron microscopy as well as in gel diffusion assays. Immobilized lectins are used for affinity chromatography during the separation of glycoproteins as they have advantage over other purification techniques because elution can be pursued with a relatively inexpensive monosaccharide and the glycoprotein, which is to be purified, is not subjected to denaturation [4].Over the past few years, Lectins have been found to have anticancer properties. Several researches have shown the use of Lectins to inhibit tumor growth, especially by causing cytotoxicity, apoptosis and, down-regulation of telomerase activity and inhibition of angiogenesis. In addition, lectins have also been found to sequester the pool of available polyamines in the body; thereby help in thwarting cancer cell growth [16].The polymerase chain reaction (PCR) is a scientific technique in molecular biology to amplify a single or a few copies of a piece of DNA across several orders of magnitude, generating thousands to millions of copies of a particular DNA sequence. The polymerase chain reaction is a powerful technique that has rapidly become one of the most widely used techniques in molecular biology because it is quick, inexpensive and simple. The technique amplifies specific DNA fragments from minute quantities of source DNA material, even when that source DNA is of relatively poor quality [17]. The basic PCR principle is simple. As the name implies, it is a chain reaction: One DNA molecule is used to produce two copies, then four, then eight and so forth. This continuous doubling is accomplished by specific proteins known as polymerases, enzymes that are able to string together individual DNA building blocks to form long molecular strands. The isolation, amplification using suitable primers and sequence of lectin gene from plants especially legumes is beneficial, to understand their properties and biological role at molecular level. Lectins of therapeutics interests can be preserved and can be used in clinical research and healthcare therapies [17].
2.1. Piliostigma Thonningii (PT)
- Piliostigma thonningii is a leguminous plant belonging to the sub family Caesalpiniacea, a family that comprises of trees, shrubs or very rarely scramblers. The tree is perennial in nature and its petals are white to pinkish colour produced between November and April. The fruits, which are hairy, hard, flattish, rusty brown and woody splits at ripening and usually persistent on the tree, are produced between June and September [18]. Locally in Nigeria, the seed is called Okpoatu in Igbo, Kalgo in Hausa and Abefe in Yoruba. P. thonningii grows in open woodland and savannah regions that are moist and wooded grassland in low to medium altitudes. It is widely distributed in Africa and Asia. It is found growing abundantly as a wild uncultivated tree in many parts of Nigeria such as Zaria, Bauchi, Ilorin, Plateau, Lagos and Abeokuta [7]. The level of dietary fibre is quite high when compared with that of most legumes and seeds [19]. The high crude protein content of P. thonningii seed (30.33± 0.31%) coupled with the fact that it is abundant in this part of the world may encourage its use as high protein sources in some food formulations [7]. The moisture content of P. thonningii (6.71 ± 0.40%) is lower than that of most legume seeds [20], [21]. This implies that the shelf life for this seed will likely be longer than that of most legumes. The ash content of 3.50 ± 0.04% for this seed is comparable with that of other legumes which has been reported to range between 3.0 and 4.8% [19]. While the carbohydrate content (23.00 ± 0.24%) is at the lower side when compared with that of other legumes ranging from 23% in groundnut to 66% in Bambara groundnut [22], [23]. The phytochemical screening of the seed showed the presence of saponins, flavonoids, phenolics, glycosides, anthraquinones as well as cardiac glycosides; while tannins, steroids, phylobatannins and triterpenes were absent. Some of these chemical compounds have been reported to have inhibitory effects on some gram-negative bacteria such as Escherichia coli and Bacillus subtilis amongst others [7]. The presence of these chemical compounds therefore suggests the pharmacological activities of P. thonningii. The seeds of P. thonningii fruits have been reported to be eaten by African antelope and elephant while farmers in the lower Savanna region grind up the seed as fodder for cattle during winter months [7].
3. Materials and Method
- All the determinations were done in triplicates and all reagents used were of analytical grades. This research was done at the Mary Hallaway Laboratory, Department of Biochemistry and the Centre for Biotechnology Research Ahmadu Bello University Zaria, Kaduna State, Nigeria.
3.1. Plant Material
- The dried seeds of Piliostigma thonningii were freshly collected from Piliostigma thonningii tree in the Botanical Garden of the National Research Institute of Chemical Technology (NARICT), Zaria, Kaduna State, Nigeria. The seeds were authenticated by taxonomist at the herbarium section of Department of Biological Sciences, Ahmadu Bello University Zaria. The voucher specimen number (171) was deposited at the herbarium.
3.2. Chemicals, Reagents and Equipments
- Sephadex G-75 was purchase from Pharmancia (USA), Sodium dihydrogen phosphate, hydrogen disodium phosphate, sodium chloride, Ammonium Sulphate, Sodium Hydroxide, Ethylenediaminetetraacatic acid (EDTA), dithiothreitol (DTT), 2-mercaptoethanol were purchase from Sigma (USA), Sodium Dodecyl Sulphate, Protein Markers, N,N,N’,N’-tetramethylethylenediamine (TEMED) where purchased from Bio-Rad laboratories (USA), Mueller Hinton Agar (MHA) , Potato Dextrose Agar (PDA) were purchased from Oxoid (USA), Zymo Research Plant/Seed DNA kit, Agarose gel, Agarose (0.75g in 1x TAE BUFFER), Ethidium Bromide (10mg/ml), 1x TAE (0.04M Tris-Acetate, 0.002M EDTA, pH8.0), 6x loading buffer were purchased from Thermo Fisher Scientific(USA). All other reagent used was of analytical grades.Ultra Violet Trans-illuminator, microcentrifuge, UV-visible spectrophotometer, electrophoretic machine, Eppendorf centrifuge 5430R, weighing balance, pH meter, incubator, ultracentrifuge, Techgene Amplifier, Power source, UV protected Glasses were obtained at the Centre for Biotechnology Research, Ahmadu Bello University Zaria.
3.3. Sample Preparation and Extraction of Lectin
- The freshly collected dry seeds of Piliostigma thonningii were crushed to powder using Philip blender. 10g of the powdered seed was soaked separately in 30mls of 0.06M phosphate buffer saline at 4°C for 4 hours. The extract was dispensed into 5ml centrifuge tubes and centrifuged at 7500rpm, at 4°C for 20 minutes. The supernatant was decanted into a measuring cylinder, 1ml of the supernatant was stored in an Eppendorf tube and stored at 4°C, this served as the crude extract. Aliquots of the crude extract were taken for salting out Process [24].
3.4. Partial Purification of Lectin
- Following the ammonium sulphate salt chart, ammonium sulphate was added to 10mls of the crude extract with continuous stirring using a glass rod, until 80% saturation was obtained. The sample was allowed to stand for 1hr at 4°C; afterwards, it was centrifuged at 10000×g for 30minutes. The supernatant was discarded and the precipitate suspended in minimum volume of PBS and kept at 4°C [24]. Preparation of Sephadex G75 Matrix Column4.5g of Sephadex G75 was soaked in 100ml of 0.005M phosphate buffer saline pH 7. It was allowed to swell overnight. After swelling the top layer of buffer was decanted and the gel was packed into the column gradually to prevent formation of air bubbles. After packing the gel, it was washed with 100ml of the same buffer and the tap locked. Before loading the sample, the absorbance was taken at 280nm using Ultra Violet Spectrophotometer, the absorbance was <0.005 [25]. Gel Chromatography The 80% ammonium sulphate saturated seed extract sample was loaded on the column and eluted with Phosphate Buffered Saline pH 7. The eluates were collected in 50 fractions of 2ml each at a flow rate of 400µl per minutes and its O.D was determined at 280nm.The fractions containing lectin were pulled together. The lectin-containing fractions were determined on the basis of Abs280 and a detectable hemagglutination activity against chicken erythrocyte [25]. Molecular Mass Determination by Sodium Dodecyl Sulfate- Polyacrylamide Gel Electrophoresis Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out according to the method described by [26], using a 12% resolving gel and a 5% stacking gel. At the end of electrophoresis, the gel was stained with Coomassie brilliant blue. Sample Preparation for SDS-PAGE The sample containing lectin was prepared for SDS-PAGE by adding 20µl of the loading buffer (2.5ml of 1M Tris buffer pH 6.8, 4ml Glycerol, 0.8g SDS, 2ml β-mercaptoethanol, Bromophenolblue, and 10ml distilled water) to 5 µl of the partially purified lectin protein in a ratio of 1:4. The mixture was boiled for 10min and kept on ice until needed for loading. Preparation of SDS-PAGE Gel The separating gel (12%) was prepared to a final volume of 10 ml by adding; 3.35 ml deionized water, 2.5 ml of 1.5M Tris buffer pH 8.8, 0.1% SDS, 4ml of 30% Bis-acrylamide solution, 50µl of 10% Ammoniumpersulphate (APS) (0.1g/ml; freshly prepared), and 15µl N, N, N’, N’-tetramethylethylenediamine (TEMED). The solution was swirled to mix and the mixture was loaded immediately into the gel casting tray to prevent polymerization in the beaker. The stacking gel (4%) was prepared to a final volume of 5ml by adding; 3ml deionized water, 1.25ml of 0.5M Tris buffer pH 6.8, 50µl of 10% SDS, 665µl of 30% acrylamide, 25µl of 10% Ammoniumpersulphate (APS), and 10µl N, N, N’, N’-tetramethylethylenediamine (TEMED). It was layered carefully on the separating gel while ensuring there wasn’t any bubble formation and allowed to cast [27]. Loading, Running and Staining of the Sample The electrophoretic tank was filled with 1500ml of running buffer working solution consisting of 0.025M Tris (pH 8.3), 1.92M Glycine, and 0.1% SDS. Afterwards, the prepared sample (20µl) was dispensed into the gel well. Electrophoresis was carried out at 50V for 7hours. The gel was removed and stained overnight with Coomassie blue in a staining tray. The staining solution constitutes the following; 0.1% Coomassie blue G-250 in 50% methanol, and 10% acetic acid. This was followed by rinsing with destaining solution (10% methanol and 10% acetic acid) until the proper destaining of the gel was obtained, the protein bands were then visualized and the molecular weight estimated by comparing the electrophoretic mobility of the sample against the molecular mass marker protein [27]. The composition of the molecular weight markers used were Phosphorylase b 97,400 daltons, Serum albumin 66,200 daltons, Ovalbumin 45,000 daltons, Carbonic anhydrase 31,000 daltons, Trypsin inhibitor 21,500 daltons and Lysozyme 14,400 daltons.
3.5. Hemagglutination Assay
- The hemagglutination activity of Piliostigma thonningii lectin was determined by the addition of erythrocytes to the sample. The assay was carried out in a 96 well round bottom micro titre plate. The first well of the micro titre row served as positive control to which 50μl of plant sample and 50μl of blood was added and the last well served as negative control which contained 50μl of blood and 50μl of PBS solution. Between the positive and negative control each well contains blood, PBS and lectins. Initially, 50ul PBS was added to all the wells. Then 50μl of crude extract was pipetted to the first well and it was serially diluted till the negative control. Similar procedure was followed for the other samples. Finally 50μl of 5% washed red blood cells was pipetted into each well. The plate was then placed quietly on the laboratory bench for 2hrs, after which the haemagglutination was observed in the wells containing the crude extract [28], [29].
3.6. Antibacterial and Antifungal Assay
- The test organisms used were clinical isolates of bacteria and fungi obtained from the Department of Microbiology, Ahmadu Bello University, Zaria. The antibacterial and antifungal activity was carried out against the following isolates: Staphylococcus aureus, Bacillus subtilis (Gram-positive), Escherichia coli, Salmonella typhi (Gram-negative), and Candida albicans (Fungus). Various concentration of the crude sample ranging from 100% to 12.5% was prepared using sterile distilled water as a diluent [30]. Sensitivity test of the Protein was done using agar well diffusion. The bacterial and fungal isolates were standardized using 0.5 Macfarland turbidity standard. The standardized innocula were uniformly streaked into sterile petri-dishes containing sterile Mueller Hinton Agar (MHA) for bacteria and sterile Potato Dextrose Agar (PDA) for fungi growth. Four wells were punched on each agar plates with the aid of a sterile cork borer (8mm in diameter). The wells were properly labelled according to the number of different concentrations of the crude prepared (100%, 50%, 25%, and 12.5%). The punched wells were then filled up with the different concentration of the protein. The plates were allowed to stand on the bench for one hour for the protein to diffuse into the agar. The bacteria plates were incubated at 37°C for 24hrs, while the fungi plates containing Potato Dextrose Agar were incubated at room temperature for 3 days. After the incubation period, the plates were observed for zone of inhibition. The diameter of the zones was measured using a transparent ruler calibrated in millimetre [31].
 | Figure 1. Piliostigma thonningii showing leaves, pods and seeds |
3.7. DNA Extraction
- The Piliostigma thonningii lectin DNA was extracted with Zymo Research (ZR) Plant/Seed kit, following manufacturer’s protocol. Initially, beta mercaptoethanol was added to the plant/seed DNA Binding buffer to a final dilution volume of 0.5% (v/v). The zymo-spin IV-HRC Spin Filters was prepared by snapping its base, it was inserted into a collection tube and spinned in a microcentrifuge at 8000×g for 3mins. 750μl of the lysing solution was added to 150g of the powdered seed sample in the ZR bashing bead lysing tube. The sample cells in the bashing bead tube were disrupted by vortexing for 20mins, it was spinned at 10000×g for 1min. 400μl of the supernatant was pipetted into the zymo-spin IV Spin Filter and centrifuged at 10000×g for 1minute. 1200μl of the plant/seed DNA binding buffer was added to the filtrate and mixed together. 800μl of the mixture was transferred into Zymo-Spin IIC Column and centrifuged at 10000×g for 1minute. 200μl DNA pre-wash buffer was added to the Zymo-Spin IIC Column in a new collection tube and centrifuged at 10000×g for 1minute. 500μl plant/seed DNA wash buffer was added to the Zymo-Spin IIC Column and centrifuged at 10000×g for 1minute. The zymo-spin IIC Column was transferred to a new collection tube; 100μl of DNA Elution buffer was added directly to the column matrix and centrifuged at 10000×g for 30sec to elute the DNA. The eluted DNA was transferred into an already prepared Zymo-Spin IV-HRC Spin Filter and centrifuged at 8000×g for 1min. The filtered DNA was collected into a 500μl centrifuge tube and stored at -20°C. The DNA was eluted in 50μl of elution buffer.Electrophoretic Characterization of DNA by Agarose Gel ElectrophoresisPreparation of Agarose Gel: According to the Treseder laboratory protocol [32], the edges of the electrophoretic tank were sealed and the comb was placed near the edges of the tray, with the fingers of the comb slightly above the plate. Agarose Gel was made by weighing 0.75g of agarose in a conical flask and added to 50ml of 1x TAE (Tris-acetate-EDTA) buffer. The agarose was solubilized by heating in a microwave oven at 80°C for 50 sec; the solution was allowed to cool to 60°C. 10μl of ethidium bromide was added to the solution, and the whole solution was poured into the taped tray (gel slab) in a horizontal position with the comb inserted at one end before polymerization was completed to make the gel. After the gel was polymerized, 300ml of 1x TAE buffer was prepared and poured into the electrophoretic tank, the gel slab was turned horizontally and the combs gently removed. The gel was totally submerged in the buffer but was not covered more than 1cm above the gel. Loading of samples: 2µl of loading dye was mixed with 10μl of the eluted DNA and then loaded into the well. The electrophoresis was ran at 100volts for 45minutes. When the dye front travelled approximately 80% of the gel length, the power source was switched off and the gel tray removed. The ethidium bromide stained bands in the gel were viewed with the UV Trans-illuminator.Estimation of the quality and quantity of the extracted genomic DNA by UV Spectrophotometry: 10μl of the DNA sample was diluted with 90μl of TE Buffer (IDTechnologies). 100μl of the buffer was used as blank. The spectrophotometer reading for the diluted DNA sample was recorded at 260nm and 280nm. The quality and concentration was calculated by the formula below [33].
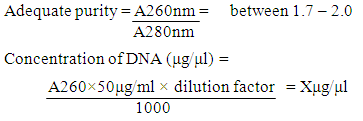
3.8. Polymerase Chain Reaction (PCR)
- PCR was carried out with a slight modification of the method of Vural, [33] and Maltas [34] to a final volume of 20μl. The tubes contained 2μl of genomic DNA, 3.8μl of 0.5mM primer mix (forward and reverse), 10μl of 10x PCR master mix Buffer, 1.2μl of 1.5mmol/l MgCl2, and 1.0μl of 5% Dimethylsulfoxide (DMSO) and 2μl of nuclease free water. The program was initiated on a TechGene amplifier. It was denatured at 94°C for 5min, followed by 40 cycles of amplification with denaturation for 60 sec at 94°C, annealing for 60 sec at 66°C, and an extension at 72°C for 60 sec. Elongation was done at 72°C for 5 min. The primers used were LEC5 Forward:5’-TCAACGAAAACGAGTCTGGTG-3’ LEC 6 Reverse:5’-GGTGGAGGCATCATAGGTAAT-3’ The amplification products (10μl) were electrophoresed in 1.5% (w/v) TAE agarose gel containing 10mg/ml ethidium bromide. The electrophoresis ran in TAE buffer at 100V for 40minutes. The DNA bands were observed under ultraviolet light and photographed by an Image System.
4. Results
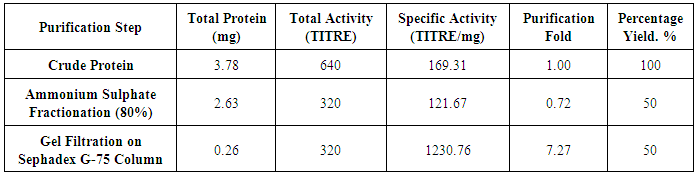
4.1. Purification Profile of PT Lectin Gene
- The purification profile of Piliostigma thonningii lectin gene is summarised in table 1. The crude protein of P. thonningii seeds contained approximately 3.78 mg/ml total protein with a specific activity of 169.31HAU/mg. Precipitation of the crude protein with ammonium sulphate resulted to a purification fold of 0.72 and a yield of 50%. The result obtained following gel filtration on Sephadex G-75 column revealed a total yield of 50% lectin with a specific activity of 1230.76HAU/mg. Figure 2 shows the elution profile of the protein lectin from Piliostigma thonningii after gel filtration on Sephadex G-75 column. From the plot of Piliostigma thonningii, two peaks was observed The fractions 6, 7 and 8 was pooled together to form Piliostigma thonningii partially purified protein 1(P1) while the fraction 16,17 and 18 was pooled together to form Piliostigma thonningii partially purified protein 1(P2), Hemagglutination assay was carried out on the fractions collected in both peaks separately and the peak with agglutinating activity (P2) had about 0.261 mg/ml of protein concentration with a total activity of 16 hemagglutinating unit.One Haemaglutinating unit (HAU) (TITRE) is the highest dilution of 50ul giving a complete hemagglutinating activity.
|
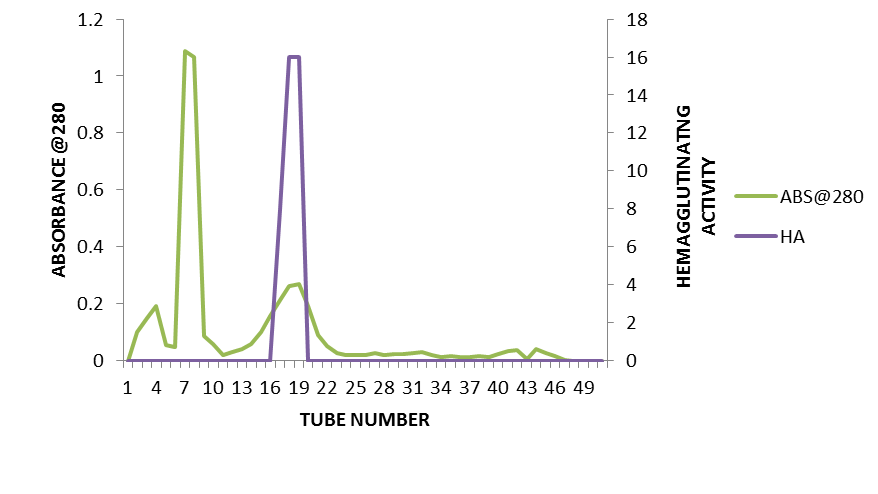 | Figure 2. Elution Profile of Piliostigma thonningii seed lectin on Sephadex G-75 Column Chromatography (Elution rate at 400µl per min) |
4.2. Antimicrobial Activity
- The result obtained revealed that there was no antimicrobial activity against the micro-organisms tested. The photomicrograph shows the absence of a zone of inhibition around the wells containing different concentration of partially purified fractions of Piliostigma thonningii inoculated with Eschericia coli labelled EC. The same absence of zone of inhibition was observed for the other organisms Bacillus subitils (BC), Salmonella typhi (ST), and Staphylococcus aureus (SA). There was no zone of inhibition around the well for the fungi Candida albicans. The minimum inhibitory concentration (MIC) and minimum bactericidal/fungicidal concentration (MBC/MFC) were not determined since there was no zone of inhibition observed during sensitivity test.
4.3. DNA Isolation and PCR
- The result shown in figure 3 is the genomic DNA (gDNA) of Piliostigma thonningii represented in the second well. The gDNA were of adequate quality, quantity and purity for applying polymerase chain reaction (PCR). The amplification of gene of interest (lectin) using the LEC5/LEC6 Primer pair yielded a 195bp product, represented in lane 5. Lane 3 contains the 1000kb DNA ladder from which the size of the amplicons gotten for both plant gDNA was estimated.
5. Discussion
- In this study, precipitation of the lectin with ammonium sulphate resulted in a 0.72-fold. This low purification fold may be as a result of the effects of the ammonium sulphate salt on the hemagglutinating ability of the lectin protein or instability resulting from protein separation in the solution. Maricel [35] reported the purification of lectin from Moringa oleifera seeds on a 2-step purification process, gel filtration using Sephadex G-75 column gave an approximate molecular Weight of 12,200 Da for the isolated lectin which is lower than the molecular weight reported in this study, though the purification process is the same, the molecular weight difference may be as a result of the specie differences. Lectin purified from seeds of Caesalpinoideae plant, Bauhinia variegata, as reported by [6] using affinity chromatography on lactose-agarose, analysis by MALDI-TOF spectrometry indicated a MW of 32 817 Da. The results weren’t similar to the estimated molecular weight for the lectin from Caesalpinoideae, P. thonningii, a member of this family in this study. In another study [36], Swartzia laevicarpa, a member of the sub-family Faboideae, resulted in a lectin fraction that was examined by electrophoresis, and a major protein band with an apparent molecular mass between 27 and 30 kDa was observed.The antimicrobial activity test of lectin protein was measured against selected bacteria Staphylococcus aureus, Escherichia coli, Salmonella typhi, Bacillus subitilis and a fungus, Candida albicans. The antimicrobial activities of the lectin protein at different dilution did not reveal inhibition zone for the purified plant lectin at the different lectin concentration. In a previous research [37] it was reported that the successive ethyl acetate extract of Albizia lebbeck leaves are found to have inhibitory effect against Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa and Bacillus cereus, the extract showed sensitivity for both gram positive and gram negative bacteria with maximum against Pseudomonas aeruginosa and minimum against Escherichia coli. This is different from the results obtained from this study because the lectin studied by Rahul [37] was from the leaves of the plant, hence, the anti-microbial activity observed could be as a result of the phytochemical properties of the leave and the method used for the isolation. In our present study, inactivity against the micro-organisms could be attributed to a probable loss of active principles during the isolation and purification processes. It has been proposed [38] that the proteins with antibacterial action form a channel on cell membrane and the cell dies as a result of the out flowing of cellular contents, this mechanism being different from that of antibiotics. This channel that is formed on the membrane is as a result of the specificity of lectins for carbohydrates, it could be that the absence of a zone of inhibition in this study is as a result of the lectin protein not being specific for any of the carbohydrate moiety on the cell membrane of the tested micro-organisms and so didn't lead to the disruption of the cell membrane and eventual death of the organism. Antibacterial activity on Gram-positive and Gram-negative bacteria occurs through the interaction of lectin with components of the bacterial cell wall including teicoic and teicuronic acids, peptidoglycans and lipopolysaccharides; study revealed that the isolectin I from Lathyrus ochrus seeds bind to muramic acid and muramyl dipeptide through hydrogen bonds between ring hydroxyl oxygen atoms of sugar and carbohydrate binding site of lectin and hydrophobic interactions with the side chains of residues Tyr100 and Trp128 of isolectin I [39].There are only a few lectins known to possess antifungal activity such as the lectins from the seeds of Phaseolus vulgaris and Pisum sativum, from the pepper seed Capsicum frutescens and from the mushroom Astragalus mongholicus [40-43]. The inhibition of fungal growth can occur through lectin binding to hyphas resulting in poor absorption of nutrients as well as by interference on spore germination process, the lectin of Pisum sativum seeds inhibited the growth of Aspergillus flavus, Fusarium oxysporum and Trichoderma viride [43], the authors suggested the use of lectin for clinical microbiology and therapeutic purposes.DNA quality is determined by its fragment length and its degree of damage due to exposure to heat, low pH and/or nucleases that cause hydrolysis, depurination and/or enzymatic\degradation. Therefore, DNA quality varies according to the material under examination, the degree of processing of the sample and the applied DNA extraction method. The plants seed used were dried and proper precautions were taken towards its preservation before its use. The DNA of the plant were successfully isolated using the Zymo Research Plant/Seed Kit as it had the crushing beads that assisted in the crushing of the plant cell wall exposing the plant nucleic material and also the reagents were prepared by the manufacturer which helped in reducing to a large extent any interference from other inhibitory materials, agarose gel electrophoresis of the DNA isolated using ethidium bromide staining indicated a DNA of good integrity, good enough for polymerase chain reaction. A sensitive qualitative molecular detection was developed using Polymerase Chain Reaction, an optimized amplification reaction that was carried out on the plant seed gDNA using the primer LEC5 (Forward) 5’-TCAACGAAAACGAGTCTGGTG-3’ and LEC6 (Reverse) 5’-GGTGGAGGCATCATAGGTAAT-3’ produced a 195bp product confirming the presence of lectin gene in both plant seed. The primer pairs LEC5/LEC6 was reported for identification of the galactose specific lectin gene present in soybean [33], [34], because the plant used in this study was a legume of the same family with soybean the same primer with a little optimization of reaction condition produced an amplification product of 195bp which indicates an evolutionary relationship, and a more sensitive and specific method of identification. Although the 195bp amplicon size is supposed to translate for a protein of an approximate molecular weight of between 119- 126kDa, the molecular weight after SDS-PAGE gotten in this study for the fraction with hemagglutinating activity after gel filtration on Albizia lebbeck seeds extract resulted in a protein with an estimated molecular weight of 21kDa, this difference observed could be related to the different phases in the life cycle of the plant, the process of translating the genetic information in the mRNA to protein and the possible loss of amino acid due to the post translational modification carried out on the protein [44]. The N-terminal amino acid sequence and subsequent full length cDNA sequence may be able to shed more light on this observed difference.The present study on the molecular detection and development of the protocol for the optimization of the primer pair conditions is the first report on Piliostigma thonningii and Albizia lebbeck growing in Nigeria.
6. Conclusions
- This study presented novel lectin gene from Nigeria-indigenous Piliostigma thonningii using the hemagglutination reaction on chicken erythrocyte and an optimized polymerase chain reaction detected the lectin gene using Lec5/Lec6 primer pairs. Finally, these findings could scientifically harnessed for large scale production of lectin for industrial applications.
ACKNOWLEDGEMENTS
- We acknowledge the effort of Dr. Mrs. Bolatito Afolabi-Nusrah; the Director, Centre for Biotechnology Research & Training (CBRT) Ahmadu Bello University Zaria, and the staff. We thank Dr. Ms Gloria Chechet, Department of Biochemistry Ahmadu Bello University Zaria for assisting with the molecular aspect of this work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML