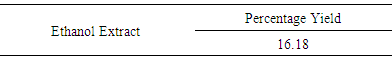
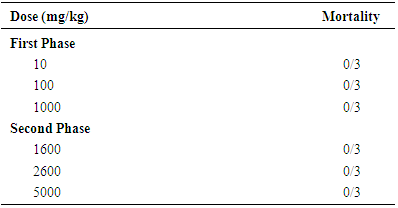
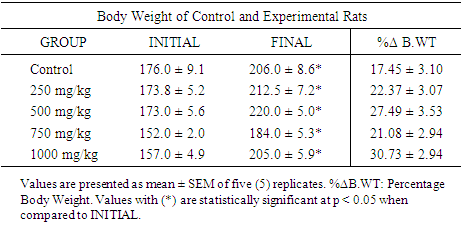
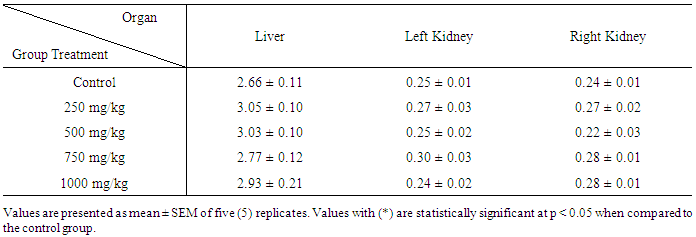
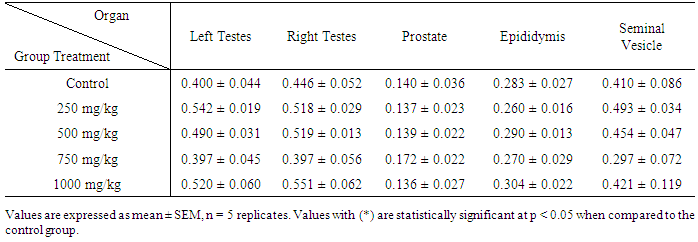
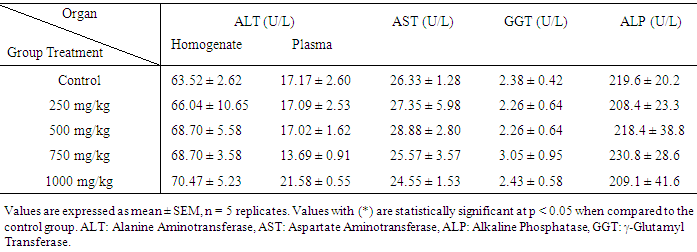
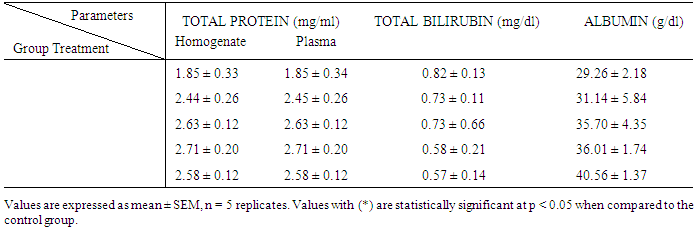
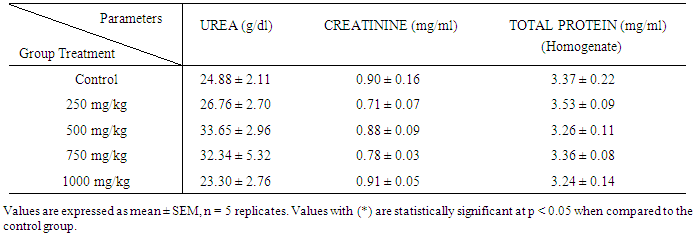
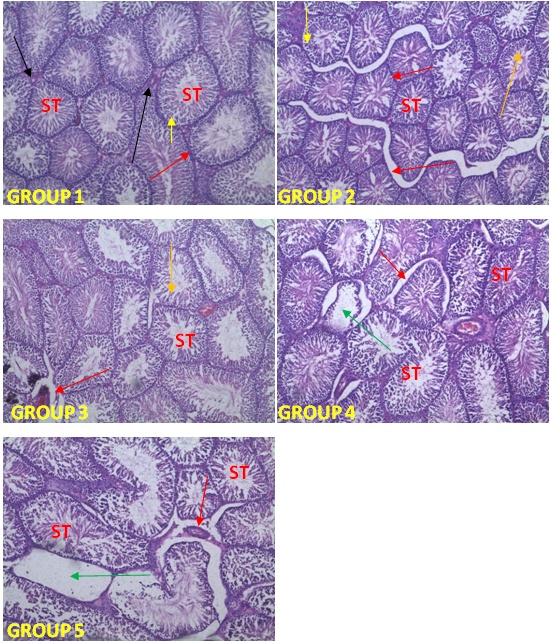
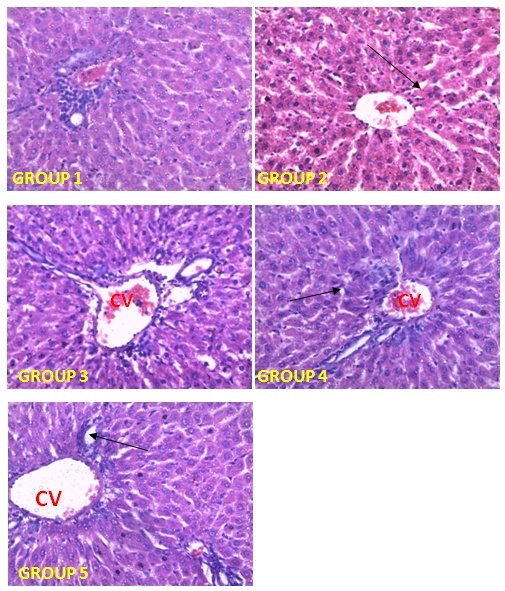
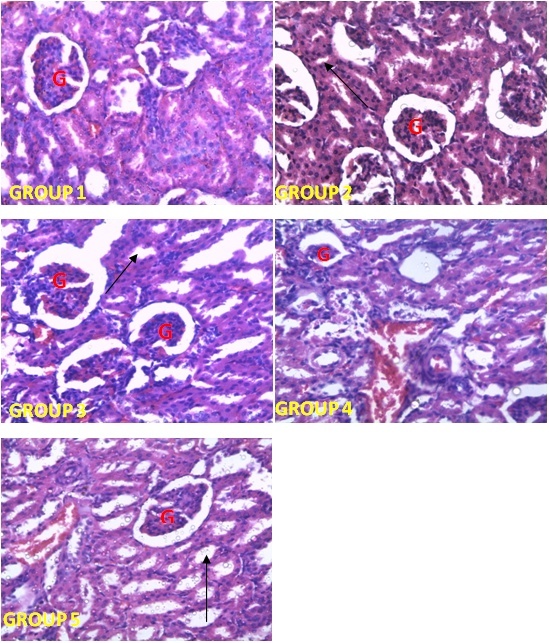
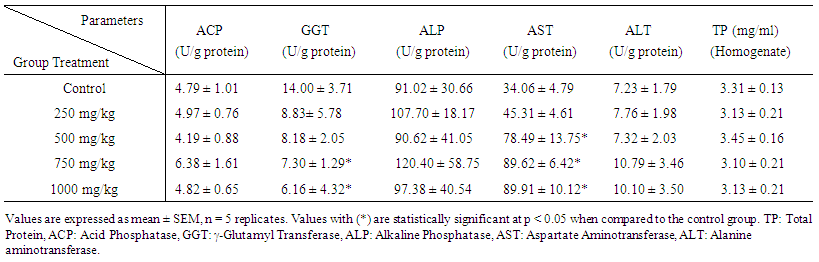
| [1] | Schmidt, E., Lutter, M. and McCleland, W. (2002). Trees and shrubs of Mpumalanga and Kruger National Park, Jacana, South Africa, pp. 80. |
| [2] | Njoronge, G. N. and Kibunga J. W. (2007). Herbal medicine acceptance, sources and utilisation for diarrhoea management in a cosmopolitan urban area (Thika, Kenya). African Journal of Ecology. 45, 65-70. |
| [3] | Cousins, D. and Huffman, M. A. (2002). Medicinal properties in the diets of gorillas: an ethno-pharmacological evaluation. African Study Monographs. 23, 65-89. |
| [4] | Igoli, J. O, Ogaji, O.G, Tor-Anyiin T. A, Igoli N. P. (2005) Traditional Medical Practices Amongst the Igede People of Nigeria. African Journal of Traditional, Complementary and Alternative Medicine. 2(2):134–152. |
| [5] | Alawa, J. P., Jokthan, G. E. and Akut, K. (2002). Ethnoveterinary medical practice for ruminants in the sub-humid zone of the northern Nigeria. Preventive Veterinary Medicine. 54, 79-90. |
| [6] | Moshi, M. J., Otieno, D. F., Mbabazi P. K. and Weisheit. A. (2009). The ethnomedicine of the Haya people of Bugabo ward, Kagera Region, North-western Tanzania. Journal of Ethnobiology and Ethnomedicine. 5, 24. |
| [7] | Arbonier, M. A. (2004). Trees, Shrubs and Lianas of West African Dry Zones. 2nd edition, Margraf publishers, Netherlands, pp. 412. |
| [8] | Mali, R. G. and Mehta, A. A. (2007). A review on anthemintic plants. Natural Product Radiance. 7, 466-475. |
| [9] | Gelfand, M., Mavi, S., Drummond, R. B. and Ndemera, B. (1985). The Traditional Medicinal Practitioner. His principles of practice and pharmacopoeia. Mambo Press, Gweru, Zimbabwe, pp. 411. |
| [10] | Teklehaymanot, T. and Gidday, M. (2007). Ethnobotanical study of medicinal plants used by people in Zegie Peninsula, North Western Ethiopia. Journal of Ethnobiology and Ethnomedicine. 3, 12. |
| [11] | Obata, O. O. and Aighokhan, E. I. (2012). Ethnobotanical practices among the people of Oka-Akoko, Nigeria. Plant Archive.12 (2), 627-638. |
| [12] | Cowan, M. M. (1999). Plant products as antimicrobial agents. Clinical Microbiology Reviews. 12, 564-582. |
| [13] | Jansen, O., Angenot, L., Tits, M., Nicolas, J. P., De Mol, P., Nikiema, J. B. and Frederich, M. (2010). Evaluation of 13 selected medicinal plants from Burkina Faso for their antiplasmodial properties. Journal of Ethnophamacology. 13, 143-150. |
| [14] | Oyelana, O. A., Durugbo, E. U., Olukanni, O. D., Ayodele, E. A., Aikulola, Z. O. and Adewole, A. I. (2011). Antimicrobial activity of Ficus leaf Extracts on some fungal and bacterial pathogens of Dioscorea rotundata from Southwest Nigeria. Journal of Biological Sciences. 11, 359-366. |
| [15] | Krief, S., Hladik, C. M. and Haxaire, C. (2005). Ethnomedicinal and bioactive properties of plants ingested by wild chimpanzees in Uganda. Journal of Ethnopharmacology. 101, 1-15. |
| [16] | Olas, B., Wachowicz, B., Nowak, P., Stochmal, A., Oleszek, W., Glowacki, A. and Bald, E. (2008). Comparative studies of the antioxidant effects of a naturally occurring resveratrol analogue trans 3, 3’, 5, 5-tetrahydroxy-4-methoxystilbene and resveratrol- against oxidation and nitration of biomolecules in blood platelets. Cell Biology and Toxicology. 24, 331-340. |
| [17] | Grassi, D., Desideri, D. and Ferri, C. (2010). Flavonoids antioxidants against atherosclerosis. Nutrients. 2, 889-902. |
| [18] | Otimenyin, S. O., Uguru, M. O. and Atang, B. L. (2004). Anti-inflammatory and analgesic activities of Ficus thonningii and Pseudocedrela kotschyi extracts. Nigerian Journal of Pharmaceutical Research. 3, 82-85. |
| [19] | Maiha, B. B., Mohammed, B. and Magaji, M. G. (2013). Psychopharmacological potential of methanol leaf extract of Ficus thonningii (Blume) in mice. Nigerian Journal of Pharmaceutical Science. 12 (2), 30-34. |
| [20] | Coker, M. E., Emikpe, B. O., Adeniyi, B. A. and Budale, B. A. (2009). The inflammatory potential, haematological and histological changes induced in rats due to the administration of methanol extracts of Ficus thonningii leaves. African Journal of Pharmacology. 3, 273-276. |
| [21] | Aniagu, S. O., Nwinyi, F. C., Akumka, D. D., Agbain, E. O., Dzarma, S., Ajoku, P., Adelusola, K..A., Ibe, J., Inyang, U. S. and Gamaniel, K. S. (2008). Short term toxicity studies of Ficus thonningii Blume Moraceae leaf extracts in rats. International Journal of Food Science and Technology. 43, 456-464. |
| [22] | Musabayane, C. T., Gondwe, M., Kadyamaapa, D. R., Chuturgoon, A. A. and Ojewole, J. A. O. (2007). Effects of F. thonningii (Blume) Moraceae stem bark ethanol extract on blood glucose, cardiovascular and kidney cell lines of the proximal (LLC-PK1) and distal tubules (MDBK). Renal Failure. 29, 389-397. |
| [23] | Trease, G. E. and Evans, W. C. (1978). A Textbook of Pharmacognosy. 11th Edition, Bailliere Tindall, London. Pp. 530. |
| [24] | Sofowora, E. A. (2006). Phytochemical screening: In “Medicinal plants and Traditional Medicine in Africa”, 2nd edition, Spectrum Books Limited Ibadan, Nigeria. Pp. 150-153. |
| [25] | Lorke, D. A. (1983). A new approach to practical acute toxicity testing. Archives of Toxicology. 54, 275-287. |
| [26] | Tchamadeu, M. C., Dzeufiet, P. D. D., Nana, P., Kouambou Nouga, F., Allard, J., Blaes, N., Siagat, R., Zapfack, L., Girolami, J. P., Tack, I., Kamtchouing, T. and Dimo, T. (2011). Acute and sub-chronic oral toxicity studies of an aqueous stem bark extract of Pterocarpus soyauxii Taub (Papilionaceae) in rodents. Journal of Ethnopharmacology. 133, 329-335. |
| [27] | Chawla, R. (1999). Biochemical tests for total proteins, albumin and pyruvate. In: Practical Clinical Biochemistry Methods and Interpretations, 2nd Edition, JAYPEE. pp 107-134. |
| [28] | Weatherburn, M. W. (1967) Phenolhypochlorite reaction for the determination of ammonia. Analytical Chemistry. 39, 971. |
| [29] | Lowry, O. H., Rosebrough, N. J., Farr, A. L. and Randall, K. J. (1951). Protein measurement with the Folin phenol reagent. Journal of Biologic Chemisry. 193, 265-275. |
| [30] | Pinnell, A. E. and Northam, B. E. (1978). New automated dye-binding method for serum albumin determination with bromocresol purple. Clinical Chemistry. 24, 80-86. |
| [31] | Jendrassisk, L. and Grof, P. (1938). Simplified photometric methods for the determination of blood bilirubin. Biochemistry Zeitschrift. 297, 81-89. |
| [32] | Deutsche Gesellschaft fur klinische Chemie, (1972). Empfehlungen der deutschen Gesellschaft fur Klinische Chemie (DGKC). Standardisierung von Methoden zur Bestimmung von Enzymaktivit ten in biologischen Flussigkeiten. (Recommendation of the German Society of Clinical Chemistry. Standardization of methods for measurement of enzymatic activities in biological fluids). Journal of Clinical Chemistry and Clinical Biochemistry. 10, 182-192. |
| [33] | Seiler, D. (1983). Saure Phosphatase in Serum (Substrate: α-Naphthylphosphat) Referenczwerte und Diagnostische Aussage. Journal of Clinical Chemistry and Clinical Biochemistry. 21, 519-525. |
| [34] | Reitman, S. and Frankel, S. (1957). A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. American Journal of Clinical Pathology. 28, 58-63. |
| [35] | Szasz, G. A. (1969). Kinetic photometric method for serum γ-glutamyl transpeptidase. Clinical Chemistry. 15(2), 124-137. |
| [36] | Akusu, M. O., Akpokodje, J. U., Ogwnegbu, S. O. and Oke, B. O. (1985). Differences in morphology of Bull spermatozoa from normal and pathological testis during epididymal transit. Nigerian Veterinary Journal. 14(2), 30-33. |
| [37] | Oyeyemi, M. O. and Ubiogoro, O. (2005). Spermiogram and morphological chacteristics in testicular and epididymal spermatozoa of large white boar in boar in Nigeria. International. Journal of. Morphology. 23(3), 235-239. |
| [38] | Pant, N. and Srivastava, S. P. (2003). Testicular and spermatotoxic effect of quinaphos in rats. Journal of Applied Toxicology. 23, 271-274. |
| [39] | Wells, M. E. and Awa, O. A. (1970). New technique for assessing acrosomal characteristics of spermatozoa. Journal of Diary Science. 53, 227. |
| [40] | Harnack, L. J., Rydell, S. A. and Stang, J. (2001). Prevalence of use of herbal products by adults in the Minneapolis/St Paul, Minn, metropolitan area. Mayo Clinic Proceedings. 76, 688–694. |
| [41] | Ogle, B. M., Tuyet, H. T., Duyet, H. N. and Dung, N. N. (2003). Food, Feed or Medicine: The Multiple Functions of Edible Wild Plants in Vietnam. Economic Botany. 57(1), 103-117. |
| [42] | Olson, H., Betton, G., Robinson, D., Thomas, K., Monro, A., Kolaja, G., Lilly, P., Sanders, J., Sipes, G., Bracken, W., Dorato, M., Van Deun, K., Smith, P., Berger, B. and Heller, A. (2000). Concordance of the toxicity of pharmaceuticals in humans and in animals regulatory. Toxicology and Pharmacology. 32, 56-57. |
| [43] | Rhiouani, M., Al-Shabanah, O. A. and Al-Majed, A. A. (2002). Effect of prolonged vigabatrin treatment on haemaological and biochemical parameters in plasma, liver and kidney of Swiss albino mice. Scientia Pharmaceutica. 70, 135-145. |
| [44] | Lahlou, S., Zafar, H. I. and Badiaa, L. (2008). Acute and chronic toxicity of a lyophilized aqueous extract of Tanacetum vulgare leaves in rodents. Journal of Ethnopharmacology. 117, 221-227. |
| [45] | Ahur, V. M., Madubunyi, I., Adenkola, A. Y. and Udem, S. C. (2010). The effect of acetyl acetate extract of Ficus thonningii (Blume) leaves on erythrocyte osmotic fragility and haematological parameters in acetaminophen treated rats. Comparative Clinical Pathology. 10, 1107-1111. |
| [46] | Raza, M., Al-Shabanah, O. A., El-Hadiya, T. M. and Al-Majed, A. A. (2002). Effect of prolonged vigabatrin treatment on haematological and biochemical parameters in plasma, liver and kidney of Swiss albino mice. Science Pharmaceutica. 70, 135-145. |
| [47] | Teo, S., Stirling, D., Thomas, S., Hoberman, A., Kiorpes, A. and Khetani, V. (2002). A 90-day oral gavage toxicity study of D-methylphenidate and D, L methyphenidate in Sprague Dawley rats. Toxicology. 179, 183-196. |
| [48] | Ezeja, M. I., Anaga, A. O. and Asuzu I. U. (2014). Acute and sub-chronic toxicity profile of methanol leaf extract of Gouania longipetala in rats. Journal of Ethnopharmacology. 151, 1155–1164. |
| [49] | Bass, N. M. and Ockner, B. A. (1996). Drug-induced liver disease. In: Zakin, D. and Boyer, T. D. (Eds.), Hepatology, a Text Book of Liver Diseases, 3rd edition W. B. Saunders, Philadephia, pp.962–1017. |
| [50] | Mukinda, J. and Syce, J. A. (2007). Acute and chronic toxicity of aqueous extract of Artemisia afra in rodents. Journal of Ethnopharmacology. 112, 138–144. |
| [51] | Ramaiah, S. K. (2011). Preclinical safety assessment. Current gaps, challenges and approaches in identifying translatable biomarkers of drug-induced liver damage. Clinics in Laboratory Medicine. 31, 161–172. |
| [52] | Ozer, J., Ratweb, M., Shawc, M., Baileya, W. and Schomaker, S. (2008). The current state of serum biomarker of hepatoxicity. Toxicity. 245, 194–205. |
| [53] | Burtis, C. A. and Ashwood, E. R. (2001). Enzymes: In Tietz Fundamentals of Clinical Chemistry, 5th edition W. B. Saunders Company, New York, USA, pp.352–369. |
| [54] | Mauro, P., Renze, B. and Wouter, W. (2006). In Tietz text book of clinical chemistry and molecular diagnostics. 5th edition, editors: Carl A.B., Edward, R, David E.B, Elsevier; Enzymes. Pp. 604-616. |
| [55] | Thapa, B. R. and Walia, A. (2007). Liver function tests and their interpretation. Indian Journal of Pediatrics. 74, 663-671. |
| [56] | Whitby, L. G., Smith, A. F. and Becket, B. J. (1989). Lecture notes on clinical chemistry, 4th edition. Blackwell Scientific Publications, London. pp 38-178. |
| [57] | Mukinda, J. T. and Eagles, F. K. (2010). Acute and sub-chronic oral toxicity profile of the aqueous extract of Pohygala fruticosa in female mice and rats. Journal of Ethnopharmacology. 128, 236–240. |
| [58] | Mukinda, J. and Syce, J. A. (2007). Acute and chronic toxicity of aqueous extract of Artemisia afra in rodents. Journal of Ethnopharmacology. 112, 138–144. |
| [59] | Ramalingam, V. and Vimaladevi, V. (2002). Effect of mercuric chloride on membrane-bound enzymes in rat testis. Asian Journal of Andrology. 4, 309–311. |
| [60] | Breton, S., Smith, P. T. S., Lui, B. and Brown, D. (1996). Acidification of the male reproductive tract by a proton pumping (H+)-ATPase. Natural Medicine. 2, 470–472. |
| [61] | Samarth, R. M. and Samarth, M. (2009). Protection against radiation induced testicular damage in Swiss albino mice by Mentha piperita (Linn.). Basic Clinical Pharmacology and. Toxicology. 104, 329–334. |
| [62] | Butterworth, P. J. and Moss, D. W. (1966). The effect of urea on human kidney alkaline phosphatase. Biochemical Journal. 99, 9–10. |
| [63] | Tate, S. S. and Rose, M. E. (1977). Human kidney γ-glutamyl transpeptidase: catalytic properties, subunit, structure and localization of the γ-glutamyl binding site on the large subunit. Journal of Biological Chemistry. 252, 6042–6045. |
| [64] | Sherin, R. J. and Hodgen, G. D. (1967). Testicular γ-glutamyl transpeptidase: an index of Sertoli cell function in man. Journal of Reproduction and Fertility. 48, 191–194. |
| [65] | Yang, J., Wu, G., Feng, Y., Lu, Q., Lin, S. and Hu, J. (2010). Effects of taurine on male reproduction in rats of different ages. Journal of Biomedical Science. 17 (Suppl 1), S9. |
| [66] | Garner, D. L. and Hafez, E. S. E. (1993). Spermatozoa and seminal plasma. In Hafez E.S.E (eds.). Reproduction in Farm animals. (6th ed.). Lea and Febiger, Philadelphia, USA. Pp. 165-187. |
| [67] | Moss, J. A., Melrose, D. R., Reed, H. C. B. and Vanderplassche, M. (1979). Spermatozoa, semen and artificial insemination. Baillire Tindal, London. pp. 59-91. |
| [68] | Hafez, E. S. E. (1987). Advances in reproductive biology and semen evaluations. In: Reproduction in farm animals. 5th edition. Lea and Febiger, Philadelphia, USA. pp. 649. |
| [69] | Thomas, M. J. and Thomas, J. A. (2001). Toxic responses of the reproductive system. In: Casarett and Doull’s Toxicology. The basic science of poisons (6th edition), McGraww-Hill Medical Publishing Division, New York, pp. 677- 681. |
| [70] | Farombi, E. O., Adedara, I. A., Akinrinde, S. A., Ojo, O. O. and Eboh, A. S. (2012). Protective effects of kolaviron and quercetin on cadmium-induced testicular damage and endocrine pathology in rats. Andrologia. 44(4), 273-284. |
| [71] | Ishihara, M., Itoh, M., Miyamoto, K., Takeuchi, Y., Takenaka, I. and Jitsunari, F. (2000). Spermatogenic disturbance induced by di-(2-ethylhexyl) phthalate is significantly prevented by treatment with antioxidant vitamins in rat. International Journal of Andrology. 23, 85-94. |
| [72] | Adimoelja, A., Setiawan, L. and Djojotananjo, T. (1995). Tribulus terrestris (protodioscin) in the treatment of male infertility with idiopathic oligoasthenoterato-zoospermia. In: First International Conference of Medical Plants for Reproductive Medicine in Taipei, China. |
| [73] | Chinoy, N. J. and Padman, P. (1996). Anti-fertility investigation on the benzene extract of Carica papaya seeds in male albino rats. Journal of Medicinal and Aromatic Plant Science. 19(2), 422-426. |
| [74] | Parveen, S., Das, S., Kundra, C. P. and Pereira B. M. (2003). A comprehensive evaluation of the reproductive toxicity of Quassia amara in male rats. Reproductive Toxicology. 17(1), 45-50. |
| [75] | Abdel-Magnied, E. M, Abdel-Rahman, H. A. and Hanaz, F. M. (2001). The effect of Aqueous extracts of Cynomorium coccineum and Withania somnifera on testicular development in immature Wistar rats. Journal of Ethnopharmacology. 75, 1-4. |
| [76] | Yakubu, M. T., Akanji, M. A. and Oladiji, A. T. (2008). Effects of oral administration of aqueous extract of Fadogia agrestis (Schweinf. Ex Hiern) stem on some testicular function indices of male rats. Journal of Ethnopharmacology. 115, 288–292. |



 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML