-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2016; 6(2): 51-58
doi:10.5923/j.ajb.20160602.05

Alterations of Phospholipids Synthesis in Mitochondrial Membrane of the Liver Cells in the Dynamics of Chronic Emotional Painful Stress Development
Karim T. Almatov 1, Gaffurjon R. Abdullaev 2
1Physiology and Biophysics Department, National University of Uzbekistan Named after Mirzo Ulugbek, Tashkent, Uzbekistan
2Physical Education Faculty, Namangan State University, Namangan, Uzbekistan
Correspondence to: Karim T. Almatov , Physiology and Biophysics Department, National University of Uzbekistan Named after Mirzo Ulugbek, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

At chronic emotional painful stress, regardless of the duration of stress, content of lysophosphatidylcholine, phosphatidic acid and phosphatidylserine increases in liver mitochondria. In this case, content of phosphatidylinositol, conversely, decreases slightly in early stress, and then depending on the duration of the stress, the reduction of the phospholipid increases.
Keywords: Phospholipid, Mitochondrial membrane, The liver cells, Oxidative phosphorylation, Chronic emotional painful stress, ATP level, Cytochrome c
Cite this paper: Karim T. Almatov , Gaffurjon R. Abdullaev , Alterations of Phospholipids Synthesis in Mitochondrial Membrane of the Liver Cells in the Dynamics of Chronic Emotional Painful Stress Development, American Journal of Biochemistry, Vol. 6 No. 2, 2016, pp. 51-58. doi: 10.5923/j.ajb.20160602.05.
1. Introduction
- Mitochondria produce more than 90% of the cell energy by oxidative phosphorylation. About 60% of the energy released during the oxidation in mitochondria substrates dissipated as heat, maintaining a stable body temperature [1]. Mitochondria are the major regulators of calcium homeostasis, cells acid-base balance, the level of production of cellular activity regulators, as free radicals and nitric oxide [2-7]. Signal interactions in "cell — mitochondria" system carried out at the level of membrane potentials, metabolites (energy-rich bond, substrata, etc.) substances participating in neuroendocrine regulation (catecholamines, indolamines, thyroid hormones, etc.), calcium cations. Recently it was shown that the reduction in intracellular ATP levels by only 15-20% the intensity of all volatile cell function falls to 75-80% of the initial value, which leads to the development of multisystem pathologies [8]. In close dependence on intracellular ATP is the cells’ ability to maintain its specific volatile functions. Thus, mitochondria are organelles not only provide the body with energy, but also maintain a dynamic relationship of metabolism.Damaged mitochondria are a trigger of cytochrome c release through mitochondrial pores [9]. Released cytochrome c is a "death sentence" for the living cells [12]. Cytochrome c entails the transfer of the apoptotic signal, the result of which is often a variety of diseases. Thus, in many apoptotic neurons during development of the nervous system of vertebrates a loss of cardiolipin takes place, it is an easy-oxidized phospholipid, which is part of the inner mitochondrial membrane. It was showed, that cardiolipin content is reduced to the disappearance of mitochondria in these cells, and by the time of the loss of mitochondrial cardiolipin mass decreases slightly. The fact that disappearance of cardiolipin competitively connected with the production of mitochondrial reactive oxygen species and an increase in lipid peroxidation points on participation of oxygen free radicals in these processes [13]. From what has been said it is well correlated a report about the fundamental importance of the two staging process of cytochrome c release from mitochondria of apoptotic cells. In the first stage there is separation of cytochrome c from the inner mitochondrial membrane where it is associated with cardiolipin by electrostatic and / or hydrophobic interactions. This separation takes place after the oxidative modification of cardiolipin. The subsequent increase in the outer membrane permeability of mitochondria under the influence of Bax oncoprotein results in the release of cytochrome c from mitochondria [14]. Other researchers reported about the loss of the molecular interaction between cytochrome c and cardiolipin due to lipid peroxidation (LPO) [15]. This modification of cardiolipin in a lipid peroxidation induced a release of cytochrome c from mitochondria to the cytosol, which is one of the important early stages of apoptosis.It is well known that phospholipids of cell membranes are not only part of the membrane, but also they are needed for other important functions of cell membranes and organelles. The phospholipid composition of the membrane is regulated in three ways: 1) biosynthesis, 2) peroxidation and 3) enzymatic hydrolysis (phospholipids trans alkylation or trans acylation). Mitochondria possess lipolytic systems besides autonomous lipid synthesizing system [14, 15]. Regardless of microsomal synthesis phosphatidic acid, cardiolipin form in them, some stages of phosphatidylcholine biosynthesis occur. An essential element of phospholipid metabolism in mitochondria is transferase reactions. They are the only way of major phospholipids synthesis, such as phosphatidylserine and phosphatidylinositol, as well alkyl and acyl residues and other phospholipids regeneration and metabolism take place. Lipolytic system of mitochondria plays the most important role in the transferase reaction (transalkylation - phospholipase D; transacylation – PLA2 and lysophospholipase A1). Phospholipid fatty acid is a key structural component of cell organelles and cells of the human body and human organs and cells play a vital role in the functioning of the membrane. System transport paths, and particularly transportation of molecules through the cell membrane, and intracellular membranes made by using phospholipids. Thus, the operation of the body's cells, tissues and organs occurs due phospholipid fatty acids.It should be noted that the extension of the long period of adaptation to stress and thus the prevention of diseases of different organs is relevant and promising trend in cell physiology and bioenergetics. In connection with this the study of lipid metabolism alteration is a topical problem in the dynamics of chronic emotional painful stress development.The aim of this work was to study the characteristics of the phospholipid metabolism in the rat liver mitochondria in the dynamics of chronic emotional painful stress development.
2. Materials and Methods
- Experiments were carried out on sterile white male rats with an initial body weight of 180-200, the animals were kept under standard vivarium conditions, on a normal laboratory diet, in terms of free movement and 12 - hour light regime. Approximately two weeks before the start of the experiment the rats were determined by emotional test of "open field". To do this, the animals suddenly placed in a dark box in the center of the field, which is the arena of a diameter of 1.5 m, divided into squares with sides of 20 cm, illuminated mirror incandescent 500 watt, suspended in the central part at the height of 60 cm from the floor (in the center "open field" is created illumination 1,000 lux) [16, 17, 18]. Emotional stress is called emotional processes that accompany stress and lead to adverse changes in the body. During stress an emotional reaction develops before others, activating the autonomic nervous system and its endocrine support. At prolonged or repeated stress an emotional arousal can stagnate and functioning of the body - takes a bad turn.For 5 days for 2 min locomotor activity of animals was evaluated in meters (the number of squares crossed, multiplied by a factor of 0.3), and the number of fecal pellets was counted, urination, outputs to the center of the "open field", washing and rising to the back paws. All these indicators serve as a criterion of rats’ emotionality. The animals with a lot of urination and defecation, which is considered a particular type of response to an unusual situation, refer to the more emotional. Orientative- investigatory forms of behavior, manifested in horizontal and vertical (rise on hind legs) locomotor activity of animals are closely correlated with this parameter.The rats were immobilized, placed in narrow cages and carried electrocutaneous irritation paws and tail (current frequency - 50 Hz, the power of - 30 V, the pulse frequency - 7 per minute, pulse width - 0.5 seconds) for 30 minutes daily. Depending on the group animals were subjected to electrocutaneous irritation within 1, 2 and 3 weeks, 1, 2 and 3 months [19, 20]. Sacrifice of animals was carried out 10 minutes after the last electrocutaneous irritation. The control group consisted of animals, which weren’t exposed to electrical stimulation.Mitochondria were isolated from the brain and liver tissues of animals on the conventional method of differential centrifugation [21] with some modifications [22].Determination of total phospholipid content of mitochondria in the extract was determined after perchloric acid burning on phosphorus using Vaskovsky reagent [23]. Lipid extract (600 ul) was evaporated on a rotary evaporator. To the residue was poured 700 microliter mixture of equal volumes of sulfuric and perchloric acids and burned in a sand bath to bleaching. Cool to room temperature and poured in 4 ml of freshly prepared 1% solution of ammonium molybdate and 0.3 mL of the reducing agent, mixed and placed for 10 minutes in a boiling water bath. Chilled samples were colorimetered against control at 820 nm in a spectrophotometer. The calibration graph was built on an aliquot part of a standard KH2P04 solution containing from 2 to 10 mg phosphorus to determine the inorganic phosphorus. At calculation of phospholipid concentration result of phosphorus determination was multiplied 1,39X25 millimole / l.Bligh-Dyer [24] extracted phospholipids of the liver mitochondria. The phospholipid composition was analyzed by two-dimensional microfine-layer chromatography on glass plates measuring 6x9 cm, coated with silica gel [25]. Before use, the plate was activated for 20 minutes at a temperature 110°C. Chloroform solution of phospholipids was applied to plate with glass capillary in an amount of 10-15 microliter and was separated in following solvent systems: in the first direction - chloroform - methanol - 28% of ammonia (65: 25: 5), and in the second direction - chloroform - acetone - methanol - acetic acid - water (6: 8: 2: 2: 1).Specific detectors identified mitochondrial phospholipids: (26) ninhydrin reagent for amine containing phospholipids, Dragendorff reagent for cholin phospholipids and by witnesses. Ammonium silver nitrate was used for the detection of glycerol and inositol. Presence of sphingolipid detected with a mixture of sulfuric and acetic acid (1:1). Lysophosphatidylcholine, lysophosphatidylethanolamine and lysocardiolipin were isolated by treating the corresponding diacyl analogues of PLA2.Mitochondrial phospholipids were quantitatively identified by phosphorus. Thereto plates were developed in iodine chamber, phospholipid spots encircled with a fine needle, and then the plates were heated for removing iodine for 15 min at 100°C. Silica gel, containing phospholipids, was scraped into glass test tubes (silica gel with each spot in a separate tube), added 0.2 ml of perchloric acid (72%) and phospholipids in 190-200°C burned for 20 minutes. After cooling, 1 ml of Vaskovsky reagent [27] was added, the tubes vortexed, and heated in a boiling water bath for 15 minutes. After cooling, the silica gel was centrifuged to separate and spectrophotometered at 830 nm. The calibration curve was created for the standard KH2P04 solution. The sensitivity of the method was 1-20 micrograms of phosphorus in the spot.
3. Results and Discussion
- Alterations in phospholipids exchange of rat liver mitochondria in the dynamics of the chronic emotional painful stress development given in Figure 1.At the beginning of the stress (Week 1) phosphatidylcholine content is not changed in liver mitochondria, phosphatidylethanolamine increased by 34.8% of the normal level. After 2 weeks of stress phosphatidylcholine content increases to 32.3%. This increase of the phosphatidylethanolamine content (59.6%) was accelerated. After 3 weeks of the stress phosphatidylcholine and phosphatidylethanolamine content was closer to the normal indicators. Thus, if after 1 month phosphatidylcholine and phosphatidylethanolamine content reduced by 22.3 and 19.4% respectively of the control level, for 2 months - 24.6 and 16.5% for 3 months - 22 and 18.4 3%. All these changes are happening against the background of increasing the content of lysophosphatidylcholine (1, 2, and 3 weeks to 47.1, 75.3 and 64.7% respectively of the norm, at 1, 2 and 3 months - by 55.3, 59.2 and 65.5%) increased. Previously, we [28] reported that PLA2 hydrolytic activity increases during the stress.It is known that phosphatidylcholine is located on the outside, and phosphatidylethanolamine - on the inner surface of biological membranes and constitute the bulk of their lipids [29]. Accordingly, it is easy to increase the amount of the value of phosphatidylcholine and phosphatidylethanolamine in membrane permeability disorders and metabolic processes in the cell. Phospholipid metabolism in biological membranes includes three processes: 1) the lipid synthesis de novo; 2) transferase reaction; 3) exchange with serum phospholipids and other membrane phospholipids via lipid-transporting proteins. It is known that the synthesis of phosphatidylcholine and phosphatidylethanolamine in animal tissues occurs during the reaction SDR-choline or ethanolamine SDR (formed in the reaction with MFR phosphorylation choline and ethanolamine) and 1,2-diglycerides [30, 31]. Cholinephosphate transferase availability in mitochondria, realizing the synthesis of phosphatidylcholine in this way, was shown in investigations [32, 33]. However, the existence of a complete set of mitochondrial enzymes for the phosphatidylcholine and phosphatidylethanolamine biosynthesis de novo is still disputed. Phosphatidylcholine may also be formed from phosphatidylethanolamine by three-stage methylation. A number of studies [34, 35] shows microsomal localization of the process. It was showed, that phospholipids are capable of entering into transalkylation reaction, i.e. the bases exchange [36, 37]. Bases exchange with phosphatidylcholine or phosphatidyl ethanolamine is the only way for phosphatidylserine and phosphatidylinositol formation in animal cells [38]. Simultaneously with transalkylation of phosphatidylethanolamine to phosphatidylserine, phosphatidylserine decarboxylation occurs anew to phosphatidylethanolamine, so the overall process is substantially catalytic cycle of serine decarboxylation to ethanolamine. The latter reacts with the CTP initiating synthesis of new molecules of phospholipids.Thus, the process can regulate the exchange rate and biosynthesis of phospholipids in the cell. In our opinion, in the initial stage of stress in liver cells phosphatidylethanolamine synthesis increases firstly, after 2 weeks phosphatidylcholine synthesis increases from phosphatidylethanolamine three-step methylation. Such a high rate of phospholipids synthesis can not be saved for a long time. In the future, the synthesis of phospholipids due to lack of substrates slows down and approaches the performance standards, and the synthesis was reduced from 1 month experiment.At the beginning of the stress (Week 1) phosphatidylserine content was not changed in liver mitochondria, and phosphatidylinositol slightly decreased. With increasing duration of the stress abovementioned transformation of phosphatidylserine to phosphatidylethanolamine and phosphatidylcholine to phosphatidylethanolamine gradually normalized, and in the future - slowed. At the same time the synthesis of phosphatidylserine remains elevated, phosphatidylinositol synthesis slows and correlates of stress duration. It is known that phosphatidylserine and phosphatidylinositol are formed in the transferase reaction [39]. Phosphatidylserine decarboxylase in a cell is associated with the inner membrane of mitochondria [40], although there is no direct evidence of this fact, the enzyme performs transalkylation of phospholipids can also be localized in the mitochondria. The observed changes, in our opinion, are the direct result of enzymatic reactions of methylation and decarboxylation, occurring in liver tissue [41]. It is believed that enzymes of a base exchange [42] can catalyze the transalkylation reaction in the animals. It was shown in animal tissues, that phospholipase D is capable to catalyze the transalkylation reaction of forming alcoholic phosphatidylethanol in animal organs is catalyzed by phospholipase D, rather than an enzyme of bases exchange [43].
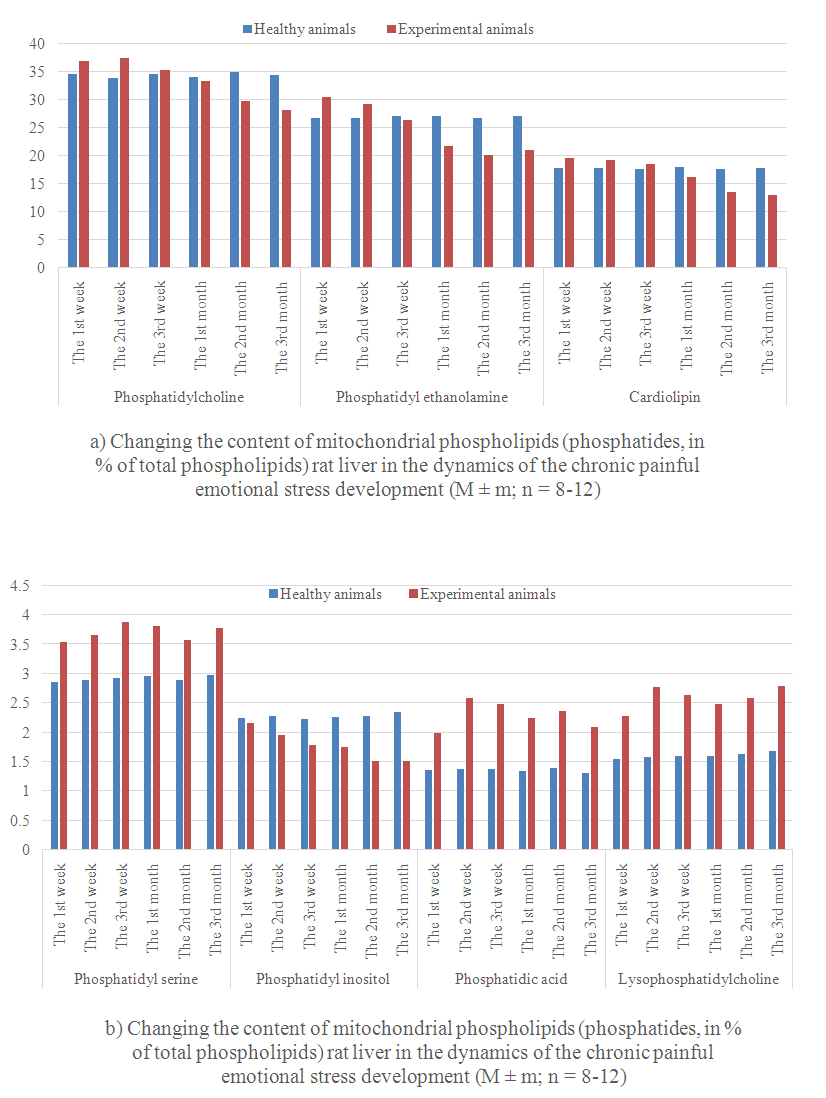 | Figure 1. |
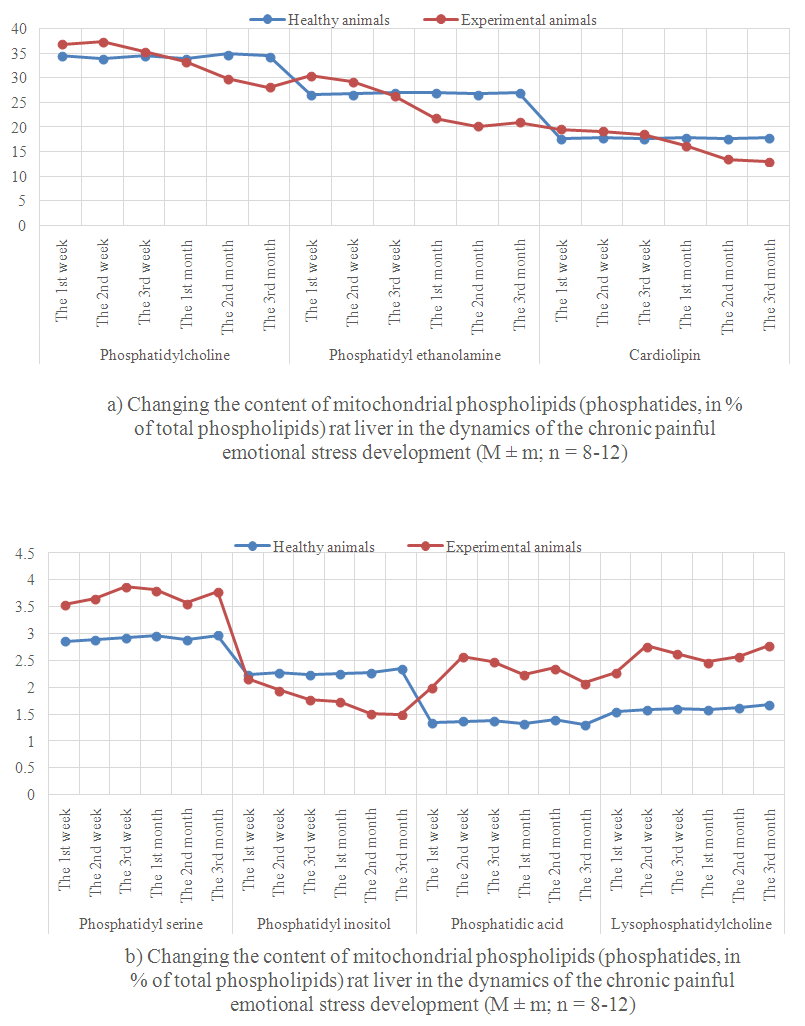 | Figure 2. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML