-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Biochemistry
p-ISSN: 2163-3010 e-ISSN: 2163-3029
2016; 6(2): 46-50
doi:10.5923/j.ajb.20160602.04

Evaluation of Some Lichen Extracts for β-Glucosidase Inhibitory as a Possible Source of Herbal Anti-diabetic Drugs
Hengameh Parizadeh 1, Rajkumar H. Garampalli 2
1Department of Studies in Microbiology, Manasagangotri, University of Mysore, Mysore, Karnataka, India
2Department of Studies in Botany, Manasagangotri, University of Mysore, Mysore, Karnataka, India
Correspondence to: Rajkumar H. Garampalli , Department of Studies in Botany, Manasagangotri, University of Mysore, Mysore, Karnataka, India.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In the present study, lichen species; Herpothallon sp., Parmotrema tinctorum, Leptogium sp., Heterodermia leucomelos, Parmotrema crinitum, Ramalina sinensis, Parmotrema reticulatum and Cladonia subradiata were evaluated for their β-glucosidase inhibitory, which is linked with anti-diabetic effect. Lichens had proved to be promised source of dug discovery in many researches, but this particular aspect of enzyme inhibitory is still not studied properly, so this study was performed in order to find out new natural source of anti-diabetic drugs by inhibition of β-glucosidase enzyme. Overall, All species showed inhibition in one or all their solvents extract. Ethanol was the most suitable solvent to extract glucosidase inhibitory components. R. sinensis (91%), Leptogium sp. (87%), and H. leucomelos (85%) showed highest inhibition which was higher than standard glucosidase inhibitor; acarbose (65%). There was a linear correlation (r=0.5360) between inhibitory activity and phenolic contents. So we can emphasize that Lichen phenolic compounds can be a great natural source of β-glucosidase inhibitors.
Keywords: Anti-diabetic, Lichen, β-glucosidase, Enzyme inhibition
Cite this paper: Hengameh Parizadeh , Rajkumar H. Garampalli , Evaluation of Some Lichen Extracts for β-Glucosidase Inhibitory as a Possible Source of Herbal Anti-diabetic Drugs, American Journal of Biochemistry, Vol. 6 No. 2, 2016, pp. 46-50. doi: 10.5923/j.ajb.20160602.04.
Article Outline
1. Introduction
- β-Glucosidases (β-D-glucoside glucohydrolases) is the key enzyme (Krisch et al., 2010) component present in cellulase and completes the final step during cellulose hydrolysis by converting the cellobiose to glucose. This reaction is always under control as its product (glucose) inhibits it (Singhania et al., 2013). But, a metabolic disorder makes the conversion uncontrollable, so high blood glucose occurs which calls Diabetes. At 2013, diabetes had caused 5.1 million deaths and cost USD 548 billion in healthcare spending. Without concerted action to prevent diabetes, in less than 25 years’ time there will be 592 million people living with the disease. Most of those cases would be preventable (Hirst, 2013). Among the two principal form of diabetes, insulin dependent (type-1) and non insulin dependent (type-2), Non insulin dependent diabetes accounts for 90% of all cases worldwide, due to the body’s inability to respond properly to the action of insulin produced by the pancreas (Notkins, 2002, Malathi et al., 2010). Non-insulin dependent diabetes (NIDDM) is becoming a pandemic and despite the recent surge in new drugs to treat and present the condition, its prevalence continues to soar. In spite of great strides that have been made in understanding and in the management of this disease, serious problems like diabetic retinopathy, diabetic nephropathy and low extremely amputation continues to confront patients and physicians. The graph of diabetes related mortality is rising and reducing the life expectancy to 5 to 15 years (Malathi et al., 2010). In recent years, much interest has been focused on biologically active compounds occurring in natural resources. Wide arrays of plant derived active principles representing numerous chemical compounds have demonstrated activity consistent with their possible use in the treatment of Non-insulin dependent diabetes mellitus (NIDDM) (Bailey and Day, 1989, Malathi et al., 2010). Digestive enzymes have been targeted as potential avenues for modulation of blood glucose concentration through inhibition of the enzymatic breakdown of complex carbohydrates to glucose (Abeysekera et al., 2007). The key enzymes involve in glucose formation are pancreatic amylase and intestinal glucosidase (Abeysekera et al., 2007). Beta Glucosidases catalyze the hydrolysis of alkyl and aryl-β-glycosides as well as disaccharides and short chain oligosaccharides. Many of them show also synthetic activity via reverse hydrolysis or transglycosylation. Alpha-glucosidase (EC 3.2.1.20) enzyme is involved in the digestion of dietary carbohydrates in humans. Inhibition of α-glucosidase activity reported to be involved in the decrease of glucose levels in plasma, as a result suppression of postprandial hyperglycemia (Gao et al., 2008, Verma N, et al., 2012).Beta-glucosidase (EC 3.2.1.21) is another enzyme of hydrolases group, reported to be involved in the processing of glycoproteins. Since many animal viruses including human immunodeficiency virus (HIV-1), human hepatitis B virus (HBV), human cytomegalovirus virus (HCMV) and influenza virus contains an outer envelope composed of glycoproteins, which is important to complete their cycle and infectivity. Beta-glucosidase reported to be involved in the processing of glycoproteins. Therefore, the study of inhibitors for β-glucosidase enzyme is of great importance for the treatment of type 2 diabetes and also treatment of viral diseases (Sánchez-Medina et al., 2001, Simoes-Pires et al., 2009, Mehta et al., 1998, Verma N, et al., 2012). So in this study, β-Glucosidase was chosen as target as its inhibition results in anti-diabetic activity as well as anti-viral activity. Acarbose is an anti-diabetic drug used to treat Diabetes mellitus. It inhibits glycoside hydrolases enzymes which breakdown complex carbohydrates and currently is a common anti-diabetic drug. But it has lots of gastrointestinal side effects. Hepatitis also has been reported with acarbose use (Prescrire and Groc 1998). So still there is urgent need for natural products with fewer side effects to prevent and control diabetes. Many medicinal herbal extracts have been found to inhibit these enzymatic activity and still researches are going on. About 60% of the world population use Traditional medicines derived from medicinal plants. In the past few years, there has been an exponential growth in the field of herbal medicine (Jothi and Brindha, 2014). Herbal extracts are easily available, more affordable and have less side effects as compared to the synthetic antidiabetic drugs. Antidiabetic herbal formulations (AHF) are considered to be more effective for the management of diabetes (Wais et al., 2012). So, natural compounds cab be feasible alternatives for the treatment of diabetes or reinforcements to currently used treatments. They may even reduce the risk of the disease. Large amounts can be consumed in everyday diet, which is a positive aspect (Coman et al., 2012). A great source of medicinally active natural products is Lichen. Lichens are known for their wide biological activates because of their unique medicinally active secondary metabolites (Boustie and Grube 2005). There are many reports on antidiabeteic activity of lichens (Rashmi et al., 2015; Zhang et al., 2012; Karunaratne et al., 2014). In spite of the wide spectrum of biological activities shown by lichens, they have long been neglected by mycologists and overlooked by pharmaceutical industries (Behera et al., 2006), hence this study targeted lichens as a possible herbal source for anti-diabetic activity. β-glucosidases are ubiquitous in the nature and can be found in bacteria, fungi, plants and animals. Their activity is fundamental in many biological pathways, such as degradation of structural and storage polysaccharides, host-pathogen interactions, cellular signalling, and oncogenesis (Krisch, et al., 2010, Bhatia et al., 2002). Beta-Glucosidase inhibitors have been the subject of extensive interest, because of their potential as drugs for the treatment of diabetes, cancer, viral infection and hereditary lysosomal storage diseases (Salazar et al., 2007). But more research was carried out on anti α-glucosidase action of natural products and investigation on their anti β–Glucosidases properties are quit neglected despite their important role in diabetes. So in this study, evaluation of some natural lichen extracts against β–Glucosidases enzyme was performed.
2. Materials and Methods
2.1. Preparation of Lichen Extracts
- Lichen samples were collected from Ooty, Tamil Nadu, India and were identified on the basis of morphology and chemical analysis and literature references (Awasti, 1988; Orange, 2001) as Herpothallon sp., Parmotrema tinctorum, Leptogium sp., Heterodermia leucomelos, Parmotrema crinitum, Ramalina sinensis, Parmotrema reticulatum and Cladonia subradiata. They were washed for removal of debris and were air-dried, then were powdered and subjected to Soxhelt extractor by Hexane, Ethyl acetate, Methanol and Ethanol solvent. After 8 hours of extraction process, Lichen extracts were evaporated completely and the powder forms were stored at 4°C for further study.Anti β-glucosidase activity of lichen extracts:In order to evaluate extracted lichen species for anti glucosidase propertiy, a spectrophotometry method was followed according to Verma et al., 2012. In this method p-nitro phenyl-β-D-glucopyranoside (PNGP) was used as substrate and enzyme inhibitory was measured by % inhibition based on turbidity of each sample. The concept behind O.D measurement here is, more inhibition cause less product conversion, hence sample contains more substrate and more turbidity. A 2 mM p-nitro phenyl-β-D- glucopyranoside (0.5 mL), 0.2 mL lichen extract (20μg/mL) and 50 mM potassium phosphate buffer (0.3 mL) pH 5 were placed in a test tube and incubated at 37° for 10 min in a water bath. A 20 mU of enzyme β-glucosidase (Enzyme activity: 3500U/mg) was added in the mixture and incubated at 37° for 30 min. After completion of incubation period, the enzymatic reaction was terminated by addition of 2.6 mL of potassium phosphate buffer pH 10. The same was done with Acarbose, A commercially available enzyme inhibitory drug to control Diabetes mellitus and final results were compared with this standard drug. For negative control, phosphate buffer of pH 10 was added at the beginning of the reaction to block enzyme activity. Absorbance was measured at 410 nm for each sample. The percentage inhibition calculate using this formula:
% Inhibition = [A 410 Control - A 410 Extract / A 410Control] x 100Correlation between β-glucosidase activity and phenol content:Reports had been shown that high content of phenolic components could inhibit glucosidase activity (Landete et al., 2008; Afrapoli et al., 2012, Wongsa et al., 2012). In order to investigate the effect of phenolic content on glucosidase inhibitory a correlation study was performed by comparing % of inhibition if each extraxt with its phenolic content which was quantified in the previous study on the extracts. Statistical analysis:All tests were done in triplicates and mean of each sample ± SEM was used for calculation and data interpretation. Correlation study was evaluated by GraphPad Prism software Version. 6.1 and pearson’s r near by 1 was assumed as a significant correlation. P<0.05 is considered as significance relationship between data.
3. Results
- β-glucosidase inhibitory properties of lichen extracts:Lichen extracts of four different solvents were subjected to anti β-glucosidase study, in vitro. Results indicated that among all solvent extracts, Ethanolic lichen extracts was more efficient for anti β- glucosidase activity. All ethanolic lichen extracts showed inhibition from 12% (P.reticulatum) to 88% (C.subradiata) while in other extracts P.tinctorum, P.crinitum, C.subradiata and P.reticulatum were completely ineffective. Overall, R.sinensis, Leptogium sp. and H. leucomelos had shown highest inhibitory activity respectively; highest inhibition was observed in R.sinensis by 91% in ethyl acetate extract. Acarbose (20μg/mL) as positive standard anti-diabetic drug, showed 65% inhibition, which is much less than R.sinensis (Fig 1).
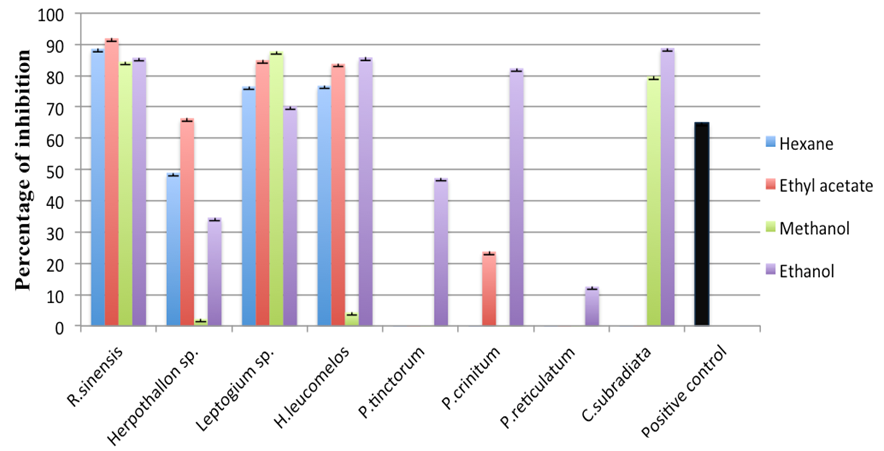 | Figure 1. Percentage of inhibition of each lichen extracts |
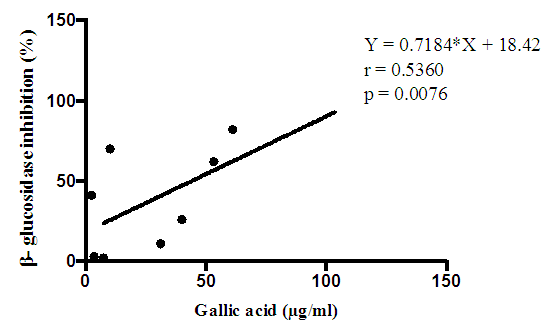 | Figure 2. Correlation between phenolic content and glucosidase inhibitory of lichen species |
4. Discussion
- In the presented study, eight lichen extracts where evaluated for their β- glucosidase inhibitory, in order to find a possible source of antidiabetic herbal drug. It was clearly shown that ethanol is a great solvent to extract lichen samples for their glucosidase activity. Overall R.sinensis with an average (from all extracts) of 87% of inhibition was the most effective extract, which is even higher than standard glucosidase inhibitory drug; Acarbose. As the previous studies hypothesized that more phenolic contents in an extract cause more glucosidase inhibitory, anti β- glucosidase activity of ethanol extract as the most effective one, was compared with their Phenolic contents and a linear relationship was appeared. In the study of Karunaratne et al., 2014, two phenolic compounds, methyl β - orcinolcarboxylate and methylorsellinate had highest glucosidase inhibitory than rest of non-phenolic compounds with 4 - 5 fold higher activity than acarbose. Verma et al., 2012 studied on some lichen extracts and R. celastri had shown α-glucosidase activity almost equivalent to the acarbose. R. nervulosa, R. pacifica and R. celastri showed 89.21%, 69.38% and 61.96% β- glucosidase activity, which was higher than the standard inhibitor (59.98%) and they had concluded that, the extract of lichen species studied here have glucosidase inhibition potential. Compare to these results, still R. sinensis of our study had shown higher inhibition (91%). In some literature its is assumed that high Phenolic compounds have lower inhibitory effect against amylase activity but a stronger inhibition activity against glucosidase and therefore, can potentially be used as effective therapy for postprandial hyperglycaemia with minimal side effects (Kwon et al., 2006). To testify this hypothesis, the correlation study was done and result showed a good relationship among phenolic contents and beta glucosidase activity.
5. Conclusions
- Lichens exhibit a huge array of remarkable biological activities; these properties of lichen substances make them possible pharmaceuticals (Molnar and Farkas, 2009). There is less report on β- glucosidase inhibitory action of herbal extracts including lichens despite key role of this enzyme on diabetes mellitus and wide biological activities of lichens. This study revealed some important features in this regards; to get maximum glucosidase inhibition Ethanol is bets to extract lichen, all studied lichen had enzyme inhibitory with ethanolic extract. Another point was correlation between phenolic contents and glucosidase inhibition. Finally we can conclude that lichen can be a great source for anti-diabetic herbal drugs as they may contain high amount of glucosidase inhibitory compounds.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML